Abstract
We have recently identified tumor necrosis factor receptor superfamily, member 9 (TNFRSF9, best known as CD137 or 4–1BB) as a biomarker of tumor-reactive T cells naturally occurring in cancer patients, and developed a rapid, accurate system to comprehensively isolate lymphocytes with tumor-rejecting properties from human biopsies. Our findings reveal a previously unappreciated role for CD137, a co-stimulatory TNFR family member, in the immunobiology of human cancer.
Tumor necrosis factor receptor superfamily, member 9 (TNFRSF9, best known as CD137 or 4–1BB) is a TNFR family member with established co-stimulatory functions, promoting the survival and proliferation of activated T cells. The expression of CD137 by mouse and human CD8+ and CD4+ T cells is upregulated upon antigen-induced activation.Citation1 This makes CD137 an attractive biomarker for the identification (and selection) of antigen-stimulated T cells. Indeed, cytomegalovirus-, Epstein-Barr virus-, influenza virus-, adenovirus- as well as tumor-associated antigen (TAA)-specific T cells can be isolated from the peripheral blood by CD137 enrichment, but an ex vivo stimulation step based on defined antigens is required to this aim.Citation2,Citation3 Although this strategy can facilitate identification of new immunogenic epitopes from pre-defined antigens,Citation2 its potential has not been fully explored in oncological settings, in which cancer cells express both shared and patient-specific antigens of unknown specificity.
The accumulation of tumor-infiltrating lymphocytes (TILs) is often associated with improved survival among patients affected by various malignancies, supporting the notion that spontaneous T cells with antitumor activity can accumulated within neoplastic lesions and control tumor growth. The beneficial impact of some types of TILs can be further improved by immunotherapy. For example, TILs with antitumor reactivity can be isolated and expanded from resected melanoma lesions in a relatively straightforward manner, and can mediate durable, complete responses upon autologous (re)-administration to preconditioned patients.Citation4 Current methods for isolating tumor-reactive TILs are limited by a relatively low throughout (a limited number of cells can be screened) and sensitivity. Thus, potentially active tumor-reactive TILs can be excluded by the final cell product, which may also contain non-reactive TILs. Of even greater concern, these methods have generally been incapable of generating TILs with a therapeutic activity in patients with neoplasms other than melanoma, suggesting that melanoma is unique in its ability to yield TILs of sufficient potency and number to mediate clinical effects upon adoptive transfer.Citation5
Given that TILs make direct contact with malignant cells, we hypothesized that, unlike their circulating counterparts, the naturally occurring tumor-reactive T cells that are found within neoplastic lesions express activation-associated molecules as the natural product of their interaction with cancer cells.Citation6 By studying primary leukocytes from patients with ovarian cancer, we observed a preferential increase in the frequency of naturally-arising CD137+ T cells at tumor sites, even in the absence of ex vivo antigenic stimulation. CD137+ T cells were found in ascites as well as within solid tumors. The frequency of CD137+ T cells was actually higher in this latest setting, perhaps since TILs are in close contact with malignant cells. To determine whether these were bona fide tumor-reactive T cells, CD137+ TILs were isolated from the tumor after enzymatic dissociation and evaluated for the ability to recognize and react against autologous cancer cells.
Upon enzymatic dissociation, tumors were first cultured overnight in the presence of homeostatic cytokines such as interleukin (IL)-7 and IL-15, which increased the frequency of CD137+CD8+ T cells. IL-2, which is quintessential for TIL expansion, had no impact on the frequency of CD137+ TILs, suggesting that IL-7 and IL-15 better support the survival of antigen-activated TILs ex vivo. CD137+ cells were then enriched from dissociated tumor specimens by cell separation techniques. Upon re-exposure to autologous tumor cells, only CD137+ TILs produced interferon- γ (IFNγ) whereas their CD137− counterparts failed to do so. Furthermore, the addition of MHC class I-blocking antibodies to dissociated tumors prevented CD137 upregulation and IFNγ production by TILs, indicating that these functions require the MHC-dependent, TCR-mediated activation resulting from the recognition of cognate TAAs. In support of this notion, all CD8+ melanoma-derived TILs specific for the MART-126–35 peptide that were stimulated with MHC-matched MART-1+ cancer cells (but not with MHC-mismatched or MART-1- cells) upregulated CD137 expression and produced IFNγ, 2 processes that were restricted to the MART-126–35/HLA-A2 tetramer+ TIL population. Thus, TILs do upregulate CD137 upon the recognition with defined TAA-derived epitopes. This said, melanoma-derived TILs not specific for MART-1 but possessing a MHC-dependent reactivity against melanoma cell lines also upregulated CD137 upon exposure to cancer cells, indicating that TILs with heterogeneous specificities can be collectively identified and enriched by CD137-based cell separation ex vivo.
