Abstract
The prognosis of patients with advanced pancreatic cancer is extremely poor and there are only a few standard treatments. Here, we report the results of a Phase I clinical trial to investigate the safety, immunostimulatory effects, and antineoplastic activity of a multi-target vaccine composed of four distinct peptides derived from cancer-testis (CT) antigens and vascular endothelial growth factor receptors (VEGFRs). Nine patients with unresectable, advanced pancreatic cancer who were refractory to standard chemotherapy were enrolled. Each patient was vaccinated with HLA-A*2402-restricted peptides derived from the CT antigens kinesin family member 20A (KIF20A) and cell division cycle-associated 1 (CDCA1) as well as from VEGFR1 and VEGFR2 subcutaneously once a week, and disease progression was evaluated up to 6 mo later. Adverse events were assessed using the Common Terminology Criteria for Adverse Events v. 3.0. Immunological responses were monitored by ELISPOT assays and flow cytometry based on peptide-specific dextramers. The clinical outcomes that were measured were tumor response, progression-free survival (PFS) and overall survival (OS). In general, the multi-peptide vaccine was well-tolerated, and no grade 3 or 4 adverse events were observed upon vaccination. Peptide-specific T-cell responses were detected in all 9 patients, and clinical benefits were observed in four of them. Median PFS and OS were 90 and 207 d, respectively. The elicitation of multiple and robust peptide-specific T-cell responses as well as the status of host lymphocytes may be useful prognostic factors among patients with advanced pancreatic cancer treated with peptide-based anticancer vaccines.
Introduction
Pancreatic cancer is a common disease worldwide and its incidence is gradually increasing. Pancreatic cancer is associated with a high mortality rate because most cases are not diagnosed until they are advanced and inoperable.Citation1 Nowadays, very few standard treatments have been established for the treatment of this deadly disease,Citation2 implying that new therapeutic modalities are urgently needed. Anticancer vaccines based on synthetic peptides have been developed several laboratories worldwide, and their safety and clinical efficacy are documented by an abundant literature.Citation3,Citation4 We have previously reported that peptide-based anticancer vaccines are capable of inducing antigen-specific cytotoxic T lymphocyte (CTL) responses in vivo and of providing clinical benefits to some patients with advanced colorectal carcinomaCitation5 or biliary tract cancer.Citation6 In the present study, we selected 4 peptides, 2 of which deriving from cancer-testis (CT) antigens and 2 of which deriving from vascular endothelial growth factor receptors (VEGFRs), that were identified by cDNA microarray technology coupled with laser microdissection to be overexpressed by close to 100% of pancreatic cancer cells and the associated endothelium. In particular, we performed a Phase I clinical study to assess the safety, immunostimulatory potential, and therapeutic profile of a multi-peptide vaccine in patients with advanced pancreatic cancer. Patients were vaccinated on a continuous basis over a long-term until their disease had progressed, at which time we assessed the safety, immunological and clinical parameters. Here, we report the immunological responses to such a multi-peptide vaccine in anticipation of a Phase II clinical trial that will evaluate the clinical profile of this immunotherapeutic anticancer intervention.
Results
Patient characteristics
Nine patients (6 men and 3 women; median age: 65.8 y; age range: 52–78 y) whose HLA type was A*2402 were enrolled in this study (). Their primary tumor site was the pancreas head in 4 cases, the pancreas body in 4 cases, and the pancreas tail in 1 case. All patients had several metastases to the liver, lymph nodes, or peritoneum. The previous therapies received by these individuals consisted of gemcitabine (GEM), tegafur plus gimeracil plus oteracil potassium (TS-1), cisplatin (CDDP), or uracil plus tegafur (UFT). One patient was also exposed to radiation therapy.
Table 1. Patient characteristics
Assessment of toxicity
We assessed toxicity using Common Terminology Criteria for Adverse Events (CTCAE) v3.0. Two of the patients developed a grade 1 injection site reaction while 7 developed a grade 2 injection site reaction. Low hemoglobin, white blood cell, neutrophil, and platelet counts were observed before the 1st vaccination, but did not worsen throughout the study period, and no other severe adverse events over grade 3 were seen in this time frame. Thus, the multi-peptide vaccine that we employed was well-tolerated up to a dose of 3 mg per peptide (9 mg total) during the 6 mo of the study.
