Abstract
Prolonged lymphopenia correlating with decreased survival commonly occurs among glioma patients undergoing radiation therapy (RT) and temozolomide (TMZ) treatment. To better understand the pathophysiology of this phenomenon, we prospectively monitored serum cytokine levels and lymphocyte subsets in 15 high-grade glioma patients undergoing combined radiation and TMZ (referred to as RT/TMZ) treatment. Sufficient data for analysis were acquired from 11 of the patients initially enrolled. Lymphocyte phenotyping data were obtained using cytofluorometric analysis and serum cytokine levels were measured using the a multiplex bead-based assays. Total lymphocyte counts (TLCs) were > 1000 cells per μL peripheral blood in 10/11 patients at baseline, but dropped significantly after treatment. Specifically, after RT/TMZ therapy, the TLCs were found to be < 500 cells/μL in 2/11 patients, 500–1000 cells/μL in 7/11 patients, and > 1000 cells/μL in the remaining 2 patients. Among residual mononuclear blood cells, we observed a proportional drop in B and CD4+ T cells but not in CD8+ T lymphocytes. Natural killer cells remained to near-to-baseline levels and there was a transient and slight (insignificant) increase in regulatory T cells (Tregs). The circulating levels of IL-7 and IL-15 remained low despite marked drops in both the total and CD4+ T lymphocyte counts. Thus, patients with malignant glioma undergoing RT/TMZ treatment exhibit a marked decline in TLCs, affecting both CD4+ T cells and B lymphocytes, in the absence of a compensatory increase in interleukin-7 levels. The failure to mount an appropriate homeostatic cytokine response may be responsible for the prolonged lymphopenia frequently observed in these patients.
Introduction
Glioblastoma multiforme (GBM) is the most common primary brain tumor and is responsible for approximately 70 000 deaths worldwide each year.Citation1 The current standard of care for GBM patients includes maximal debulking surgery followed by 6 wk of concurrent temozolomide (TMZ) and radiation therapy (RT) plus 6 mo of maintenance therapy with TMZ as a single therapeutic agent.Citation2 Patients also frequently require the management of symptomatic cerebral edema with corticosteroids. This comprehensive GBM treatment course is unfortunately associated with significant hematologic toxicity, including thrombocytopenia and lymphopenia. Lymphopenia is common and can be severe, with up to 45% of GBM patients developing grade III-IV lymphopenia 2 mo after completing this multi-modal therapy. Treatment-induced lymphopenia generally persist for at least one year after combined RT/TMZ therapy, and prior evidence suggests that lymphopenia may be detected as long as 10 y after focal external-beam RT.Citation3,Citation4
Diminished circulating lymphocytes adversely affect glioma patients with important clinical consequences. Lymphopenic patients are indeed susceptible to opportunistic pathogens such as Pneumocystis carinii, although the incidence of the associated-complications (e.g., pneumonia) has decreased with the standard use of antibiotic prophylaxis based on trimethoprim-sulfamethoxazole.Citation5 Importantly, treatment-related lymphopenia has been linked to poor disease-specific survival in a recently published prospective analysis of GBM patients receiving RT/TMZ treatment.Citation3 However, the exact mechanisms underlying the association between lymphopenia and disease-specific survival in these particular patients remain unknown. Considering that T lymphocytes are essential effector cells in the anticancer immune response, it is possible that the depletion of circulating lymphocyte populations impairs pre-existent antitumor immunity and this reduction is at least partly responsible for the inferior survival observed in lymphopenic patients.Citation6
Overall, very little is known about the physiological response to lymphopenia in patients with solid tumors. Interleukin (IL)-7 and IL-15 have been identified as key cytokines in the compensatory reaction to declining lymphocytes.Citation7 The levels of IL-7, a T-cell growth and anti-apoptotic factor, increase in response to HIV- and chemotherapy-induced lymphopenia.Citation7 In fact, IL-7 is crucial for the generation and homeostasis of T cells, such that humans with congenital defects in the IL-7 receptor exhibit severe combined immune deficiency and are unable to produce T lymphocytes, whereas the B and natural killer (NK) cells are maintained.Citation7 In two Phase I clinical trials enrolling patients with advanced cancer (mostly melanoma and sarcoma), recombinant human IL-7 successfully increased the size of both CD4+ and CD8+ T-cell populations.Citation8,Citation9 IL-15 is another T-cell growth factor that appears to selectively stimulate CD8+ T cells, at least in mice.Citation10
In this study, we compared the circulating levels of IL-7 and IL-15 with quantitative changes in various lymphocyte subpopulations in malignant glioma patients subjected to RT/TMZ treatment. We found that RT/TMZ results in significant lymphopenia unaccompanied by an appropriate compensatory cytokine response, likely driving prolonged disruptions in lymphocyte homeostasis. These findings may have important ramifications for clinical immunotherapeutic strategies, including approaches to prevent or ameliorate treatment-induced lymphopenia in cancer patients.
