Abstract
Blood clotting specifically supports the metastatic dissemination of malignant cells to the lung. We have recently demonstrated that 2 tumor types that are prone to form lung metastases, renal cell carcinoma and soft tissue sarcoma, share specific adhesive mechanisms that support the invasion and colonization of blood clots in the pulmonary vasculature.
Keywords: :
Blood clotting is a critical step during the metastatic dissemination of malignant cells, as it protects them from the cytotoxic activity of circulating immune cells.Citation1 In addition, thrombi have been shown to support the epithelial-mesenchymal transition, promote the transmigration, and sustain the colony-forming activity of blood-borne cancer cells.Citation2-Citation4 In line with this notion, the metastatic potential of several tumors is strongly reduced in transgenic mice that exhibit defects in blood coagulation or platelet activation.Citation1 Interestingly, the formation of thrombi has been shown to specifically support the metastatic colonization of lungs but has no apparent effects on the dissemination of malignant cells to the liver.Citation5 This difference could originate from the specialized structure of pulmonary blood vessels, which, unlike their hepatic counterparts, are lined with a tight layer of endothelium to avoid fluid leakage into the alveolar space.Citation6,Citation7 Thus, the metastatic colonization of the lungs depends on specific factors that induce the retraction of the endothelium and promote cancer cell extravasation, such as angiopoietin-like 4 (ANGPTL4).Citation7 Interestingly, the formation of clots around blood-borne cancer cells serves a similar purpose, mediating endothelial retraction through platelet-secreted ATP.Citation4 Additional clot components, such as fibrin and fibronectin, have been shown to promote the activation of integrins, causing malignant cells to generate long, axon-like invadopodia as a means to penetrate the clot and navigate the narrow inter-endothelial spaces of the pulmonary vasculature.Citation5
To follow up on the question whether clot formation is relevant for the metastatic colonization of the lungs, we focused on renal cell carcinoma (RCC) and soft tissue sarcoma (STS), mostly because these 2 tumor types are known to metastasize predominantly to the lung.Citation8 Upon intravenous injection into mice, RCC and STS cells lodge into the lung vasculature, where they become surrounded by fibrin. This mechanism is important for lung colonization because co-injecting the anticoagulant hirudin significantly reduced experimental lung invasion by STS cells. To further analyze the role of blood clotting in lung metastasis, we embedded a panel of RCC and STS cell lines into a 3 dimensional matrix of clotted plasma, side by side with malignant cell lines that predominantly metastasize to other organs such as the liver or bone (i.e., melanoma, breast, prostate, and pancreatic cancer cells). Inspection by phase contrast microscopy revealed a stark difference in cell morphology: while clot-embedded RCC and STS cells developed a spread phenotype with extensive invadopodia, the overwhelming majority of melanoma, breast, prostate, and pancreatic cancer cells maintained their initial, roundish shape. This discrepancy in invadopodia formation was not due to a general inability to invade tissues, as a majority of non-clot invasive tumor cells display a normal metastatic potential. Moreover, the capacity of RCC and STS cells to generate invadopodia proved to be specific for clotted plasma or its main component fibrin, both of which were much more permissive for cell spreading than matrigel.
Studying transgenic mice lacking plasma fibronectin, we have recently demonstrated that fibrin-fibronectin complexes promote the activation of integrin αvβ3 and that this mechanism correlates with clot invasion and lung metastasis.Citation5 While plasma fibronectin was not necessary for RCC and STS cells to generate invadopodia in fibrin, we found that these cells expressed a constitutively active form of integrin αvβ3 and that knocking down the β3 subunit markedly reduced invadopodia formation, fibrin invasion, as well as lung colonization. To further elucidate the function of integrin αvβ3 in this setting, we tested fibronectin matrix formation, which has been shown to depend on integrin activation.Citation9 We detected elaborate fibronectin meshworks in STS and RCC cells, whereas non-clot invasive cell lines as well as RCC cells depleted of integrin β3 by RNA interference lacked the capacity to form a fibronectin matrix. The interaction between integrin β3 and fibronectin, in turn, turned out to be critical for the formation of stress fibers, suggesting that the binding of integrin αvβ3 to fibrin alone does not provide the tensile strength to sustain actomyosin contraction. The formation of stress fibers is a prerequisite for the maturation of focal adhesions, large signaling hubs that promote cell invasion, survival and proliferation.Citation10 In line with this notion, we demonstrated that both integrin β3 and fibronectin are necessary for invadopodia and colony formation by cancer cells in fibrin. Moreover, we found that integrin αvβ3 and fibronectin cooperate in maintaining high expression levels of snail family zinc finger 2 (SNAI2, best known as SLUG), which is relevant for invadopodia formation in vitro and lung colonization in vivo.