Antigen-specific immune responses
Peptide-specific CTL responses were documented by ELISPOT assays in all 9 patients enrolled in the study. We determined the response to each specific antigen in every patient using the algorithm described in Figure S1. The results of this study are summarized in Figure S2. The number of peptide-specific interferon γ (IFNγ) spots per section increased with the number of vaccinations, a trend that continued for the entire duration of the study. CDCA1-specific CTLs were shown to increase upon vaccination by HLA*A2402/CDCA1 dextramers and flow cytometry. (). The number of CDCA1-specific IFNγ spots increased according to a similar trend (). The same applied to VEGFR2-specific CTLs and IFNγ spots (). The immune responses elicited by our multi-peptide vaccine were not the same for all antigens in a specific patient, nor for the same antigen across different patients. Strong CTL responses against KIF20A-, CDCA1-, and VEGFR2-derived peptides were indeed more frequent than robust responses to VEGFR1-derived peptides. The ability of the vaccine to induce a strong T-cell response seemed to linked not only to the nature of the epitope but also the status of the host immune system.
Figure 1. Immunological monitoring of the response of one patient to CDCA1-targeting vaccination. (A) Pre-vaccination lymphocytes were analyzed by flow cytometry using HLA-A2402/CDCA1 dextramers in combination with anti-CD8 monoclonal antibodies. (B) Interferon γ (IFNγ) secretion by lymphocytes isolated from patient n° 2 before vaccination and exposed to TISI cells pulsed with CDCA1-derived peptides, as monitored by ELISPOT assays. (C) Lymphocytes isolated from patient n° 2 after the 4th cycle of vaccination were analyzed by flow cytometry using HLA*A2402/CDCA1 dextramers in combination with anti-CD8 monoclonal antibodies. (D) IFNγ secretion by lymphocytes isolated from patient n° 2 after the 4th cycle of vaccination and exposed to TISI cells pulsed with CDCA1-derived peptides, as monitored by ELISPOT assays. In B and D, responder-to-stimulator (R/S) cell ratios were 1, 0.5, 0.025, and 0.13.
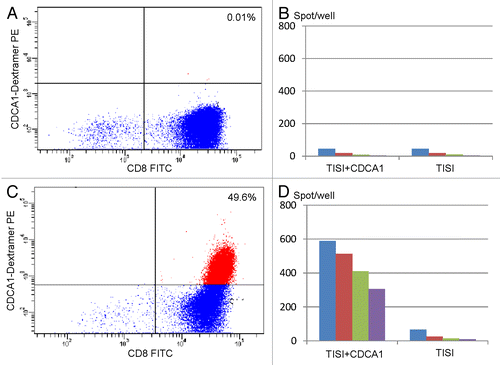
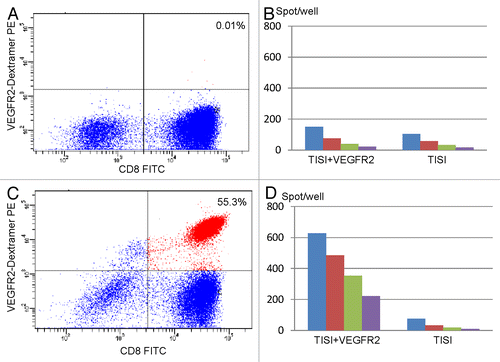
Figure 2. Immunological monitoring of the response of one patient to VEGFR2-targeting vaccination. (A) Pre-vaccination lymphocytes were analyzed by flow cytometry using HLA*A2402/VEGFR2 dextramers in combination with anti-CD8 monoclonal antibodies. (B) Interferon γ (IFNγ) secretion by lymphocytes isolated from patient n° 1 before vaccination and exposed to TISI cells pulsed with VEGFR2-derived peptides, as monitored by ELISPOT assays. (C) Lymphocytes isolated from patient n° 1 after the 4th cycle of vaccination were analyzed by flow cytometry using HLA-A2402/VEGFR2 dextramers in combination with anti-CD8 monoclonal antibodies. (D) IFNγ secretion by lymphocytes isolated from patient n° 2 after the 4th cycle of vaccination and exposed to TISI cells pulsed with VEGFR2-derived peptides, as monitored by ELISPOT assays. In B and D, responder-to-stimulator (R/S) cell ratios were 1, 0.5, 0.025, and 0.13.