Results
Patient demographics, baseline, and treatment characteristics
Fifteen patients with World Health Organization (WHO) Grade III–IV gliomas were initially enrolled in this study. Eleven of these patients completed at least 2 out of 3 scheduled study evaluations and are included in this report. presents basic demographic and clinical information about this patient cohort. In summary, median age was 63 y (range, 32–74 y), 8 patients were male and 3 were female, and 8 had Grade IV glioblastoma, whereas 3 had Grade III glioma. The median baseline TLC in the peripheral blood was 1320 cells/µL and the individual baseline TLC (in all patients except one) was typically > 800 cells/µL. Similarly, the median baseline CD4+ T lymphocyte count was 714 cells/µL, and all patients had baseline CD4+ T-cell counts > 200 cells/µL. All patients completed concurrent RT/TMZ-based therapy and received between 1 and 6 cycles of adjuvant TMZ.
Table 1. Patient demographics
RT/TMZ decreases total lymphocyte counts, affecting CD4+ but not CD8+ T cells
As shown in , RT/TMZ resulted in a significant decrease in TLCs, with a median TLC drop from 1320 cells/µL to 800 cells/µL (P = 0.002) by week 10, 4 wk after completion of bimodal radiation and TMZ therapy. Of note, TLCs did not rebound upon completion of radiation treatment, considering that at week 18, 12 wk after the termination of RT, median TLCs remained inferior than pretreatment TLCs, at 900 cells/µL (P = 0.001). As shown in , these data correspond to an approximately 40% drop in baseline TLCs (P = 0.0003 for week 0 vs. week 10 and P = 0.001 for week 0 vs. week 18). To understand which particular lymphocyte subsets were most affected by RT/TMZ-based therapy, we analyzed the composition of CD4+ and CD8+ T-cell subsets in the blood of treated patients over time by flow cytometry (). Interestingly, the percentage of CD3+ lymphocytes did not drop appreciably over the study period (; P = 0.17), suggesting that both CD4+ and CD8+ subsets might be relatively unaffected. However, further analyses showed this assumption to be incorrect. On a percentage basis, CD8+ T-cell levels remained relatively constant, or even increased to slight extents (). However, we observed a proportional decrease in the percentage of CD3+CD4+ T cells of approximately 50%, for example from 65% to 35% in the representative patient shown (, dot plots on left), with similar trends in the mean value (, middle panel). These data demonstrate the selective inhibitory effect of radiation/TMZ therapy on the CD4+ T-cell compartment as compared with its CD8+ counterparts. These trends were reflected in absolute CD4+ and CD8+ T-cell counts as well, with a significant decrease in the total number of CD4+ (P = 0.02) but not CD8+ (P = 0.45) CD3+ T cells (, far right panels). Similar results were obtained with CD3+CD4+FOXP3-CD45RA+ cells () and reflected by the increased CD45RA-/CD45RA+ cell ratio (). These changes were also reflected in the CD4+/CD8+ T-cell ratio, which was found to be significantly decreased at 18 wk (P = 0.02), 3 mo after the completion of radiation therapy ().
Figure 1. Concurrent RT/TMZ effects on total peripheral lymphocyte count over time. (A and B) Total lymphocytes were counted in the peripheral blood of Grade III and IV glioma patients prior to (baseline) and following 6 wk of radiation therapy (RT) and temozolomide (TMZ) treatment, at the indicated time points. (A) Absolute total lymphocyte counts (TLCs) at baseline, week 10 (4 wk after completing RT/TMZ), and week 18 (12 wk after completing RT/TMZ). (B) Percent changes in TLC, plotted individually for each patient in the study.
