Abstract
Preclinical studies have established that CD8+ T cells are necessary for efficient immunotherapeutic regimens targeting v-erb-b2 avian erythroblastic leukemia viral oncogene homolog 2 (ERBB2, best known as HER2/Neu). Recently, we extended upon these findings by demonstrating that anti-HER2/Neu therapy also requires CD4+ T cells and CD40/CD40L signaling within the tumor microenvironment. Our results add to mounting evidence demonstrating that adaptive immunity is crucial to the efficacy of conventional and targeted anticancer chemotherapeutics.
Therapeutic agents targeting v-erb-b2 avian erythroblastic leukemia viral oncogene homolog 2 (ERBB2, best known as HER2/Neu) are an essential component of the standard treatment of HER2+ breast carcinoma patients.Citation1 However, the mechanisms by which HER2/Neu-targeting interventions mediate the antitumor response have not yet been completely revealed. Although endogenous adaptive immune responses targeting HER2/Neu were described in breast cancer patients over 15 y ago,Citation2 the role of adaptive immunity in HER2/Neu-targeting therapy has only recently become appreciated. This new understanding originates, in large part, to preclinical studies demonstrating that Rag1−/− and CD8-deficient mice bearing HER2/Neu+ tumors are significantly impaired in their ability to respond to anti-HER2/Neu therapy.Citation3,Citation4 Given the profound therapeutic implications of these findings, determining the mechanisms through which HER2/Neu-targeting agents elicit or boost endogenous antitumor immune responses is of tremendous importance. To this end, we have recently demonstrated that CD4+ T cells as well as the interaction between CD40 and its ligand (CD40L) within the tumor microenvironment play an essential role in the therapeutic activity of HER2/Neu-targeting agents.Citation5
In particular, we transplanted a HER2/Neu+ tumor model in immunocompetent mice to further examine the requirement for adaptive immunity in the therapeutic activity of HER2/Neu-targeting antibodies. Although depleting CD4+ regulatory T cells (Tregs) has been shown to prevent the growth of HER2/Neu+ tumors,Citation6 we observed that the depletion of CD4+ T cells significantly inhibited the efficacy of anti-HER2/Neu antibodies. These results suggested that after the administration of HER2/Neu-targeting antibodies, the positive role of CD4+ effector T cells in antitumor immune responses is more prominent than that of CD4+ Tregs. Anti-HER2/Neu therapy was also less efficient when CD4+ T cell-depleting antibodies were administered after the cessation of HER2/Neu-targeting antibodies. Thus, the requirement for CD4+ T cells was not limited to early phase immune responses, which are traditionally associated with helper T-cell activity, nor was the effect consistent with alleviation of immunosuppression by CD4+ Tregs. Rather, our data suggested that CD4+ T cells might exert relatively direct antitumor effects ().
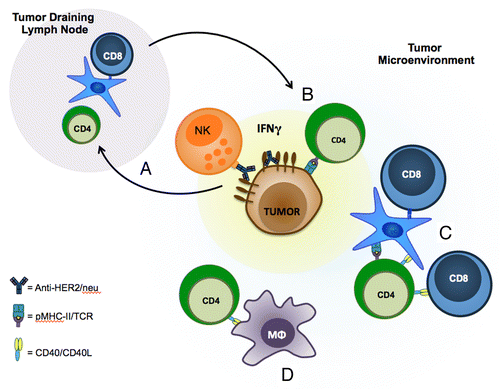
Figure 1. CD4+ T cells and CD40/C40L interactions in the tumor microenvironment are necessary for the therapeutic efficacy of anti-HER2/Neu antibodies. (A) In addition to antibody-dependent cell-mediated cytotoxicity (ADCC), the binding of anti-HER2/Neu antibodies to HER2 promotes adaptive immune responses, resulting in increased tumor infiltration by CD4+ and CD8+ T cells. (B) CD4+ T cells are capable of mediating direct antitumor responses by engaging MHC class II molecules on the surface of cancer cells that has been induced by interferon γ (IFNγ). In line with this notion, the intratumoral depletion of CD4+ T cells inhibits the therapeutic efficacy of anti-HER2/Neu antibodies. (C and D) The intratumoral blockade of CD40/CD40L interactions also reduces the therapeutic potential of anti-HER2/Neu antibodies. CD40/CD40L interactions may promote antigen presentation by dendritic cells (DCs), activate CD8+ T cells (C) and/or promote macrophage activation (D).