Clinical responses
The clinical responses of patients enrolled in this study are summarized in . Four patients manifested stable disease (SD) and 5 progressive disease (PD). The 4 patients who achieved SD plus of those who exhibited PD wished to receive optional rounds of vaccination and continued the study for up to 6 mo. Eventually, the disease progressed in all 9 patients and they all succumb to pancreatic cancer within 3 y. The median progression-free survival (PFS) of these patients upon vaccination was 90 d (95% CI: 11–169 d), while 1-y PFS was 0% (). The median overall survival (OS) of this cohort was 207 d (95% CI: 93–321 d) and the 1-y OS was 22.2% (). According to the univariate analysis of prognostic factors, patients who developed multiple and robust CTL responses to the vaccine exhibited an improved prognosis (). Patients with a relatively high lymphocyte counts also exhibited improved disease outcome as compared with individuals with a poor lymphocytic compartment.
Table 2. Clinical outcomes and immunological responses
Figure 3. Progression-free and overall survival of the patients enrolled in this study. (A) Progression-free survival (PFS) after the 1st cycle of vaccination. The median survival time (MST) was 90 d (95% CI: 11–169 d) and the 1-y PFS ratio was 0%. (B) Overall survival (OS) after the 1st cycle of vaccination. The MST was 207 d (95% CI: 93–321 d) and the 1-y OS ratio was 22.2%.
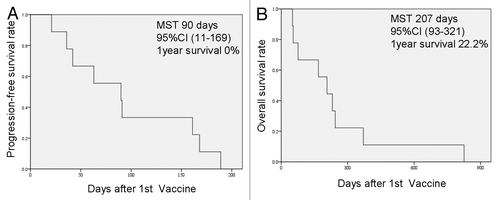
Table 3. Prognostic factors for progression-free and overall survival
Discussion
Pancreatic cancer is well known as a neoplasm associated with an extremely poor prognosis. Surgery in the early stages of disease is the only curative treatment for pancreatic cancer patients, but unfortunately most of these lesions are not found until late disease stages. There are only a few standard chemotherapeutic regimens employed in this setting: GEM, TS-1, or CDDP. The PFS and OS rates achieved with these treatments are similar to those obtained with the multi-peptide vaccine presented here, though our patients were enrolled after the failure of standard chemotherapy. This observation suggests that peptide-based anticancer vaccines might improve the PFS and OS of pancreatic cancer patients. Similarly to recent reports on the therapeutic activity of peptide-based anticancer vaccination, we observed no complete remissions or partial responses in the present study, but an apparent improvement in OS. We should now plan a Phase II clinical study to assess the therapeutic profile of our multi-peptide vaccine in a randomized setting.
Here we focused on the induction of CTL responses targeting not only CT antigens, but also VEGFRs, which are highly expressed by cancer-associated endothelial cells. One of the crucial factors in the escape of neoplastic cells from immunosurveillance is the downregulation of HLA antigens. CTLs are not able to react against malignant cells that do not express HLA, and this frequently occurs in the course of oncogenesis or tumor progression. In our approach, CTLs are able to respond to VEGFR expressed by the tumor vasculature even if cancer cells do not express HLA molecules. Our multi-peptide vaccine should therefore work in any HLA situation. Our findings demonstrate that multi-peptide anticancer vaccines are able to elicit CTL responses specific for each of the vaccine components in all patients. Thus, multi-peptide vaccines might represent a valuable candidate for the treatment of pancreatic cancer.
So far, anticancer vaccination has been tested in several clinical trials, but only one vaccine, namely sipuleucel-T (trade name Provenge®) is available for clinical use. This preparation has been approved by the US FDA in 2011.Citation7 Many Phase III clinical trials testing anticancer vaccines have failed for a variety of reasons.Citation8 It is thought that the efficacy of therapeutic anticancer vaccines is largely influenced by the conditions of the host immune system, and that a new classification for candidate patients is therefore needed to ensure the clinical success of such an approach.Citation9,Citation10 Our results indicate that pancreatic patients with relatively good lymphocyte counts achieve a better prognosis that patients with a poor lymphocyte status. Thus the conditions of the host immune system are crucial for anticancer vaccines to elicit robust immune responses and mediate clinically-relevant effects. In an attempt to further elucidate the relationship between immune parameters of the hosts and the therapeutic profile of anticancer vaccine, data from a Phase II study to be analyzed with a multivariate regression model is required.