CD137+ TILs also inhibited tumor growth in vivo, in xenograft tumor models, whereas CD137- TILs did not. Accordingly, the antitumor efficacy of TIL preparations was markedly enhanced upon CD137-based selection. Thus, TILs with tumor-rejecting functions can be identified (and selected) as they express CD137. Our findings define a new method for the rapid and efficient isolation of tumor-reactive TILs from various types of cancer that does rely upon ex vivo stimulation with defined antigens ().
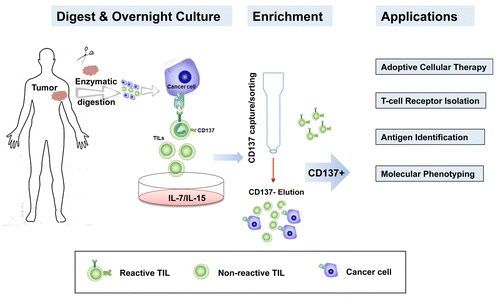
Figure 1. Isolation of CD137+ T cells from clinical tumor specimens. CD137 is selectively expressed on tumor-infiltrating lymphocytes (TILs) that have been activated upon encounter with tumor-associated antigens (TAAs). Upon the enzymatic dissociation of tumor samples, the expression of CD137 among TILs can be increased by overnight exposure to interleukin (IL)-7 and IL-15. Strategies that allow for the enrichment of CD137+ TILs represents the next step toward the improvement of adoptive CD137+ T-cell transfer and downstream translational investigations, including the sequencing of T-cell receptor (TCR)-coding genes, the identification of novel TAAs and the molecular phenotyping of specific TIL subsets.
While the expression of programmed cell death 1 (PDCD1, best known as PD-1) may also allow for the selection of natural tumor-reactive TILs,Citation7 PD-1+ TILs encompass distinct CD137+ and CD137− subsets. In our hands, the reactivity of TILs against TAAs was primarily restricted to the PD-1+CD137+, as opposed to the PD-1+CD137-, subpopulation. We therefore conclude that while a fraction of PD-1+ TILs may exert antineoplastic functions, the expression of CD137 on TILs better identifies recently activated tumor-specific T cells.
Our results identify CD137 as an activation-dependent biomarker of naturally-occurring, TAA-reactive TILs, rationalizing investigations that harness the selectivity and signaling of CD137 for cancer immunotherapy, for instance by means of agonist antibodiesCitation8 or natural ligands.Citation9 Our enrichment strategy is also positioned to support downstream translational investigations, including the profiling of immunoregulatory elements in tumor-reactive TILs as well as the sequencing of T-cell receptor (TCR)-coding genes for use in antigen discovery and immunotherapy. Notably, the isolation and expansion of CD137+ TILs for therapeutic purposes could be conducted over a one-week period, corresponding to the CD137-based enrichment followed by one week of culture in the presence of IL-2. Methods minimizing the duration of culture but not involving the selection of tumor-reactive T cells have been explored with encouraging clinical results in melanoma.Citation10 Our technique for the rapid enrichment of tumor-reactive TILs may be applied to a large panel of tumor types. The development of a reliable, closed chamber-based method for the enrichment and outgrowth of CD137+ lymphocytes represents the next logical step toward the use of these cells as anticancer immunotherapeutics.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
This research was supported by Sandy Rollman Ovarian Cancer Foundation (DP), Ovarian Cancer Research Fund (DP) and NIH RO1-CA168900 and P50-CA083638 (DP).
