Abstract
Targeting the epithelial-to-mesenchymal transition (EMT) is emerging as a novel intervention against tumor progression and metastatic dissemination, as well as the resistance to chemo- and radiotherapy displayed by multiple carcinomas. We have recently developed an immunotherapeutic approach to target a major driver of EMT, the T-box transcription factor T (also known as brachyury). This therapeutic paradigm is currently being tested in patients with advanced carcinomas in the context of a Phase I clinical trial.
The process known as epithelial-to-mesenchymal transition (EMT) has gained much attention within the field of cancer research as it plays a critical role in the progression of human carcinomas.Citation1 A normal occurrence in the course of embryogenesis, the EMT may aberrantly take place in epithelial neoplasms, resulting in the loss of cell polarity and cell-to-cell contacts. At the level of the neoplastic cell, the EMT manifests with several features, including the loss of E-cadherin expression, an increase in motility, invasive potential and mesenchymal characteristics, as well as an enhanced propensity to metastatic dissemination.Citation2 The EMT is also known to increase the resistance of malignant cells to multiple treatments, including chemotherapy, radiation therapy, and some targeted antineoplastic agents. It has been shown, for example, that breast tumors recurring upon conventional therapy contain an increased proportion of cells exhibiting EMT-associated features,Citation3 suggesting that carcinoma cells with a mesenchymal-like phenotype might be selected rather than eliminated by standard therapeutic interventions ().
Figure 1. Targeting the epithelial-to-mesenchymal transition to block metastatic dissemination and alleviate resistance to therapy. (A) The epithelial-to-mesenchymal transition (EMT) promotes the invasive potential of cancer cells, their propensity to generate distant metastases and their resistance to chemo- and radiotherapy. (B) A vaccine directed against a driver of EMT could elicit an immune response that effectively targets malignant cells undergoing the EMT. Combining this immunotherapeutic approach with conventional treatments targeting epithelial cancer cells might result in effective tumor eradication.
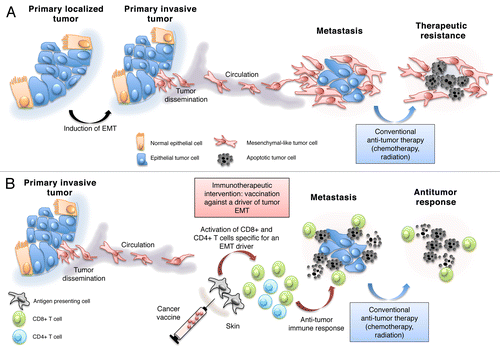
Despite the widespread recognition of the role of the EMT in tumor progression, specifically targeting the molecular drivers of this phenomenon has not yet been exploited as a means for preventing metastasis and, possibly, eliminating cancer cells that would otherwise resist most currently available therapies. This is not surprising as most of the master regulators of the EMT are transcription factors that cannot be targeted with antibodies and are difficult to inhibit with conventional pharmacological approaches. One alternative method to inhibit the EMT is to immunize patients against one of the EMT-relevant transcription factors. This approach is expected to generate an effective T-cell response that selectively eradicates tumor cells expressing the EMT driver of choice and undergoing the epithelial-to-mesenchymal switch. When employed in combination with conventional therapeutics that are capable of eliminating epithelial tumor cells, the immunological targeting of mesenchymal cancer cells may thus lead to effective tumor eradication ().
One of the molecules that orchestrates the EMT in neoplastic lesions is the T-box transcription factor T (also known as brachyury). Brachyury is able to drive the epithelial-to-mesenchymal switch of human carcinoma cell lines in vitro and to promote the metastatic dissemination of human tumor xenografts in vivo.Citation4,Citation5 In addition, the levels of brachyury have been shown to positively correlate with the resistance of malignant cells to various chemotherapeutic as well as to irradiation,Citation6,Citation7 and several studies have demonstrated the association between robust brachyury expression and poor clinical outcome in patients with various types of carcinomas. Brachyury has also been extensively characterized as a tumor-associated antigen (TAA). Indeed, brachyury is highly expressed by various carcinomas, including lung and breast (primary and metastatic) cancer lesions, but not by the majority of normal adult tissues, with the exception of the testis and thyroid.Citation8,Citation9 Being predominantly associated with tumors and playing a relevant role in disease progression as well as in chemo- and radioresistance, brachyury represents as an attractive target for anticancer interventions.
Because of its immunogenicity, brachyury is also a viable molecule for immunotherapy. Brachyury-specific cytotoxic CD8+ T cells can be expanded in vitro from the blood of cancer patients, as shown by means of a nonameric brachyury-derived peptide. In addition, these brachyury-specific T cells are able to lyse carcinoma cells that present epitopes of brachyury in the context of MHC class I molecules.Citation8,Citation9 Another demonstration of the immunogenicity of brachyury came from the observation that patients receiving a prostate-specific antigen (PSA)-targeting vaccine in combination with monoclonal antibodies specific for cytotoxic T lymphocyte-associated protein 4 (CTLA4) or a carcinoembryonic antigen (CEA)-targeting vaccine can develop brachyury-specific T cells post-vaccination. Most likely this originates from the cross-presentation of TAAs released by malignant cells succumbing to the antitumor immune response elicited by the vaccine. These studies demonstrated the immunogenicity of brachyury in humans and its potential to serve as a target for anticancer vaccination.