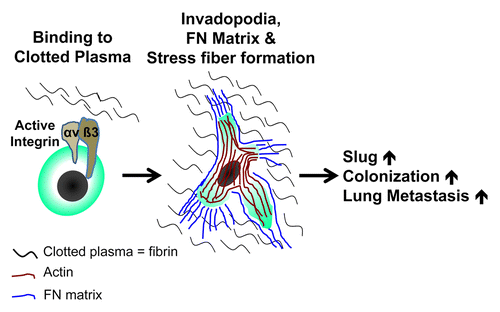
Together, our data define a mechanism that could explain the propensity of RCC and STS cells for lung colonization (). This mechanism is based on the specialized interaction of these cells with blood clot and depends on activated integrin αvβ3 as well as fibronectin, which together support the formation of stress fibers and the expression of SLUG. In turn, SLUG promotes clot invasion and metastatic dissemination into the lung. Interestingly, this model is also operational in a clinical setting. Indeed, SLUG expression, clot invasion and fibronectin matrix formation are hallmarks of primary cancer cells from RCC patients with lung metastases. Taken together, our data indicate that SLUG is a marker for metastatic RCC. Moreover, they suggest that inhibiting fibrin or stress fiber formation could represent a valuable strategy for the prevention and treatment of metastatic RCC and STS.
Figure 1. Role of integrin αvβ3 in lung colonization by cancer cells. Activated integrin αvβ3 on renal cell carcinoma (RCC) and soft tissue sarcoma (STS) cells facilitates their binding to blood clots. This in turn sustains the formation of invadopodia along with the assembly of an elaborate fibronectin (FN) matrix, the formation of actin stress fibers and upregulation of snail family zinc finger 2 (SNAI2), best known as SLUG. Altogether, these factors contribute to the metastatic colonization of the lung by malignant cells.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Citation: Knowles LM, Maranchie JK, Pilch J. Invadopodia formation in blood clots: Not so SLUGgish after all. OncoImmunology 2013; 2:e27338; 10.4161/onci.27338
References
- Palumbo JS, Talmage KE, Massari JV, La Jeunesse CM, Flick MJ, Kombrinck KW, Jirousková M, Degen JL. Platelets and fibrin(ogen) increase metastatic potential by impeding natural killer cell-mediated elimination of tumor cells. Blood 2005; 105:178 - 85; http://dx.doi.org/10.1182/blood-2004-06-2272; PMID: 15367435
- Labelle M, Begum S, Hynes RO. Direct signaling between platelets and cancer cells induces an epithelial-mesenchymal-like transition and promotes metastasis. Cancer Cell 2011; 20:576 - 90; http://dx.doi.org/10.1016/j.ccr.2011.09.009; PMID: 22094253
- Liu J, Tan Y, Zhang H, Zhang Y, Xu P, Chen J, Poh YC, Tang K, Wang N, Huang B. Soft fibrin gels promote selection and growth of tumorigenic cells. Nat Mater 2012; 11:734 - 41; http://dx.doi.org/10.1038/nmat3361; PMID: 22751180
- Schumacher D, Strilic B, Sivaraj KK, Wettschureck N, Offermanns S. Platelet-derived nucleotides promote tumor-cell transendothelial migration and metastasis via P2Y2 receptor. Cancer Cell 2013; 24:130 - 7; http://dx.doi.org/10.1016/j.ccr.2013.05.008; PMID: 23810565
- Malik G, Knowles LM, Dhir R, Xu S, Yang S, Ruoslahti E, Pilch J. Plasma fibronectin promotes lung metastasis by contributions to fibrin clots and tumor cell invasion. Cancer Res 2010; 70:4327 - 34; http://dx.doi.org/10.1158/0008-5472.CAN-09-3312; PMID: 20501851
- Sarin H. Physiologic upper limits of pore size of different blood capillary types and another perspective on the dual pore theory of microvascular permeability. J Angiogenes Res 2010; 2:14; http://dx.doi.org/10.1186/2040-2384-2-14; PMID: 20701757
- Padua D, Zhang XH, Wang Q, Nadal C, Gerald WL, Gomis RR, Massagué J. TGFbeta primes breast tumors for lung metastasis seeding through angiopoietin-like 4. Cell 2008; 133:66 - 77; http://dx.doi.org/10.1016/j.cell.2008.01.046; PMID: 18394990
- Knowles LM, Gurski LA, Engel C, Gnarra JR, Maranchie JK, Pilch J. Integrin αvβ3 and fibronectin upregulate Slug in cancer cells to promote clot invasion and metastasis. Cancer Res 2013; 73:6175 - 84; http://dx.doi.org/10.1158/0008-5472.CAN-13-0602; PMID: 23966293
- Wu C, Keivens VM, O’Toole TE, McDonald JA, Ginsberg MH. Integrin activation and cytoskeletal interaction are essential for the assembly of a fibronectin matrix. Cell 1995; 83:715 - 24; http://dx.doi.org/10.1016/0092-8674(95)90184-1; PMID: 8521488
- Provenzano PP, Keely PJ. Mechanical signaling through the cytoskeleton regulates cell proliferation by coordinated focal adhesion and Rho GTPase signaling. J Cell Sci 2011; 124:1195 - 205; http://dx.doi.org/10.1242/jcs.067009; PMID: 21444750