Citation: Ye Q, Song D, Powell Jr. DJ. Finding a needle in a haystack: Activation-induced CD137 expression accurately identifies naturally occurring tumor-reactive T cells in cancer patients. OncoImmunology 2013; 2:e27184; 10.4161/onci.27184
References
- Watts TH. TNF/TNFR family members in costimulation of T cell responses. Annu Rev Immunol 2005; 23:23 - 68; http://dx.doi.org/10.1146/annurev.immunol.23.021704.115839; PMID: 15771565
- Wolfl M, Kuball J, Ho WY, Nguyen H, Manley TJ, Bleakley M, Greenberg PD. Activation-induced expression of CD137 permits detection, isolation, and expansion of the full repertoire of CD8+ T cells responding to antigen without requiring knowledge of epitope specificities. Blood 2007; 110:201 - 10; http://dx.doi.org/10.1182/blood-2006-11-056168; PMID: 17371945
- Zandvliet ML, van Liempt E, Jedema I, Kruithof S, Kester MG, Guchelaar HJ, Falkenburg JH, Meij P. Simultaneous isolation of CD8(+) and CD4(+) T cells specific for multiple viruses for broad antiviral immune reconstitution after allogeneic stem cell transplantation. J Immunother 2011; 34:307 - 19; http://dx.doi.org/10.1097/CJI.0b013e318213cb90; PMID: 21389867
- Rosenberg SA, Yang JC, Sherry RM, Kammula US, Hughes MS, Phan GQ, Citrin DE, Restifo NP, Robbins PF, Wunderlich JR, et al. Durable complete responses in heavily pretreated patients with metastatic melanoma using T-cell transfer immunotherapy. Clin Cancer Res 2011; 17:4550 - 7; http://dx.doi.org/10.1158/1078-0432.CCR-11-0116; PMID: 21498393
- Rosenberg SA, Restifo NP, Yang JC, Morgan RA, Dudley ME. Adoptive cell transfer: a clinical path to effective cancer immunotherapy. Nat Rev Cancer 2008; 8:299 - 308; http://dx.doi.org/10.1038/nrc2355; PMID: 18354418
- Ye Q, Song D, Poussin M, Yamamoto T, Best A, Li C, Coukos G, Powell DJ Jr.. CD137 accurately identifies and enriches for naturally-occurring tumor-reactive T cells in tumor. Clin Cancer Res 2013; Forthcoming http://dx.doi.org/10.1158/1078-0432.CCR-13-0945; PMID: 24045181
- Inozume T, Hanada K, Wang QJ, Ahmadzadeh M, Wunderlich JR, Rosenberg SA, Yang JC. Selection of CD8+PD-1+ lymphocytes in fresh human melanomas enriches for tumor-reactive T cells. J Immunother 2010; 33:956 - 64; http://dx.doi.org/10.1097/CJI.0b013e3181fad2b0; PMID: 20948441
- Ascierto PA, Simeone E, Sznol M, Fu YX, Melero I. Clinical experiences with anti-CD137 and anti-PD1 therapeutic antibodies. Semin Oncol 2010; 37:508 - 16; http://dx.doi.org/10.1053/j.seminoncol.2010.09.008; PMID: 21074066
- Ye Q, Loisiou M, Levine BL, Suhoski MM, Riley JL, June CH, Coukos G, Powell DJ Jr.. Engineered artificial antigen presenting cells facilitate direct and efficient expansion of tumor infiltrating lymphocytes. J Transl Med 2011; 9:131; http://dx.doi.org/10.1186/1479-5876-9-131; PMID: 21827675
- Besser MJ, Shapira-Frommer R, Treves AJ, Zippel D, Itzhaki O, Hershkovitz L, Levy D, Kubi A, Hovav E, Chermoshniuk N, et al. Clinical responses in a phase II study using adoptive transfer of short-term cultured tumor infiltration lymphocytes in metastatic melanoma patients. Clin Cancer Res 2010; 16:2646 - 55; http://dx.doi.org/10.1158/1078-0432.CCR-10-0041; PMID: 20406835