Based on these observations, we developed a brachyury-targeting vaccine based on a heat-killed recombinant strain of Saccharomyces cerevisiae expressing full-length human brachyury. This vaccine has been successfully used to activate and promote the maturation of human dendritic cells in vitro. These cells could be used to expand human brachyury-specific CD8+ and CD4+ T cells from the peripheral blood of healthy donors and cancer patients.Citation10 The vaccine was also evaluated in vivo. In particular, the administration of heat-killed brachyury-expressing yeast to mice was shown to elicit brachyury-specific CD4+ and CD8+ T-cell responses that were capable of reducing tumor burden in an experimental model of brachyury-driven metastasis.Citation10 In light of the role of the EMT in tissue remodeling, wound healing and embryonic development, mice vaccinated with our recombinant vaccines were also evaluated for signs of potential toxicity. Of note, immune responses against brachyury developed in the absence of any interference with wound healing, any effect on pregnancy and birth rates, and any other general side effect.Citation10 Based on these results, a Phase I clinical trial was initiated to test heat-killed brachyury-expressing yeast in patients with advanced tumors. To our knowledge, this is the first vaccine targeting a driver of the EMT that has successfully entered clinical development. It is tempting to hypothesize that, if employed at an early stage of disease, a brachyury-targeting anticancer vaccine could prevent or inhibit the establishment of metastatic lesions and potentially limit the acquisition of chemo- or radioresistance.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Citation: Fernando R, Hamilton D, Palena C. An immunotherapeutic intervention against tumor progression: Targeting a driver of the epithelial-to-mesenchymal transition. OncoImmunology 2013; 2:e27220; 10.4161/onci.27220
References
- Nieto MA. The ins and outs of the epithelial to mesenchymal transition in health and disease. Annu Rev Cell Dev Biol 2011; 27:347 - 76; http://dx.doi.org/10.1146/annurev-cellbio-092910-154036; PMID: 21740232
- Thiery JP. Epithelial-mesenchymal transitions in tumour progression. Nat Rev Cancer 2002; 2:442 - 54; http://dx.doi.org/10.1038/nrc822; PMID: 12189386
- Creighton CJ, Li X, Landis M, Dixon JM, Neumeister VM, Sjolund A, Rimm DL, Wong H, Rodriguez A, Herschkowitz JI, et al. Residual breast cancers after conventional therapy display mesenchymal as well as tumor-initiating features. Proc Natl Acad Sci U S A 2009; 106:13820 - 5; http://dx.doi.org/10.1073/pnas.0905718106; PMID: 19666588
- Fernando RI, Litzinger M, Trono P, Hamilton DH, Schlom J, Palena C. The T-box transcription factor Brachyury promotes epithelial-mesenchymal transition in human tumor cells. J Clin Invest 2010; 120:533 - 44; http://dx.doi.org/10.1172/JCI38379; PMID: 20071775
- Fernando RI, Castillo MD, Litzinger M, Hamilton DH, Palena C. IL-8 signaling plays a critical role in the epithelial-mesenchymal transition of human carcinoma cells. Cancer Res 2011; 71:5296 - 306; http://dx.doi.org/10.1158/0008-5472.CAN-11-0156; PMID: 21653678
- Huang B, Cohen JR, Fernando RI, Hamilton DH, Litzinger MT, Hodge JW, Palena C. The embryonic transcription factor Brachyury blocks cell cycle progression and mediates tumor resistance to conventional antitumor therapies. Cell Death Dis 2013; 4:e682; http://dx.doi.org/10.1038/cddis.2013.208; PMID: 23788039
- Larocca C, Cohen JR, Fernando RI, Huang B, Hamilton DH, Palena C. An autocrine loop between TGF-β1 and the transcription factor brachyury controls the transition of human carcinoma cells into a mesenchymal phenotype. Mol Cancer Ther 2013; 12:1805 - 15; http://dx.doi.org/10.1158/1535-7163.MCT-12-1007; PMID: 23783250
- Palena C, Polev DE, Tsang KY, Fernando RI, Litzinger M, Krukovskaya LL, Baranova AV, Kozlov AP, Schlom J. The human T-box mesodermal transcription factor Brachyury is a candidate target for T-cell-mediated cancer immunotherapy. Clin Cancer Res 2007; 13:2471 - 8; http://dx.doi.org/10.1158/1078-0432.CCR-06-2353; PMID: 17438107
- Roselli M, Fernando RI, Guadagni F, Spila A, Alessandroni J, Palmirotta R, Costarelli L, Litzinger M, Hamilton D, Huang B, et al. Brachyury, a driver of the epithelial-mesenchymal transition, is overexpressed in human lung tumors: an opportunity for novel interventions against lung cancer. Clin Cancer Res 2012; 18:3868 - 79; http://dx.doi.org/10.1158/1078-0432.CCR-11-3211; PMID: 22611028
- Hamilton DH, Litzinger MT, Jales A, Huang B, Fernando RI, Hodge JW, Ardiani A, Apelian D, Schlom J, Palena C. Immunological targeting of tumor cells undergoing an epithelial-mesenchymal transition via a recombinant brachyury-yeast vaccine. Oncotarget 2013; 4:1777 - 90; PMID: 24125763
