Abstract
Oncolytic viruses are novel immunotherapeutic agents that appear to mediate potent antineoplastic effects in both preclinical and clinical settings. Recent studies demonstrate that manipulating the mechanisms whereby cancer cells die in the course of oncolytic virotherapy has potential to boost anticancer immune responses.
Conventional anticancer therapies are invasive, generally associated with severe side effects, and offer a limited impact on the survival of patients bearing disseminated tumors. Oncolytic viruses (OVs) are a novel class of multimodal biological therapies that selectively kill cancer cells, while leaving healthy cells unaffected. Moreover, OVs are advantageous over conventional forms of anticancer therapy as they target cancer stem cells, can replicate in hypoxic environments, and display and excellent clinical tolerability, even when used at high doses.Citation1 Accordingly, OVs are showing encouraging results in clinical trials.Citation1,Citation2 The anticancer activity of OVs is mediated by their ability to directly kill malignant cells, to interfere with the tumor vasculature, and to activate the immune system against cancer. The replication of OVs leads to the lysis of neoplastic cells coupled to the release of pathogen-associated molecular patterns (PAMPs) such as viral proteins and nucleic acids. PAMPs attract immune cells to neoplastic lesions, and these cells can take up tumor-associated antigens (TAAs) released along with the cytopathic effects for priming anticancer immune responses.Citation3 In addition, OVs can trigger the release of cytokines that stimulate the differentiation and maturation of antigen-presenting cells, also favoring the elicitation of T-cell responses. Traditionally, most of the effort to potentiate OVs for cancer therapy have been focused on increasing the viral replication rate within tumors, to increase the amounts of dying tumor cells. However, we have recently learned from the field of anticancer chemotherapy that the mechanisms whereby cancer cells die are essential for the elicitation of durable anticancer immune responses as they shape the early stages of tumor-associated antigen presentation. Kroemer and Zitvogel have elegantly showed that specific chemotherapeutics (including anthracyclines and oxaliplatin) as well as UV radiation potently induce a state of pre-mortem cellular stress that translates into the emission of immunomodulatory molecules known as danger-associated molecular patterns (DAMPs).Citation4 In the context of immunogenic cell death (ICD), it has been shown that the exposure of calreticulin on the surface of dying cancer cells serves as an “eat-me” signal for antigen-presenting cells,Citation5 while the release of ATPCitation6 and high-mobility group box 1 (HMGB1)Citation7 enhances the infiltration and activation of immune cells, overall resulting in the priming of potent tumor-specific immune responses.Citation8
The list of agents that induce ICD is expanding and we are starting to appreciate the diversity of the stress responses and immunological pathways activated by these agents.Citation7 OVs inherently exacerbate the antigenicity of dying cancer cells as well as their ability to release of DAMPs. In fact, a few classes of OVs including Coxsackievirus, measles virus and adenovirus expressing CD40 ligand (CD40L) kill cancer cells in vitro while stimulating the emission of the main signals associated with ICD.Citation3 To understand which features of an oncolytic strain of Herpes simplex virus 1 (HSV-1) are essential for its antineoplastic activity, we discovered that the initial stages of viral replication as well as the ability of the virus to trigger ICD are determinants of its therapeutic success. Indeed, oncolytic HSV-1 applied to subcutaneous tumors activates an immunogenic form of apoptosis characterized by the activating cleavage of caspase-3, the upregulation of heat shock 70 kDa protein (HSP70) and elevated serum levels of HMGB1.Citation9 Moreover, the OV-induced death of cancer cells is accompanied by an influx of antigen-presenting cells into neoplastic lesions, which is essential for the generation of TAA-specific CD8+ T cells. The establishment of such an immunological milieu correlated with the survival of tumor-bearing mice subjected to HSV-1-based oncolytic virotherapy.
In a related study, we combined oncolytic HSV-1 with the ICD-inducing agent mitoxantrone and found that this combinatorial approach has synergistic therapeutic effects.Citation10 Thus, the co-administration of mitoxantrone did not enhance the cytotoxic effects of HSV-1 or the type of cell death induced by HSV-1 in vitro. However, such an immunochemotherapeutic regimen resulted in changes in the dynamics of recruitment of tumor-infiltrating lymphocytes in vivo. More specifically, the combination of oncolytic HSV-1 and mitoxantrone increased the intratumoral levels of Ly6G+ neutrophils and antigen-specific CD8+ T cells, and the depletion of these immune cells abrogated the therapeutic effects of immunochemotherapy. In a clinically relevant tolerized system based on v-erb-b2 avian erythroblastic leukemia viral oncogene homolog 2 (ERBB2, a prominent TAA best known as HER2) we demonstrated that this combinatorial regimens is able to break the immunological tolerance that is generally established with respect to TAAs.Citation10 We are studying in detail the immunological mechanisms by which oncolytic HSV-1 and mitoxantrone exert a synergistic immunochemotherapeutic effect. At this stage, it is tempting to speculate that chemotherapy and OVs may use different but synergistic pathways to promote the emission of DAMPs and the elicitation of anticancer immune responses. In addition, OVs release PAMPs in the tumor microenvironment, leading to secretion of type I interferons and other pro-inflammatory cytokines. These distinct features of the combinatorial immunochemotherapeutic regimen that we tested may allow for the activation of robust anticancer immune responses that also eliminate cancer cell variants that would escape chemotherapy and oncolytic virotherapy employed as standalone interventions (). Our preclinical results suggest that engaging the immune system is one promising mechanisms for which oncolytic virotherapy can be harnessed in the fight against cancer. In fact, there are several ways in which OVs are being modified or combined with other therapeutic regimens for enhancing their efficacy. These include: (1) the use of OVs as anticancer vaccines, upon the genetic engineering of OVs to express cytokines or TAAs (2) the co-administration of OVs with immunological checkpoint blockers, and (3) the combination of OVs and adoptive cell therapy.