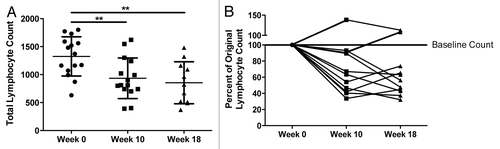
Figure 2. Concurrent RT/TMZ adversely affects total T-cell counts by selectively depleting CD4+ T-cell populations. (A–F) The peripheral blood lymphocytes of Grade III and IV glioma patients were immunophenotyped with fluorophore-conjugated antibodies specific for the indicated marker prior to (baseline) and following 6 wk of radiation therapy (RT) and temozolomide (TMZ) treatment. Post-treatment time points analyzed were week 10 (4 wk after completing RT/TMZ) and week 18 (12 wk after completing RT/TMZ). Flow cytometry was used to calculate percentages of lymphocytes with the indicated marker profile. Absolute numbers were determined by calculating cell count per blood volume. (A–D) CD3+ cells (A), CD3+CD8+ cells (B), CD3+CD4+ cells (C), CD4+CD45RA+ cells (D). Left panel: representative flow cytometry results. Middle panel: change in mean (and range) % lymphocytes with indicated marker profile. Right panel: change in absolute cell counts. (E–F) Plot of ratio of cells with indicated marker profile: CD45RA-/CD45RA+ T-cell ratio (E), CD4+/CD8+ T-cell ratio (F). Statistical analyses were performed using paired samples t-test; *P < 0.05.
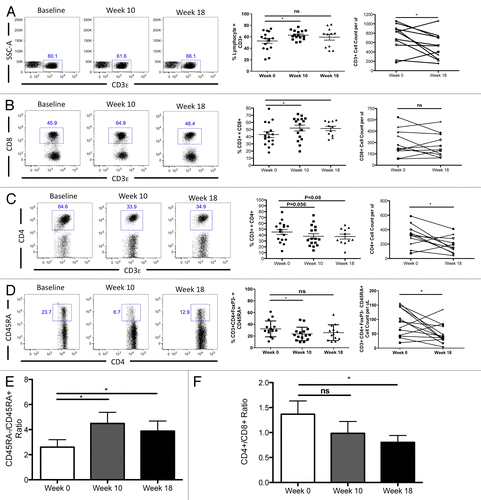
RT/TMZ treatment decreases circulating B cells
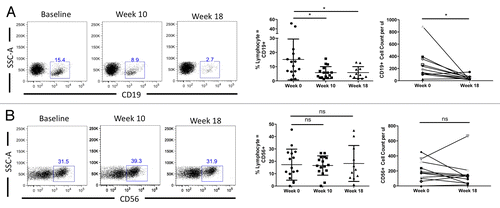
Considering the profound drop in CD4+ T-cell counts noted in GBM patients subjected to RT/TMZ treatment, we next examined the levels of circulating B cells and NK cells. As shown in , CD19+ B cells were also markedly affected by RT/TMZ, decreasing over time on both a percentage and absolute basis. Surprisingly, circulating (CD56+) NK cells appeared to be relatively resistant to treatment, with both percentages and absolute cell numbers insignificantly affected over the observation period (). Taken together with the data above, these observations suggest that the RT/TMZ treatment has an inhibitory effect on the levels of specific lymphocyte subsets.
Figure 3. Concurrent RT/TMZ depletes B cells but not NK cells. (A and B) The peripheral blood lymphocytes of Grade III and IV glioma patients were immunophenotyped with fluorophore-conjugated antibodies specific for the indicated markers prior to (baseline) and following 6 wk of radiation therapy (RT) and temozolomide (TMZ) treatment. Post-treatment time points analyzed were week 10 (4 wk after completing RT/TMZ) and week 18 (12 wk after completing RT/TMZ). Flow cytometry was used to calculate the percentage of (CD19+) B lymphocytes (A) and (CD56+) natural killer (NK) cells (B). Absolute numbers were determined by calculating cell count per blood volume. Left panel: representative flow cytometry results. Middle panel: change in mean (and range) % lymphocytes with indicated marker profile. Right panel: change in absolute cell count. Statistical analyses were performed using paired samples t-test; *P < 0.05.