Although multi-peptide vaccines are valuable candidate for the treatment of pancreatic cancer, their clinical efficacy is currently limited. One of the major obstacles against the efficacy of such an immunotherapeutic strategy is related to immunosuppression. Regulatory T cells are well known to play a critical role in this setting. Accordingly, non-myeloablative chemotherapy to deplete regulatory T cells is a promising approach to overcome immunosuppression.Citation11 Chemokine (C-C motif) receptor 4 (CCR4) antagonists as well as anti-CCR4 monoclonal antibodies, one of which have already been approved in Japan for use in cancer patients, might also constitute useful tool against immunosuppression, as regulatory T cells express CCR4.Citation12,Citation13 Another method to circumvent this issue, based on the antineoplastic agent denileukin diftitox, has also been examined in animal and human models.Citation14,Citation15 Finally, the blockade of immunological checkpoint is crucial for obtaining robust anticancer immune responses. Ipilimumab (an monoclonal antibodies specific for cytotoxic T lymphocyte-associated protein 4, CTL4),Citation16 as well as antibodies targeting programmed cell death 1 (PDCD1, best known as PD-1)Citation17,Citation18 and its major ligand (CD274, best known as PD-L1)Citation19 showed very promising results in clinical studies. Combining these agents with an anticancer vaccine may constitute an efficient means of boosting the clinical activity of the latter.Citation20
Several peptides derived from tumor-associated antigens have already been tested in clinical trials.Citation21-Citation25 In the present study, we selected peptides from 4 distinct antigens, inducing strong immune responses in vivo. KIF20ACitation26 is a conserved motor domain that binds to microtubules, while CDCA1Citation27 is a molecular linker between the kinetochore attachment site and tubulin subunits. Both KIF20A and CDCA1 are overexpressed by pancreatic cancers. Conversely, VEGFR1 and VEGFR2Citation28 are expressed on the tumor endothelium. Some of these peptides have been used separately or in different combinations for the treatment of non-small cell lung carcinoma, renal cell carcinoma, or pancreatic cancer. Our study is the first to report on the use of a four-peptide vaccine that simultaneously target cancer cells and the tumor endothelium in pancreatic cancer patients. Before this approach can be considered as a candidate for the treatment of patients with pancreatic cancer, it will be necessary to test its therapeutic potential in randomized a Phase II clinical trial.
Materials and Methods
Patient eligibility
Patients with unresectable pancreatic cancer who were refractory to standard chemotherapy were eligible for this study. All patients were required to express HLA-A molecules of the A*2402 type. Additional inclusion criteria were age between 20 and 80 y, no severe functional impairment of organs, white blood cell counts between 2000 and 10000/mm3, hemoglobin > 8 mg/dL, platelet counts > 100,000/mm3, AST and ALT < 100IU/L, and total bilirubin < 2 mg/dL. Performance status as measured by the ECOG scale was 0 to 2. An interval of at least 4 weeks since the last chemotherapy was required. Exclusion criteria encompassed pregnancy, serious infections, severe underlying diseases, severe allergic diseases and a judgment of unsuitability by the principal investigator.