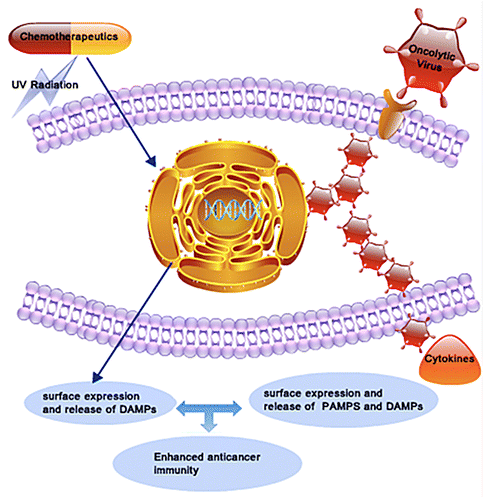
Figure 1. Combinatorial immunochemotherapy based on immunogenic cell death inducers and oncolytic viruses exerts synergistic anticancer activity. Conventional immunogenic cell death (ICD) inducers such as anthracyclines and UV radiation indirectly provoke an endoplasmic reticulum (ER) stress, leading to the release of damage-associated molecular patterns (DAMPs) within the tumor microenvironment. Oncolytic viruses (OVs) overload the protein translation machinery of malignant cells to directly cause an ER stress and potentially release DAMPs. In addition, the replication of OVs within neoplastic lesions leads to release of foreign viral proteins and nucleic acids that activate immune cells to release cytokines. At least theoretically, the combined administration of ICD inducers and OVs might activate synergistic immunological cascades culminating in improved anticancer immune responses.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Citation: Workenhe ST, Mossman KL. Rewiring cancer cell death to enhance oncolytic viro-immunotherapy. OncoImmunology 2013; 2:e27138; 10.4161/onci.27138
References
- Russell SJ, Peng KW, Bell JC. Oncolytic virotherapy. Nat Biotechnol 2012; 30:658 - 70; http://dx.doi.org/10.1038/nbt.2287; PMID: 22781695
- Heo J, Reid T, Ruo L, Breitbach CJ, Rose S, Bloomston M, Cho M, Lim HY, Chung HC, Kim CW, et al. Randomized dose-finding clinical trial of oncolytic immunotherapeutic vaccinia JX-594 in liver cancer. Nat Med 2013; 19:329 - 36; http://dx.doi.org/10.1038/nm.3089; PMID: 23396206
- Workenhe ST, Mossman KL. Oncolytic virotherapy and immunogenic cancer cell death: sharpening the sword for improved cancer treatment strategies. [Epub ahead of print] Mol Ther 2013; Forthcoming http://dx.doi.org/10.1038/mt.2013.220; PMID: 24048442
- Casares N, Pequignot MO, Tesniere A, Ghiringhelli F, Roux S, Chaput N, Schmitt E, Hamai A, Hervas-Stubbs S, Obeid M, et al. Caspase-dependent immunogenicity of doxorubicin-induced tumor cell death. J Exp Med 2005; 202:1691 - 701; http://dx.doi.org/10.1084/jem.20050915; PMID: 16365148
- Obeid M, Tesniere A, Ghiringhelli F, Fimia GM, Apetoh L, Perfettini JL, Castedo M, Mignot G, Panaretakis T, Casares N, et al. Calreticulin exposure dictates the immunogenicity of cancer cell death. Nat Med 2007; 13:54 - 61; http://dx.doi.org/10.1038/nm1523; PMID: 17187072
- Michaud M, Martins I, Sukkurwala AQ, Adjemian S, Ma Y, Pellegatti P, Shen S, Kepp O, Scoazec M, Mignot G, et al. Autophagy-dependent anticancer immune responses induced by chemotherapeutic agents in mice. Science 2011; 334:1573 - 7; http://dx.doi.org/10.1126/science.1208347; PMID: 22174255
- Ma Y, Adjemian S, Mattarollo SR, Yamazaki T, Aymeric L, Yang H, Portela Catani JP, Hannani D, Duret H, Steegh K, et al. Anticancer chemotherapy-induced intratumoral recruitment and differentiation of antigen-presenting cells. Immunity 2013; 38:729 - 41; http://dx.doi.org/10.1016/j.immuni.2013.03.003; PMID: 23562161
- Krysko DV, Garg AD, Kaczmarek A, Krysko O, Agostinis P, Vandenabeele P. Immunogenic cell death and DAMPs in cancer therapy. Nat Rev Cancer 2012; 12:860 - 75; http://dx.doi.org/10.1038/nrc3380; PMID: 23151605
- Workenhe ST, Simmon GJ, Pol JG, Lichty BD, Halford WP, Mossman KL. HSV induced immunogenic oncolysis shapes the adaptive immune response and survival after oncolytic HSV-1 treatment. Mol Ther 2013; PMID: 24048442
- Workenhe ST, Pol JG, Lichty BD, Cummings DT, Mossman KL. Combining oncolytic HSV-1 with immunogenic cell death-inducing drug mitoxantrone breaks cancer immunotolerance and improves therapeutic efficacy. Cancer Immunol Res 2013; 1:1 - 11