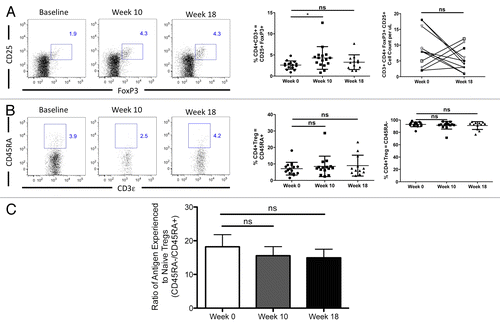
RT/TMZ causes a transient increase in circulating regulatory T cells
In light of the well-documented role of regulatory T cells (Tregs) in the inhibition of adaptive immune responses to cancer,Citation6,Citation11-Citation13 we also investigated whether RT/TMZ affects the percentage or absolute amount of these cells in the peripheral blood of GBM patients. For the purposes of this analysis, we defined Tregs as CD4+ (and CD3+) T cells that co-express both forkhead box P3 (FOXP3) and CD25. As shown in , on a percentage basis, circulating Tregs increased slightly at week 10, but returned to near-to-baseline levels by week 18. On an absolute basis, changes in Treg levels were variable, with some patients exhibiting a moderate increase and others a decrease in the numbers of circulating Tregs at the 18-wk time point (, far right panel). Finally, we also tested whether the percentage of Tregs with a naïve phenotype (CD45RA+) was affected by treatment. As shown in , the balance of circulating Tregs with a naïve phenotype was not significantly altered by the combinatorial RT/TMZ treatment. Taken together, these data paint an intriguing picture of RT and TMZ mediating a profound decrease in TLCs that is primarily due to declines in CD4+ T lymphocytes and CD19+ B cells.
Figure 4. Concurrent RT/TMZ treatment transiently expands Tregs despite overall depletion of CD4+ T cell counts. (A–C) The peripheral blood lymphocytes of Grade III and IV glioma patients were immunophenotyped with fluorophore-conjugated antibodies specific for the indicated markers prior to (baseline) and following 6 wk of radiation therapy (RT) and temozolomide (TMZ) treatment. Post-treatment time points analyzed were week 10 (4 wk after completing RT/TMZ) and week 18 (12 wk after completing RT/TMZ). Flow cytometry was used to calculate the percentage of T lymphocytes with the indicated profiles. Absolute numbers were determined by calculating cell count per blood volume. (A and B) CD4+/CD3+/FOXP3+ cells (A) and CD4/CD45RA+ cells (B). Left panel: representative flow cytometry results. Middle panel: change in mean (and range) % lymphocytes with indicated marker profile. Right panel: change in absolute cell count. (C) Ratio of antigen-experienced (CD45RA-) to antigen-naïve (CD45RA+) T cells. Statistical analyses were performed using paired samples t-test; *P < 0.05.
RT/TMZ-associated lymphopenia is not accompanied by homeostatic increases in IL-7 or IL-15
The homeostatic maintenance of circulating CD4+ and CD8+ T cells within a relatively tight window is primarily achieved by the lymphocyte-regulatory cytokines IL-7 and IL-15.Citation14 Thus, we next evaluated the serum levels of these cytokines, expecting a compensatory increase in IL-7 levels as total lymphocyte and CD4+ T-cell counts decreased over time.Citation15,Citation16 As shown in and , this was not the case. Mean baseline IL-7 levels in the GBM patients enrolled in our study was 2.59 pg/mL, not significantly different from normal levels reported in several series.Citation14,Citation17 As the TLCs and the levels of CD4+ T cells dropped (Figs. One and 2), a compensatory increase in the concentration of IL-7 was not observed at either the 10-wk or 18-wk time point following RT/TMZ treatment (). A similar trend was noted for IL-15, although IL-15 levels increased slightly (but not significantly) at the 18-wk time point (Figs. S1 and S2). Considering that IL-7 levels have previously been shown to inversely correlate with CD4+ T-cell counts, we also tested for such a reciprocal relationship. As shown in , no correlation between serum IL-7 levels and circulating CD4+ T-cell counts was observed among patients at 10 and 18 weeks after RT/ TMZ treatment. Similar results were obtained when IL-7 levels and TLCs were studied. Taken together, these findings suggest that lymphopenia in RT/TMZ-treated patients might be mediated, at least in part, by their failure to mount a compensatory IL-7 response to decreased levels of T and B lymphocytes.