Study design and endpoints
This was a Phase I study. Patients who received standard chemotherapy under a diagnosis of inoperable pancreatic cancer between May 2009 and August 2009 were invited to participate after providing their informed consent. The HLA-A genotypes of these patients were examined, and 9 patients with HLA-A*2402 were enrolled. Four peptides were used for the vaccine, which were derived from KIF20A (KVYLRVRPLL), CDCA1 (VYGIRLEHF), VEGFR1 (DYLNEWGSRF), and VEGFR2 (RFVPDGNRI). These peptides were chosen among antigens identified by a cDNA microarray technology coupled with laser microdissection as highly overexpressed by pancreatic cancer cells or the associated endothelium. We determined the purity (> 97%) of the peptides by analytical high-performance liquid chromatography (HPLC) coupled to mass spectrometry. We tested both the endotoxin levels and bioburden of these peptides and found them to be within acceptable levels based on GMP grade vaccines (PolyPeptide or NeoMPS Inc.). Peptides were mixed with incomplete Freund's adjuvant (IFA, also known as Montanide ISA51, from SEPPIC) which has been used in many clinical studies, and were injected subcutaneously (at doses of 1, 2, or 3 mg per peptide) once a week into the inguinal or the axillar site before the judgment of disease progression, for up to 6 mo. The endpoints of the study were the assessment of toxicities caused by vaccination based on CTCAE v.3.0, immunological responses, tumor responses, progression-free survival (PFS) and overall survival (OS) from the first administration of the vaccine. Assessments were performed every 4 vaccinations. This study was approved by the institutional review board at Tokyo Women's Medical University and was registered with the University Hospital Medical Information Network Clinical Trials Registry (UMIN-CTR number, 000004337). Informed consent was obtained from all patients, and all procedures were in accordance with the Declaration of Helsinki.
Lymphocyte preparation for immunomonitoring
Immunological assays were periodically standardized and validated by Clinical Laboratory Improvements Amendments (CLIAs) and the International Conference on Harmonization of Technical Requirements for Registration of Pharmaceuticals for Human use (ICH) guidelines. Peripheral blood lymphocytes (PBLs) were obtained from each patient before and after every 4th vaccination. Peripheral blood was taken by venipuncture, collected in an EDTA-containing tube and maintained at room temperature until transfer to the laboratory (within 24 h). PBLs were then isolated on a Ficoll-Paque Plus density gradient (GE Healthcare Bio-sciences) and stored at −80°C in serum-free storage medium (Juji Field) at a concentration of 5 × 106 cells/mL. After thawing, cell viability was confirmed to be more than 90% by trypan blue exclusion.
ELISPOT assays
Peptide-specific CTL responses was estimated by ELISPOT assays upon in vitro CTL sensitization. Frozen peripheral blood mononuclear cells (PBMCs) derived from the same patient were thawed and their viability was confirmed to be more than 90%. PBMCs (at a concentration of 5 × 105 cells/mL) were cultured in the presence of 10 mg/mL of the respective peptide and 100 IU/mL interleukin-2 (IL-2, from Novartis, Emeryville, CA) at 37°C for 2 wks. Peptides were added to cell cultures on days 0 and 7. Following CD4+ T-cell depletion by a Dynal CD4 Positive Isolation Kit (Invitrogen), an IFNγ ELISPOT assay was performed using Human IFNγ ELISpot PLUS kits (MabTech), according to the manufacturer’s instructions. Briefly, HLA-A*2402+ TISI B lymphoblasts (IHWG Cell and Gene Bank) were incubated with 20 μg/mL of peptides overnight, followed by the washout of residual peptides in media, resulting in the generation of peptide-pulsed TISI cells as stimulating cells. CD4− cells were then cultured with peptide-pulsed TISI cells (2 × 104 cells/well) at 1:1, 1:2, 1:4, or 1:8 responder to stimulator (R/S) cell ratios in 96-well plates (Millipore) at 37°C overnight. Unpulsed TISI cells were used as negative control for stimulation. To confirm IFNγ secretion, we stimulated responder cells with phorbol 12-myristate 13-acetate (PMA) and ionomycin (3 μg/mL) overnight, and then tested them by an ELISPOT assay (2.5 × 103 cells/well) in the absence of stimulator cells. All ELISPOT assays were performed in triplicate wells. Plates were analyzed by the automated ELISPOT reader ImmunoSPOT S4 (Cellular Technology) and ImmunoSpot Professional Software v. 5.0 (Cellular Technology). The number of peptide-specific spots was calculated by subtracting the number of spots in control wells from the number of spots in each of the wells containing peptide-pulsed TISI cells. The sensitivity of our ELISPOT assay was estimated to be at an average level by an ELISPOT panel of the Cancer Immunotherapy Consortium (CIC, http://www.cancerresearch.org/consortium/assay-panels/).
