Abstract
Both the amplification of the gene coding for wild-type (wt) epidermal growth factor receptor (EGFR) and the overexpression of the EGFR deletion mutant, commonly known as EGFRvIII, are hallmarks of glioblastoma. We have recently reported a novel, recombinant immunotoxin, D2C7-(scdsFv)-PE38KDEL, that targets both wt EGFR and EGFRvIII, exhibiting potent antineoplastic effects against established murine gliomas.
Gliomas, originating from glial cells, are the most common neoplasms affecting the central nervous system and glioblastoma multiforme is the most malignant subtype of glioma. Surgery, radiotherapy, and chemotherapy based on the alkylating agent temozolomide, only extend the median overall survival of glioblastoma patients by less than 15 mo.Citation1 The dismal prognosis associated with currently approved treatment modalities is generally attributed to the highly heterogeneous nature of glioblastoma. Based on gene expression signature alone, Verhaak and colleagues have classified glioblastomas into classical, mesenchymal, proneural and neural subtypes, each subtype being identified by specific genetic aberrations.Citation2 Studies such as this underscore the importance of characterizing glioblastoma at the molecular level and devising specific and effective therapeutic regimens for glioblastoma patients.
The increased understanding of the molecular pathways that underlie the surge and progression of glioblastoma has led to the development of innovative therapeutic approaches that specifically target malignant cells. A major approach in this sense is represented by genetically engineered single-chain variable fragments (scFv), consisting of the heavy- and light-chain variable regions (VH and VL) of an antibody, fused to plant or bacterial toxins. This therapeutic strategy is being widely evaluated for the treatment of brain tumors.Citation3 Unlike traditional chemotherapeutics, scFv-toxin fusions specifically kill malignant, as opposed to non-transformed, cells, hence causing tumor regression while preserving adjacent normal tissues.
We have recently described the generation of a novel recombinant immunotoxin, D2C7-(scdsFv)-PE38KDEL, that is specific for both wild-type (wt) epidermal growth factor receptor (EGFR) and for its deletion mutant commonly known as EGFRvIII.Citation4 The classical subtype of glioblastoma is defined by EGFR gene amplification,Citation2 and glioblastomas bearing EGFR amplifications often express EGFRvIII.Citation5 This suggest that wt EGFR and EGFRvIII may operate as critical drivers in the genesis of glioblastoma, hence representing ideal targets for targeted anticancer therapies. Of note, D2C7 is a unique monoclonal antibody that recognizes both wt EGFR and EGFRvIII.Citation6
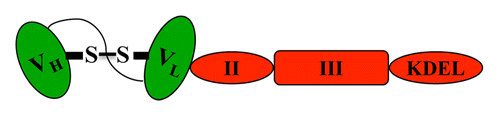
D2C7-(scdsFv)-PE38KDEL was constructed by fusing a 15-amino acid peptide linker, disulfide-stabilized VH and VL fragments derived from D2C7 (D2C7-scdsFv), and the Pseudomonas exotoxin (PE38KDEL) (). D2C7-(scdsFv)-PE38KDEL exhibited potent cytotoxic activity (IC50 = 0.18–2.5 ng/mL) against cultured glioblastoma cells expressing wt EGFR only or co-expressing wt EGFR and EGFRvIII. In vitro, the efficacy of our bispecific fusion protein exceeded that of the wt EGFR-specific transforming growth factor α-based immunotoxin TP-38 and the EGFRvIII-specific immunotoxin MR1–1-PE38KDEL, two Pseudomonas exotoxin-based agents that are currently being evaluated in Phase I clinical trials for glioblastoma therapy.Citation3
Figure 1. Structure of D2C7-(scdsFv)-PE38KDEL. S−S depicts the disulfide bond between the D2C7 heavy (VH) and light (VL) fragments (in green), which are connected by a 15-amino acid peptide linker. The PE38KDEL toxin is depicted in red. II, translocation domain; III, domain that mediates ADP-ribosylation of elongation factor 2; KDEL, leader sequence for increased retention within the endoplasmic reticulum.