Figure 5. Serum IL-7 levels after RT/TMZ treatment for malignant glioma. (A and B) The sera of Grade III and IV glioma patients were analyzed for cytokine levels via Bioplex200 multiplexed bead-based immunoassay prior to (baseline) and following 6 wk of radiation therapy (RT) and temozolomide (TMZ) treatment. (A) Serum interleukin (IL)-7 levels (pg/mL) at baseline, week 10 (4 wk after completing RT/TMZ) and week 18 (12 wk after completing RT/TMZ). (B) Scatterplot of serum IL-7 levels (y-axis; pg/mL) and absolute peripheral CD4+ T lymphocyte counts from (x-axis; cells/μL). Statistical analyses were performed using paired samples t-test; *P < 0.05.
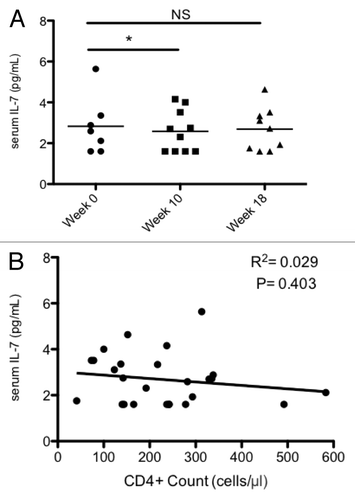
Table 2. Median (range) of observed serum levels of IL-7 and IL-15 at baseline and at 10 and 18 wk after starting RT. All units are pg/mL
Discussion
Here, we describe the results of a prospective study correlating changes in the circulating levels of total lymphocytes and specific lymphocyte subtypes with the serum levels of immunomodulatory cytokines in glioma patients receiving focal external-beam RT. These malignant glioma patients, who also received TMZ in combination with RT, developed lymphopenia but failed to mount a compensatory increase in IL-7 and IL-15 levels, despite the significant decline in total and CD4+ lymphocyte counts. This observation has potential significance for the management of GBM patients, in whom treatment-induced lymphopenia has been prospectively associated with poor disease-specific survival as well as with the development of opportunistic infections.Citation3,Citation5
Our lymphocyte phenotyping data are consistent with those of prior reports, in which prolonged decreases in the CD4+: CD8+ T-cell ratio were primarily driven by sharp drops in circulating CD4+ T cells following RT.Citation18 Similar patterns in the levels of circulating lymphocytes have been observed following focal irradiation, as shown in a series of 90 breast and testicular cancer patients treated with external-beam radiation.Citation19 This group of patients had normal CD4+: CD8+ T-cell ratios at baseline that decreased immediately after treatment initiation, an effect persisting for as long as 5 y. Long-term decreases in the CD4+: CD8+ T-cell ratio and alterations in the phenotypes of circulating lymphocytes have also been observed in patients receiving RT for benign diseases, e.g., total nodal irradiation for multiple sclerosis or rheumatoid arthritis.Citation20,Citation21
The CD4+ T cell-specific lymphopenia observed in our group of patients was profound, approaching the severity seen in HIV1-infected patients,Citation22 with all patients experiencing CD4+ T-cell counts < 500 cells/µL and over 50% exhibiting CD4+ T-cell counts < 300 cells/µL after RT/TMZ treatment. The decrease in CD4+ T-cell numbers paralleled the drop in total lymphocyte counts, but CD8+ T cells appeared to be relatively unaffected, leading to a decrease in the CD4+: CD8+ T-cell ratio following treatment. The mechanisms underlying the differential sensitivity of these 2 cell populations to RT/TMZ are unclear, particularly since both populations are presumably regulated by similar cytokine feedback loops.Citation14 Moreover, the radiosensitivity of CD4+ and CD8+ T cells in vitro appears to be similar, despite multiple clinical studies suggesting that CD4+ T lymphocytes are more radiosensitive than their CD8+ counterparts in vivo.Citation23