Flow cytometry
We analyzed the expression of peptide-specific T-cell receptors on a FACSCantoII cytofluoromter (Becton Dickinson) using CDCA1-, VEGFR1-, or VEGFR2-derived peptide-HLA dextramers coupled to phycoerythrin (PE) (Immudex), according to the manufacturer's instructions. A PE-conjugated dextramer involving a HIV1-derived epitope (RYLRDQQLL) was used as a negative control. In brief, cells were incubated with peptide- HLA PE-conjugated dextramers for 10 min at room temperature, then treated with fluorescein isothiocyanate (FITC)-conjugated anti-CD8 antibodies, allophycocyanin (APC)-conjugated anti-CD3 antibodies, PE-Cy7-conjugated anti-CD4 antibodies, and 7-aminoactinomycin D (7-AAD; from BD PharMingen) at 4°C for 20 min.
Statistical analyses
PFS and OS were analyzed done using the Kaplan-Meier method and statistical significance was evaluated by log-rank tests. A p value < 0.05 was considered as statistically significant. All statistical analyses were conducted using the SSPS statistics software v. Twenty-one (IBM).
| Abbreviations: | ||
| CDCA1 | = | cell division cycle-associated 1 |
| CT | = | cancer-testis |
| CTL | = | cytotoxic T lymphocyte |
| KIF20A | = | kinesin family member 20A |
| OS | = | overall survival |
| PFS | = | progression-free survival |
| VEGFR | = | vascular endothelial growth factor receptor |
Additional material
Download Zip (96.9 KB)Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
We thank Prof Yusuke Nakamura, Dr Takuya Tsunoda, and Dr Koji Yoshida of the Laboratory of Molecular Medicine, Human Genome Center, Institute of Medical Science, The University of Tokyo, for their excellent advice and cooperation and for providing all of the peptides.
Citation: Okuyama R, Aruga A, Hatori T, Takeda K, Yamamoto M. Immunological responses to a multi-peptide vaccine targeting cancer-testis antigens and VEGFRs in advanced pancreatic cancer patients. OncoImmunology 2013; 2:e27010; 10.4161/onci.27010
Supplemental Materials
Supplemental materials may be found here: http://www.landesbioscience.com/journals/oncoimmunology/article/27010/
References
- Egawa S, Toma H, Ohigashi H, Okusaka T, Nakao A, Hatori T, Maguchi H, Yanagisawa A, Tanaka M. Japan Pancreatic Cancer Registry; 30th year anniversary: Japan Pancreas Society. Pancreas 2012; 41:985 - 92; http://dx.doi.org/10.1097/MPA.0b013e318258055c; PMID: 22750974
- Ueno H, Ioka T, Ikeda M, Ohkawa S, Yanagimoto H, Boku N, Fukutomi A, Sugimori K, Baba H, Yamao K, et al. Randomized phase III study of gemcitabine plus S-1, S-1 alone, or gemcitabine alone in patients with locally advanced and metastatic pancreatic cancer in Japan and Taiwan: GEST study. J Clin Oncol 2013; 31:1640 - 8; http://dx.doi.org/10.1200/JCO.2012.43.3680; PMID: 23547081
- Sangha R, Butts C. L-BLP25: a peptide vaccine strategy in non small cell lung cancer. Clin Cancer Res 2007; 13:Suppl s4652 - 4; http://dx.doi.org/10.1158/1078-0432.CCR-07-0213; PMID: 17671159
- Schwartzentruber DJ, Lawson DH, Richards JM, Conry RM, Miller DM, Treisman J, Gailani F, Riley L, Conlon K, Pockaj B, et al. gp100 peptide vaccine and interleukin-2 in patients with advanced melanoma. N Engl J Med 2011; 364:2119 - 27; http://dx.doi.org/10.1056/NEJMoa1012863; PMID: 21631324
- Matsushita N, Aruga A, Inoue Y, Kotera Y, Takeda K, Yamamoto M. Phase I clinical trial of a peptide vaccine combined with tegafur-uracil plus leucovorin for treatment of advanced or recurrent colorectal cancer. Oncol Rep 2013; 29:951 - 9; PMID: 23314271