To address this issue, we examined the functional capacity of CD8+ T cells in the absence of CD4+ T cells via interferon γ (IFNγ)-specific ELISPOT assays. The depletion of CD4+ T cells in the course of anti-HER2/Neu therapy did not impair the antitumor response of CD8+ T cells, suggesting a role for CD4+ T cells exceeding the mere provision of “help” signals. Moreover, IFNγ induced the expression of MHC class II molecules on malignant cells both in vitro and in vivo, raising the possibility that CD4+ T cells might directly operate on cancer cells. We therefore examined the antitumor response of CD4+ T cells in the absence of CD8+ T cells, and found that CD4+ T cells are capable of exerting antitumor activity in an independent manner. Taken together, these data indicated that both CD8+ and CD4+ T cells are capable targeting HER/Neu+ tumors upon the administration of anti-HER2/Neu antibodies. Because HER2/Neu-targeting antibodies can also target cancer cells for antibody-dependent cell-mediated cytotoxicity (ADCC), CD8+ and CD4+ T cells might also target tumor-associated antigens other than HER2/Neu released as a result of cancer cell death. Given that the antitumor activity of CD8+ and CD4+ T cells was assessed using whole neoplastic cells, it will be of interest to determine if responses to tumor-associated antigens other than HER2/Neu are elicited in this setting.
The contribution of both CD8+ and CD4+ T cells to the therapeutic efficacy of HER2/Neu-targeting agents supports current efforts to develop anticancer vaccines for treating HER2/Neu+ tumors. Multiple vaccines targeting HER2/Neu are currently in clinical trials, but one issue arising from these studies is the need to induce both CD4+ and CD8+ T cells.Citation7 Therefore, combining vaccination with HER2/Neu-targeting agents may be a promising strategy to enhance the endogenous immune response against HER2/Neu+ neoplasms. Currently, multiple Phase I and Phase II studies are being conducted based on this approach. Administering immune checkpoint blockers following anti-HER2/Neu therapy may represent an alternative approach to improve antitumor immune responses. Indeed, preclinical data suggest that antibodies blocking programmed cell death 1 (PDCD1, best known as PD-1) enhance the efficacy of HER2/Neu-targeting therapies.Citation4
In addition to systemic CD4+ T cell depletion, we also examined the effects of intratumoral CD4+ T cell depletion. This approach demonstrated that CD4+ T cells within the tumor microenvironment are essential, and suggested that cell-to-cell interactions may be required for the full-blown therapeutic activity of HER2/Neu-targeting therapies. One important factor for CD4+ T cell effector function, and ultimately CD8+ T cell responses, is the interaction between CD40 and CD40L.Citation8 Using intratumoral injections of specific antibodies, we observed that blocking CD40/CD40L interactions within the tumor microenvironment significantly reduced the efficacy of anti-HER2/Neu therapy. Moreover, the blockade of CD40/CD40L interactions within neoplastic lesions reduced the functional capacity of T cells in situ, but not in the periphery, suggesting a predominant role for CD40/CD40L interactions within the tumor microenvironment. Future studies based on the adoptive transfer of CD40L-deficient T cells or the provision of CD40 agonists in the absence of CD4+ T cells will clarify the essential role of CD40/CD40L interactions in this setting.