Our data also revealed significant decreases in the B-cell population upon RT/TMZ-based therapy, whereas NK cells and Tregs remained fairly stable following the therapeutic course. However, clinical data suggests that CD4+ T cell-specific lymphopenia is likely to be much more relevant for the patient than a drop in circulating B cells, as evinced by the effects of the anti-CD20 monoclonal antibody rituximab.Citation24 Rituximab treatment for lymphoma decreases circulating B-cell counts to undetectable levels without causing appreciable changes in circulating immunoglobulin profiles. Although patients receiving rituximab do exhibit an increased risk of hepatitis B reactivation, the overall rate of opportunistic infections in this setting appears to be low, and B-cell depletion among rituximab-treated lymphoma patients may actually be predictive of a positive clinical response.Citation24,Citation25 In sharp contrast, CD4+ T cell-specific lymphopenia is associated with opportunistic infections in both HIV-infected and cancer patients.Citation26,Citation27 Additionally, treatment-induced drops in circulating CD4+ T cells correlate with an approximately 2-fold increase in the risk of death among glioblastoma patients.Citation3 Finally, other studies in patients with non-small cell lung carcinoma and pancreatic cancer (whether resected or unresectable) have recently shown that treatment-induced lymphopenia is broadly associated with inferior survival.Citation28-Citation30
Although the initial decrease in lymphocyte counts in GBM patients treated with RT/TMZ may be due to direct effects of radiation on circulating T and B cells, this does not explain the fact that lymphopenia persists in patients subjected to ionizing radiation for months to years after exposure. Typically, such profound decreases in the circulating lymphocyte count are accompanied by a compensatory rise in the serum levels of IL-7, a cytokine that is essential for the normal development of the immune system and the homeostasis of circulating T lymphocytes. In humans, the congenital absence of the IL-7 receptor results in a severe combined immunodeficiency syndrome characterized by the loss of T lymphocytes but normal production of NK cells and B lymphocytes.Citation31,Citation32 IL-7 levels typically increase in response to declining amounts of T lymphocytes, such as observed in HIV-infected patients, in cancer patients responding to lymphodepleting chemotherapy, or upon the administration of T cell-specific antibodies.Citation33,Citation34 For example, in melanoma patients with chemotherapy-induced lymphopenia prior to bone marrow transplant, circulating IL-7 levels rise to a median of about 30 pg/mL.Citation16 The exogenous administration of IL-7 can increase circulating CD4+ and CD8+ lymphocyte counts, demonstrating a clear role for IL-7 in this immunoregulatory process. The T cell-expanding effect of IL-7 has been demonstrated in studies of HIV-infected patients as well as in two Phase I clinical trials enrolling patients with heavily treated solid malignancies.Citation8,Citation9,Citation35 Like IL-7, IL-15 stimulates lymphocyte proliferation and survival, although it appears to act specifically on CD8+ lymphocytes and NK cells and is currently unavailable for human use.Citation36
To our knowledge, this study is the first to report that IL-7 and IL-15 levels do not increase upon RT/TMZ treatment-related lymphopenia in glioblastoma patients. RT and TMZ are both toxic to circulating lymphocytes, and as the patients in this study received both treatments, it is difficult to discern from this particular cohort whether the effects of either RT or TMZ are primarily responsible. However, as lymphopenia is observed in patients who receive RT regardless of whether or not concurrent lymphotoxic chemotherapy or corticosteroids are given, it is likely that RT is primarily responsible for the consequent lymphopenic state. Further research in patients treated with other RT regimens is needed in order to determine whether similar effects on circulating lymphocytes and associated cytokines are observed in the absence of concurrent TMZ.
