Abstract
Cross-priming plays a major role in generating CD8+ T cell-dependent antitumor immunity through cross-presentation. However, the cross-presentation of tumor-associated antigens by dendritic cells often promotes tolerance rather than CD8+ T-cell immunity. We have now identified a β-catenin-dependent pathway of cross-priming inhibition as a novel and potentially broad mechanism whereby neoplastic cells promote immunosuppression.
As the initiators of antigen-specific immune responses, dendritic cells (DCs) play a central role in regulating the balance between CD8+ T-cell immunity and tolerance to tumor-associated antigens (TAAs). The tumor microenvironment often recruits immunosuppressive cells and releases soluble factors that attenuate the activity of DCs, such as vascular endothelial growth factor (VEGF), indoleamine 2,3-dioxygenase 1 (IDO1), arginase, transforming growth factor β1 (TGFβ1), and prostaglandins, hence limiting the therapeutic potential of DC-based anticancer vaccines.Citation1-Citation3 With the exception of sipuleucel-T (Provenge®), current DC-based vaccines remain unsuccessful, and one major obstacle in this sense is the immunosuppressive activity of host DCs. Cross-priming, the process whereby DCs activate CD8+ T cells by cross-presenting TAAs on MHC class I molecules, plays a major role in generating antitumor CD8+ T-cell immunity.Citation4,Citation5 However, the DC compartment of tumor-bearing hosts is often defective or tolerogenic, being unable to induce productive CD8+ T-cell responses upon TAA cross-presentation.Citation4 While some chemotherapeutic regiments are well known to promote the cross-presentation of TAAs,Citation2,Citation4 whether and how neoplastic cells directly interfere with cross-priming to suppress antitumor CD8+ T-cell immunity has not been elucidated until recently.
We and others have previously shown that β-catenin regulates DC-mediated CD4+ T-cell responses and promotes T-cell tolerance in murine models of autoimmune diseases,Citation6,Citation7 suggesting that β-catenin serves as a tolerizing signal that shifts the balance between CD4+ T-cell immunity and tolerance. Although these studies primarily examined the function of CD4+ T cells, tumors likely employ similar mechanisms to influence DC-mediated CD8+ T-cell immunity vs. tolerance. We thus wondered whether β-catenin signaling in DCs also suppresses antitumor CD8+ T-cell immunity, and—if so—how neoplastic cells might harness β-catenin to suppress antitumor CD8+ T-cell responses. We found elevated expression levels of β-catenin in DCs from mice bearing B16 melanomas.Citation8 The tumor-mediated upregulation of β-catenin in DCs appears to be systemic, as opposed to local, since elevated β-catenin levels were observed not only in DCs isolated from tumor-draining lymph nodes, but also in splenic DCs and DCs obtained from mesenteric lymph nodes. Exposing DCs to tumor-conditioned culture media in vitro also led to upregulation of β-catenin, suggesting that this effect is due (at least in part) to the release of one or more soluble factors by malignant cells. A genetic manipulation that resulted in the constitutive activation of β-catenin in DCs (DC-β-cateninactive mice) significantly accelerated tumor growth in multiple models of neoplasia, suggesting that the activation of β-catenin in DCs negatively regulates antitumor immunity.
Both tumor-bearing and DC-β-cateninactive mice, when vaccinated with a DC-targeting monoclonal antibody (specific for lymphocyte antigen 75, LY75, best known as DEC-205) fused with model antigen ovalbumin (OVA), exhibited impaired primary and recall OVA-specific CD8+ T-cell responses, suggesting that activation of β-catenin in DCs (be it genetic or induced by tumors) negatively regulates CD8+ T-cell immunity. Both tumor-bearing and DC-β-cateninactive mice were deficient in cross-priming but not cross-presentation, as measured by antigen presentation assays in vivo. In addition, antigen-specific CD8+ T cells primed in DC-β-cateninactive and tumor-bearing mice mediated suboptimal CD8+ memory responses when transferred into naïve wild-type mice, suggesting that deficiencies in the cross-priming phase contribute to dampened CD8+ T-cell immunity. Further studies revealed that such a deficiency in cross-priming is DC-intrinsic, as DCs isolated from immunized DC-β-cateninactive and tumor-bearing mice also exhibited impaired cross-priming when cultured with OVA-specific CD8+ T cells ex vivo. Importantly, the DC-specific deletion of the β-catenin-coding gene (DC-β-catenin−/− mice) completely abrogated tumor-induced inhibition of cross-priming, confirming that this immunosuppressive pathway is dependent on β-catenin. Thus, neoplastic cells activate β-catenin in DCs to inhibit cross-priming, hence impairing CD8+ T-cell immunity. Given the importance of cross-priming in generating antitumor CD8+ T cell-mediated immune responses, our findings suggest that the activation of β-catenin in DCs may serve as a general mechanism for tumors to evade immunosurveillance.
We further asked whether β-catenin-mediated inhibition of cross-priming is reversible, and—if so—whether enhancing cross-priming results in restored antitumor CD8+ T-cell immunity. By testing various approaches to the use of Toll-like receptor agonists as adjuvants, we were able to select an immunization protocol that restored cross-priming in DC-β-cateninactive mice. Not surprisingly, CD8+ memory responses were also restored in these mice, suggesting that the β-catenin-dependent inhibition of cross-priming can be reversed to restore impaired CD8+ immunity. As β-catenin might similarly impair the ability of DCs to activate CD8+ memory T cells during the recall phase, we reasoned that enhancing cross-priming during the recall phase might restore CD8+ memory responses. Indeed, in both DC-β-cateninactive and tumor-bearing mice, CD8+ immunity was substantially boosted upon recall with antigens that favor cross-priming. These findings indicate that strong antitumor CD8+ immunity can be achieved upon recall with agents that promote cross-priming even when the initial DC-based vaccines fail, offering a new approach to improve DC-based anticancer vaccines that elicit weak antitumor responses. Further studies are warranted to examine whether these approaches are applicable to various DC-based vaccines.