The therapeutic success of a tumor-targeting agent depends on its successful delivery to the tumor site at a sufficient concentration, as well as by its uniform distribution throughout the neoplastic lesion. In our preclinical study, we achieved this by convection-enhanced delivery. The continuous intracranial delivery of D2C7-(scdsFv)-PE38KDEL through osmotic mini-pumps resulted in complete coverage of the tumor site, as evidenced by immunohistochemical analyses. This was accompanied by strong antineoplastic effects against intracranial glioblastoma xenografts expressing wt EGFR only or co-expressing wt EGFR and EGFRvIII, increasing the survival of tumor-bearing animals.
Small tyrosine kinase inhibitors (TKIs) that target the EGFR signaling cascade, such as gefitinib, erlotinib, or lapatinib, have been unsuccessfully tested for the treatment of glioblastoma patients.Citation7 Moreover, the expression levels and activation status of relevant signal transducers including wt EGFR, EGFRvIII, AKT1 as well as phosphatase and tensin homolog (PTEN) failed to predict clinical responses to TKIs. Furthermore, TKIs turned out to be ineffective against EGFRvIII, which is constitutively active and confers resistance to wt EGFR-targeting agents.Citation8 Thus, the multifaceted regulation of the tyrosine kinase signaling cascade emanating from EGFR renders glioblastomas unresponsive to TKI-based therapy. Unlike TKIs, the antineoplastic activity of D2C7-(scdsFv)-PE38KDEL is independent of the tyrosine kinase signaling cascade triggered by EGFR, but depends solely on the expression of wt EGFR or EGFRvIII. Therefore, D2C7-(scdsFv)-PE38KDEL should be active in glioblastoma patients expressing wt EGFR only or co-expressing wt EGFR and EGFRvIII.
Several anti-EGFR antibodies that inhibit ligand binding have been developed. Nimotuzumab, a humanized EGFR-specific monoclonal antibody, demonstrated promising results in both adult and pediatric high-grade glioma patients.Citation9 However, the administration of cetuximab, which is specific for wt EGFR, only improved the progression-free survival of patients bearing EGFR amplifications but lacking EGFRvIII expression.Citation10 As the majority of gliomas expressing wt EGFR also express the constitutively active variant EGFRvIII, a combinatorial approach targeting both these tumor-associated antigens is needed to tackle this complex disease. To the best of our knowledge, D2C7-(scdsFv)-PE38KDEL is the first therapeutic agent with similar affinity and efficacy toward both wt EGFR and EGFRvIII.
D2C7 interacts with a 55-amino acid epitope present in the extracellular domain of wt EGFR (residues 583–637) and EGFRvIII (residues 292–346). Of note, this epitope persists in several EGFR deletion mutants, including C-958, Δ959–1030, Δ6–185, I, and III–VII, that are associated with glioblastoma.Citation4 This increases the number of antigenic targets for D2C7-(scdsFv)-PE38KDEL in glioblastoma patients, suggesting that this agent may be efficient against tumors that are resistant to TKIs and naked EGFR-specific monoclonal antibodies.