- Aruga A, Takeshita N, Kotera Y, Okuyama R, Matsushita N, Ohta T, Takeda K, Yamamoto M. Long-term vaccination with multiple peptides derived from cancer-testis antigens can maintain a specific T-cell response and achieve disease stability in advanced biliary tract cancer. Clin Cancer Res 2013; 19:2224 - 31; http://dx.doi.org/10.1158/1078-0432.CCR-12-3592; PMID: 23479678
- Cheever MA, Higano CS. PROVENGE (Sipuleucel-T) in prostate cancer: the first FDA-approved therapeutic cancer vaccine. Clin Cancer Res 2011; 17:3520 - 6; http://dx.doi.org/10.1158/1078-0432.CCR-10-3126; PMID: 21471425
- Ogi C, Aruga A. Clinical evaluation of therapeutic cancer vaccines. Hum Vaccin Immunother 2013; 9:1049 - 57; http://dx.doi.org/10.4161/hv.23917; PMID: 23454867
- Galon J, Pagès F, Marincola FM, Thurin M, Trinchieri G, Fox BA, Gajewski TF, Ascierto PA. The immune score as a new possible approach for the classification of cancer. J Transl Med 2012; 10:1; http://dx.doi.org/10.1186/1479-5876-10-1; PMID: 22214470
- Ogi C, Aruga A. Immunological monitoring of anticancer vaccines in clinical trials. Oncoimmunology 2013; 2:e26012; http://dx.doi.org/10.4161/onci.26012; PMID: 24083085
- Koike N, Pilon-Thomas S, Mulé JJ. Nonmyeloablative chemotherapy followed by T-cell adoptive transfer and dendritic cell-based vaccination results in rejection of established melanoma. J Immunother 2008; 31:402 - 12; http://dx.doi.org/10.1097/CJI.0b013e31816cabbb; PMID: 18391755
- Pere H, Montier Y, Bayry J, Quintin-Colonna F, Merillon N, Dransart E, Badoual C, Gey A, Ravel P, Marcheteau E, et al. A CCR4 antagonist combined with vaccines induces antigen-specific CD8+ T cells and tumor immunity against self antigens. Blood 2011; 118:4853 - 62; http://dx.doi.org/10.1182/blood-2011-01-329656; PMID: 21908423
- Ishida T, Joh T, Uike N, Yamamoto K, Utsunomiya A, Yoshida S, Saburi Y, Miyamoto T, Takemoto S, Suzushima H, et al. Defucosylated anti-CCR4 monoclonal antibody (KW-0761) for relapsed adult T-cell leukemia-lymphoma: a multicenter phase II study. J Clin Oncol 2012; 30:837 - 42; http://dx.doi.org/10.1200/JCO.2011.37.3472; PMID: 22312108
- Matsushita N, Pilon-Thomas SA, Martin LM, Riker AI. Comparative methodologies of regulatory T cell depletion in a murine melanoma model. J Immunol Methods 2008; 333:167 - 79; http://dx.doi.org/10.1016/j.jim.2008.01.012; PMID: 18295790
- Morse MA, Hobeika AC, Osada T, Serra D, Niedzwiecki D, Lyerly HK, Clay TM. Depletion of human regulatory T cells specifically enhances antigen-specific immune responses to cancer vaccines. Blood 2008; 112:610 - 8; http://dx.doi.org/10.1182/blood-2008-01-135319; PMID: 18519811
- Robert C, Thomas L, Bondarenko I, O’Day S, M D JW, Garbe C, Lebbe C, Baurain JF, Testori A, Grob JJ, et al. Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N Engl J Med 2011; 364:2517 - 26; http://dx.doi.org/10.1056/NEJMoa1104621; PMID: 21639810
- Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, Powderly JD, Carvajal RD, Sosman JA, Atkins MB, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med 2012; 366:2443 - 54; http://dx.doi.org/10.1056/NEJMoa1200690; PMID: 22658127
- Hamid O, Robert C, Daud A, Hodi FS, Hwu WJ, Kefford R, Wolchok JD, Hersey P, Joseph RW, Weber JS, et al. Safety and tumor responses with lambrolizumab (anti-PD-1) in melanoma. N Engl J Med 2013; 369:134 - 44; http://dx.doi.org/10.1056/NEJMoa1305133; PMID: 23724846