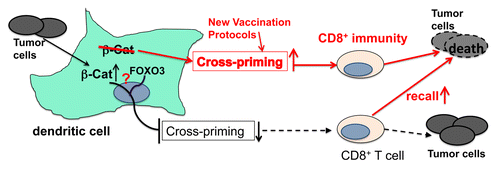
Understanding how β-catenin regulates the ability of DCs to cross-prime antigen specific CD8+ T cells requires further studies, although the maturation state of DCs and their cytokine production profile are likely involved.Citation6,Citation7 Interestingly, TADCs have recently been shown to express elevated levels of both β-catenin and forkhead box O3 (FOXO3). FOXO3 appears to render TADCs tolerogenic and to induce an immunosuppressive activity in tumor-specific CD8+ T cells, presumably as it influences the maturation state of DCs and their cytokine production pattern.Citation9 An evolutionarily conserved interaction between β-catenin and FOXO3 has been shown to regulate the transcriptional activity of both FOXO3 and β-catenin/T-cell factor (TCF),Citation10 suggesting a scenario in which the crosstalk between FOXO3 and β-catenin in DCs ultimately determines DC function ().
Figure 1. Tumors suppress CD8+ T-cell immunity by a β-catenin-dependent pathway that inhibits cross-priming. Tumors activate β-catenin in dendritic cells (DCs), most likely through the release of one or more soluble factors. The activation of β-catenin in DCs inhibits the cross-priming of antigen-specific CD8+ T cells, dampening both primary and recall CD8+ T-cell responses. The DC-specific deletion of the β-catenin-coding gene completely abrogates the ability of malignant cells to inhibit cross-priming. β-catenin-dependent inhibition of cross-priming is reversible, as cross-priming can be restored by modifying immunization protocols. Thus, CD8+ T-cell immunity can be rescued by enhancing cross-priming at the priming or recall stage. Tumor-associated dendritic cells (TADCs) also exhibit increased expression levels of forkhead box O3 (FOXO3), and the cross-talk between FOXO3 and β-catenin likely determines the function of this DC subset.
| Abbreviations: | ||
| DC | = | dendritic cell |
| TAA | = | tumor-associated antigen |
| TADC | = | tumor-associated dendritic cell |
| OVA | = | ovalbumin |
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Citation: Fu C, Jiang A. β-catenin-mediated inhibition of cross-priming: A new mechanism for tumors to evade immunosurveillance. OncoImmunology 2013; 2:e26920; 10.4161/onci.26920
References
- Mellman I, Coukos G, Dranoff G. Cancer immunotherapy comes of age. Nature 2011; 480:480 - 9; http://dx.doi.org/10.1038/nature10673; PMID: 22193102
- Apetoh L, Locher C, Ghiringhelli F, Kroemer G, Zitvogel L. Harnessing dendritic cells in cancer. Semin Immunol 2011; 23:42 - 9; http://dx.doi.org/10.1016/j.smim.2011.01.003; PMID: 21295491
- Shurin GV, Ouellette CE, Shurin MR. Regulatory dendritic cells in the tumor immunoenvironment. Cancer Immunol Immunother 2012; 61:223 - 30; http://dx.doi.org/10.1007/s00262-011-1138-8; PMID: 22065047
- Melief CJ. Cancer immunotherapy by dendritic cells. Immunity 2008; 29:372 - 83; http://dx.doi.org/10.1016/j.immuni.2008.08.004; PMID: 18799145
- Andersen BM, Ohlfest JR. Increasing the efficacy of tumor cell vaccines by enhancing cross priming. Cancer Lett 2012; 325:155 - 64; http://dx.doi.org/10.1016/j.canlet.2012.07.012; PMID: 22809568
- Jiang A, Bloom O, Ono S, Cui W, Unternaehrer J, Jiang S, Whitney JA, Connolly J, Banchereau J, Mellman I. Disruption of E-cadherin-mediated adhesion induces a functionally distinct pathway of dendritic cell maturation. Immunity 2007; 27:610 - 24; http://dx.doi.org/10.1016/j.immuni.2007.08.015; PMID: 17936032
- Manicassamy S, Reizis B, Ravindran R, Nakaya H, Salazar-Gonzalez RM, Wang YC, Pulendran B. Activation of beta-catenin in dendritic cells regulates immunity versus tolerance in the intestine. Science 2010; 329:849 - 53; http://dx.doi.org/10.1126/science.1188510; PMID: 20705860
- Liang X, Fu C, Cui W, Ober-Blöbaum JL, Zahner SP, Shrikant PA, Clausen BE, Flavell RA, Mellman I, Jiang A. β-Catenin mediates tumor-induced immunosuppression by inhibiting cross-priming of CD8+ T cells. J Leukoc Biol 2013; http://dx.doi.org/10.1189/jlb.0613330; PMID: 24023259
- Watkins SK, Zhu Z, Riboldi E, Shafer-Weaver KA, Stagliano KE, Sklavos MM, Ambs S, Yagita H, Hurwitz AA. FOXO3 programs tumor-associated DCs to become tolerogenic in human and murine prostate cancer. J Clin Invest 2011; 121:1361 - 72; http://dx.doi.org/10.1172/JCI44325; PMID: 21436588
- Hoogeboom D, Burgering BM. Should I stay or should I go: beta-catenin decides under stress. Biochim Biophys Acta 2009; 1796:63 - 74; http://dx.doi.org/10.1016/j.bbcan.2009.02.002; PMID: 19268509