In conclusion, our preclinical data suggest that the bispecific immunotoxin D2C7-(scdsFv)-PE38KDEL may provide an effective therapeutic option for glioblastoma patients expressing wt EGFR only or co-expressing wt EGFR and EGFRvIII. A good laboratory practice batch of D2C7-(scdsFv)-PE38KDEL has been prepared and further studies are ongoing to determine the maximal tolerated dose in rats, in order to submit to the US Food and Drug Administration for an investigational new drug application to launch Phase I clinical trial. Future clinical studies will determine whether D2C7-(scdsFv)-PE38KDEL can be included as a standard treatment for glioblastoma patients expressing wt EGFR only or co-expressing wt EGFR and EGFRvIII.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Citation: Chandramohan V, Bigner DD. A novel recombinant immunotoxin-based therapy targeting wild-type and mutant EGFR improves survival in murine models of glioblastoma. OncoImmunology 2013; 2:e26852; 10.4161/onci.26852
References
- Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, Belanger K, Brandes AA, Marosi C, Bogdahn U, et al, European Organisation for Research and Treatment of Cancer Brain Tumor and Radiotherapy Groups, National Cancer Institute of Canada Clinical Trials Group. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med 2005; 352:987 - 96; http://dx.doi.org/10.1056/NEJMoa043330; PMID: 15758009
- Verhaak RG, Hoadley KA, Purdom E, Wang V, Qi Y, Wilkerson MD, Miller CR, Ding L, Golub T, Mesirov JP, et al, Cancer Genome Atlas Research Network. Integrated genomic analysis identifies clinically relevant subtypes of glioblastoma characterized by abnormalities in PDGFRA, IDH1, EGFR, and NF1. Cancer Cell 2010; 17:98 - 110; http://dx.doi.org/10.1016/j.ccr.2009.12.020; PMID: 20129251
- Chandramohan V, Sampson JH, Pastan I, Bigner DD. Toxin-based targeted therapy for malignant brain tumors. Clin Dev Immunol 2012; 2012:480429; http://dx.doi.org/10.1155/2012/480429; PMID: 22400035
- Chandramohan V, Bao X, Keir ST, Pegram CN, Szafranski SE, Piao H, Wikstrand CJ, McLendon RE, Kuan CT, Pastan IH, et al. Construction of an immunotoxin, D2C7-(scdsFv)-PE38KDEL, targeting EGFRwt and EGFRvIII for brain tumor therapy. Clin Cancer Res 2013; 19:4717 - 27; http://dx.doi.org/10.1158/1078-0432.CCR-12-3891; PMID: 23857604
- Frederick L, Wang XY, Eley G, James CD. Diversity and frequency of epidermal growth factor receptor mutations in human glioblastomas. Cancer Res 2000; 60:1383 - 7; PMID: 10728703
- Zalutsky MR, Boskovitz A, Kuan CT, Pegram CN, Ayriss J, Wikstrand CJ, Buckley AF, Lipp ES, Herndon JE 2nd, McLendon RE, et al. Radioimmunotargeting of malignant glioma by monoclonal antibody D2C7 reactive against both wild-type and variant III mutant epidermal growth factor receptors. Nucl Med Biol 2012; 39:23 - 34; http://dx.doi.org/10.1016/j.nucmedbio.2011.06.005; PMID: 21958852
- Hegi ME, Rajakannu P, Weller M. Epidermal growth factor receptor: a re-emerging target in glioblastoma. Curr Opin Neurol 2012; 25:774 - 9; http://dx.doi.org/10.1097/WCO.0b013e328359b0bc; PMID: 23007009
- Tanaka K, Babic I, Nathanson D, Akhavan D, Guo D, Gini B, Dang J, Zhu S, Yang H, De Jesus J, et al. Oncogenic EGFR signaling activates an mTORC2-NF-κB pathway that promotes chemotherapy resistance. Cancer Discov 2011; 1:524 - 38; http://dx.doi.org/10.1158/2159-8290.CD-11-0124; PMID: 22145100
- Chandramohan V, Mitchell DA, Johnson LA, Sampson JH, Bigner DD. Antibody, T-cell and dendritic cell immunotherapy for malignant brain tumors. Future Oncol 2013; 9:977 - 90; http://dx.doi.org/10.2217/fon.13.47; PMID: 23837761
- Lv S, Teugels E, Sadones J, De Brakeleer S, Duerinck J, Du Four S, Michotte A, De Grève J, Neyns B. Correlation of EGFR, IDH1 and PTEN status with the outcome of patients with recurrent glioblastoma treated in a phase II clinical trial with the EGFR-blocking monoclonal antibody cetuximab. Int J Oncol 2012; 41:1029 - 35; PMID: 22752145