- Brahmer JR, Tykodi SS, Chow LQM, Hwu W-J, Topalian SL, Hwu P, Drake CG, Camacho LH, Kauh J, Odunsi K, et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med 2012; 366:2455 - 65; http://dx.doi.org/10.1056/NEJMoa1200694; PMID: 22658128
- Mkrtichyan M, Najjar YG, Raulfs EC, Liu L, Langerman S, Guittard G, Ozbun L, Khleif SN. B7-DC-Ig enhances vaccine effect by a novel mechanism dependent on PD-1 expression level on T cell subsets. J Immunol 2012; 189:2338 - 47; http://dx.doi.org/10.4049/jimmunol.1103085; PMID: 22837483
- Sawada Y, Yoshikawa T, Nobuoka D, Shirakawa H, Kuronuma T, Motomura Y, Mizuno S, Ishii H, Nakachi K, Konishi M, et al. Phase I trial of a glypican-3-derived peptide vaccine for advanced hepatocellular carcinoma: immunologic evidence and potential for improving overall survival. Clin Cancer Res 2012; 18:3686 - 96; http://dx.doi.org/10.1158/1078-0432.CCR-11-3044; PMID: 22577059
- Obara W, Ohsawa R, Kanehira M, Takata R, Tsunoda T, Yoshida K, Takeda K, Katagiri T, Nakamura Y, Fujioka T. Cancer peptide vaccine therapy developed from oncoantigens identified through genome-wide expression profile analysis for bladder cancer. Jpn J Clin Oncol 2012; 42:591 - 600; http://dx.doi.org/10.1093/jjco/hys069; PMID: 22636067
- Kono K, Iinuma H, Akutsu Y, Tanaka H, Hayashi N, Uchikado Y, Noguchi T, Fujii H, Okinaka K, Fukushima R, et al. Multicenter, phase II clinical trial of cancer vaccination for advanced esophageal cancer with three peptides derived from novel cancer-testis antigens. J Transl Med 2012; 10:141; http://dx.doi.org/10.1186/1479-5876-10-141; PMID: 22776426
- Suzuki H, Fukuhara M, Yamaura T, Mutoh S, Okabe N, Yaginuma H, Hasegawa T, Yonechi A, Osugi J, Hoshino M, et al. Multiple therapeutic peptide vaccines consisting of combined novel cancer testis antigens and anti-angiogenic peptides for patients with non-small cell lung cancer. J Transl Med 2013; 11:97; http://dx.doi.org/10.1186/1479-5876-11-97; PMID: 23578144
- Noguchi M, Kakuma T, Uemura H, Nasu Y, Kumon H, Hirao Y, Moriya F, Suekane S, Matsuoka K, Komatsu N, et al. A randomized phase II trial of personalized peptide vaccine plus low dose estramustine phosphate (EMP) versus standard dose EMP in patients with castration resistant prostate cancer. Cancer Immunol Immunother 2010; 59:1001 - 9; http://dx.doi.org/10.1007/s00262-010-0822-4; PMID: 20146063
- Taniuchi K, Nakagawa H, Nakamura T, Eguchi H, Ohigashi H, Ishikawa O, Katagiri T, Nakamura Y. Down-regulation of RAB6KIFL/KIF20A, a kinesin involved with membrane trafficking of discs large homologue 5, can attenuate growth of pancreatic cancer cell. Cancer Res 2005; 65:105 - 12; PMID: 15665285
- Harao M, Hirata S, Irie A, Senju S, Nakatsura T, Komori H, Ikuta Y, Yokomine K, Imai K, Inoue M, et al. HLA-A2-restricted CTL epitopes of a novel lung cancer-associated cancer testis antigen, cell division cycle associated 1, can induce tumor-reactive CTL. Int J Cancer 2008; 123:2616 - 25; http://dx.doi.org/10.1002/ijc.23823; PMID: 18770861
- Miyazawa M, Ohsawa R, Tsunoda T, Hirono S, Kawai M, Tani M, Nakamura Y, Yamaue H. Phase I clinical trial using peptide vaccine for human vascular endothelial growth factor receptor 2 in combination with gemcitabine for patients with advanced pancreatic cancer. Cancer Sci 2010; 101:433 - 9; http://dx.doi.org/10.1111/j.1349-7006.2009.01416.x; PMID: 19930156