Our findings might reflect either an inherent defect in cytokine production among GBM patients or, alternatively, a treatment-induced disruption of lymphocyte regulation by IL-7 and IL-15. However, an inherent defect in IL-7 and IL-15 production among glioblastoma patients is unlikely, given the normal baseline TLCs observed in these patients before treatment. It has recently been demonstrated in murine models that TMZ-induced lymphopenia stimulates a brisk rise in IL-7 levels,Citation37 further suggesting that TMZ is unlikely to account for our observations. Overall, however, the pathophysiology underlying RT/TMZ-induced lymphopenia and how this relates to immunomodulatory cytokines is presently unclear. It is possible that radiation affects the normal homeostatic relationship between serum IL-7 levels and lymphocyte counts, although the precise mechanisms by which this may occur remain obscure. It has been shown that IL-7 is produced throughout the body by stromal cells of the lymphatic endothelial lining, at least in mice.Citation38 Conceptually, it is possible that RT could damage the lymphatic endothelium, hence interfering with systemic IL-7 production. Another hypothetical scenario would involve an RT-induced release of transforming growth factor β1 (TGFβ1), a known negative regulator of IL-7 production that could systemically suppress the expected increase in IL-7 driven by lymphopenia.Citation39,Citation40 Unfortunately, TGFβ1 could not be assayed in our specimens due to sampling limitations. We plan further studies in a similar cohort of brain cancer patients to attempt to replicate these data and determine whether TGFβ1 plays a role in the suppression of IL-7 production following RT/TMZ treatment.
Although these preliminary results require replication and validation in larger prospective cohorts, they are sufficient to suggest that the efficacy of immunotherapy following RT might be tempered by disruptions in lymphocyte homeostasis. We have identified in the immunomodulatory cytokine IL-7 a potential therapeutic agent to counterbalance this phenomenon. If the prolonged lymphopenia induced by RT is indeed mediated by a loss in the innate ability to produce IL-7, the exogenous administration of IL-7 should increase lymphocyte counts in these patients. Thus, given the established correlation between treatment-induced lymphopenia and disease outcome in patients harboring various solid tumors, secondary IL-7 replacement is a promising strategy to boost the immune system, either alone or in conjunction with other immunostimulatory agents (such as CTLA4-targeting agents inhibitors).
Materials and Methods
Study patients and treatment regimen
This prospective clinical study was designed to assay lymphocyte subpopulations and serum cytokine levels among patients at the Johns Hopkins Sidney Kimmel Comprehensive Cancer Center receiving RT/TMZ treatment for high-grade (WHO Grade III–IV) gliomas. The study was approved by the institutional review board at Johns Hopkins. All patients provided informed consent for treatment and for participation in this study, according to institutional standards and the Declaration of Helsinki.
Patients received intensity-modulated RT of a total dose of 59.4 Gray (Gy) divided into 30 1.8-Gy fractions (in patients with Grade III tumors) or 60 Gy in 30 2-Gy fractions (in patients with GBM) over 6 wk. TMZ was given according to the Stupp regimen at 75 mg/m2/day during RT.Citation41 Trimethoprim-sulfamethoxazole was administered to all patients as prophylaxis against Pneumocystis infections.
Serum and peripheral blood lymphocyte samples
Baseline sera and peripheral blood lymphocyte (PBL) samples were obtained after the histologic diagnosis of either Grade III glioma or Grade IV glioblastoma but prior to the initiation of chemotherapy and RT. A second set of samples was obtained 10 wk after the initiation therapy (4 wk after the completion of concurrent RT/TMZ) and a third set at 18 wk after starting therapy (12 wk after completing RT/TMZ). Sera were aliquoted and stored at −80 °C for later analyses in parallel. PBLs were prepared by density centrifugation with Lymphoprep (Axis–Shield PoC AS) according to the manufacturer’s instruction. A hemocytometer was used to count the lymphocytes and determine TLCs per blood volume. Cells were resuspended at 10 × 106 cells/mL in freezing medium (90% heat-inactivated fetal bovine serum + 10% dimethylsulfoxide) and cryopreserved until assayed.