It should be noted that blocking CD40L intratumorally resulted in a significant, yet partial, phenotype. This implies that multiple interactions within the tumor microenvironment contribute to the antitumor response generated by anti-HER2/Neu therapy. Understanding these interactions is essential, especially in light of the fact that initially antineoplastic T cell effector functions may eventually elicit immunosuppressive mechanisms through negative feedback pathways. In this context, Spranger et al. have recently reported that IFNγ produced by CD8+ T cells not only stimulates the expression of indoleamine 2,3-dioxygenase 1 (IDO1) and CD274 (best known as PD-L1), 2 central immunosuppressive factors, but also recruits Tregs to the tumor microenvironment.Citation9
The essential role of adaptive immunity in the efficacy of HER2/Neu-targeting interventions suggests that this immunotherapeutic approach is not as passive as previously categorized.Citation7 Our findings also suggest that anti-HER2/Neu approaches may be favorably combined with other immunotherapies. Overall, our study extends previous findings suggesting that the adaptive immune system is necessary for the efficacy of anti-HER2/Neu therapy. In addition, it adds to accumulating evidence suggesting that adaptive immunity is crucial for the efficacy of conventional and targeted antineoplastic agents, and supports the ongoing effort toward the development of novel immunochemotherapeutic regimens.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
This research was supported in part by US. National Institutes of Health grants CA141975 and CA97296 to Y.X.F
Citation: Mortenson ED, Fu Y. Anti-HER2/Neu passive-aggressive immunotherapy. OncoImmunology 2013; 2:e27296; 10.4161/onci.27296
References
- De P, Hasmann M, Leyland-Jones B. Molecular determinants of trastuzumab efficacy: What is their clinical relevance?. Cancer Treat Rev 2013; 39:925 - 34; http://dx.doi.org/10.1016/j.ctrv.2013.02.006; PMID: 23562214
- Disis ML, Pupa SM, Gralow JR, Dittadi R, Menard S, Cheever MA. High-titer HER-2/neu protein-specific antibody can be detected in patients with early-stage breast cancer. J Clin Oncol 1997; 15:3363 - 7; PMID: 9363867
- Park S, Jiang Z, Mortenson ED, Deng L, Radkevich-Brown O, Yang X, Sattar H, Wang Y, Brown NK, Greene M, et al. The therapeutic effect of anti-HER2/neu antibody depends on both innate and adaptive immunity. Cancer Cell 2010; 18:160 - 70; http://dx.doi.org/10.1016/j.ccr.2010.06.014; PMID: 20708157
- Stagg J, Loi S, Divisekera U, Ngiow SF, Duret H, Yagita H, Teng MW, Smyth MJ. Anti-ErbB-2 mAb therapy requires type I and II interferons and synergizes with anti-PD-1 or anti-CD137 mAb therapy. Proc Natl Acad Sci U S A 2011; 108:7142 - 7; http://dx.doi.org/10.1073/pnas.1016569108; PMID: 21482773
- Mortenson ED, Park S, Jiang Z, Wang S, Fu Y-X. Effective anti-neu-initiated antitumor responses require the complex role of CD4+ T cells. Clin Cancer Res 2013; 19:1476 - 86; http://dx.doi.org/10.1158/1078-0432.CCR-12-2522; PMID: 23363817
- Wei W-Z, Jacob JB, Zielinski JF, Flynn JC, Shim KD, Alsharabi G, Giraldo AA, Kong YC. Concurrent induction of antitumor immunity and autoimmune thyroiditis in CD4+ CD25+ regulatory T cell-depleted mice. Cancer Res 2005; 65:8471 - 8; http://dx.doi.org/10.1158/0008-5472.CAN-05-0934; PMID: 16166327
- Milani A, Sangiolo D, Montemurro F, Aglietta M, Valabrega G. Active immunotherapy in HER2 overexpressing breast cancer: current status and future perspectives. Ann Oncol 2013; 24:1740 - 8; http://dx.doi.org/10.1093/annonc/mdt133; PMID: 23585514
- Grewal IS, Flavell RA. CD40 and CD154 in cell-mediated immunity. Annu Rev Immunol 1998; 16:111 - 35; http://dx.doi.org/10.1146/annurev.immunol.16.1.111; PMID: 9597126
- Spranger S, Spaapen RM, Zha Y, Williams J, Meng Y, Ha TT, Gajewski TF. Up-regulation of PD-L1, IDO, and T(regs) in the melanoma tumor microenvironment is driven by CD8(+) T cells. Sci Transl Med 2013; 5:200ra116; http://dx.doi.org/10.1126/scitranslmed.3006504; PMID: 23986400