Flow cytometry
Multi-parameter cytofluorometric analysis was performed to phenotype PBLs. The following conjugated monoclonal antibodies were purchased from BD Biosciences: peridinin chlorophyll protein complex–cyanine (PerCP-5–5)-anti-CD3, fluorescein isothiocyanate (FITC)-anti-CD4, allophycocyanin–cyanine (APC-Cy7)-anti-CD8, phycoerythrin–cyanine (PE-Cy7)-anti-CD25, PE–anti-FOXP3, PE-anti-CD45RO, FITC-anti-CD45RA, allophycocyanin (APC)-anti-CD45RA, and FITC-anti-Ki67. Cryopreserved cells were resuspended in a staining buffer supplied by the manufacturer (BD Biosciences, San Jose, CA) and stained for 20 min at 4 °C. The expression of FOXP3 was detected by intracellular staining of cells pre-stained with other markers and cell permeabilization was performed according to the manufacturer’s instructions (BD Biosciences). Samples were run on a FACSCanto flow cytometer (Becton Dickinson) with at least 5 × 104 events acquired per parameter. Sequential gating was used to discriminate lymphocyte subsets, as previously described.Citation42 Flow cytometry data were analyzed using the FloJo software package (Treestar).
Cytokine analysis
The Bioplex 200 (Bio-Rad) platform was used to determine the absolute concentration (in pg/mL) of IL-7 and IL-15 using banked sera samples. A multiplexed bead-based immunoassay was performed in duplicate for each serum sample following the manufacturer's protocols and using the supplied cytokine standards.
Statistical analysis
Statistical analyses were performed using Graphpad Prism (GraphPad Software) using the paired samples t-test. P values < 0.05 were considered statistically significant.
| Abbreviations: | ||
| GBM | = | glioblastoma multiforme |
| NK | = | natural killer |
| RT | = | radiation therapy |
| PBL | = | peripheral blood lymphocyte |
| TGFβ1 | = | transforming growth factor β1 |
| TLC | = | total lymphocyte counts |
| TMZ | = | temozolomide |
| Treg | = | regulatory T cell |
Additional material
Download Zip (168 KB)Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
These data were presented in abstract form at the 17th Annual Scientific Meeting of the Society for Neuro-Oncology, Washington, DC, November 2012.
Citation: Ellsworth S, Balmanoukian A, Kos F, Nirschl CJ, Nirschl TR, Grossman SA, Luznik L, Drake CG. Sustained CD4+ T cell-driven lymphopenia without a compensatory IL-7/IL-15 response among high-grade glioma patients treated with radiation and temozolomide. OncoImmunology 2013; 2:e27357; 10.4161/onci.27357
References
- Reardon DA, Rich JN, Friedman HS, Bigner DD. Recent advances in the treatment of malignant astrocytoma. J Clin Oncol 2006; 24:1253 - 65; http://dx.doi.org/10.1200/JCO.2005.04.5302; PMID: 16525180
- Stupp R, Hegi ME, Mason WP, van den Bent MJ, Taphoorn MJ, Janzer RC, Ludwin SK, Allgeier A, Fisher B, Belanger K, et al, European Organisation for Research and Treatment of Cancer Brain Tumour and Radiation Oncology Groups, National Cancer Institute of Canada Clinical Trials Group. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol 2009; 10:459 - 66; http://dx.doi.org/10.1016/S1470-2045(09)70025-7; PMID: 19269895
- Grossman SA, Ye X, Lesser G, Sloan A, Carraway H, Desideri S, Piantadosi S, NABTT CNS Consortium. Immunosuppression in patients with high-grade gliomas treated with radiation and temozolomide. Clin Cancer Res 2011; 17:5473 - 80; http://dx.doi.org/10.1158/1078-0432.CCR-11-0774; PMID: 21737504
- Meyer KK. Radiation-induced lymphocyte-immune deficiency. A factor in the increased visceral metastases and decreased hormonal responsiveness of breast cancer. Arch Surg 1970; 101:114 - 21; http://dx.doi.org/10.1001/archsurg.1970.01340260018003; PMID: 5451189
- Hughes MA, Parisi M, Grossman S, Kleinberg L. Primary brain tumors treated with steroids and radiotherapy: low CD4 counts and risk of infection. Int J Radiat Oncol Biol Phys 2005; 62:1423 - 6; http://dx.doi.org/10.1016/j.ijrobp.2004.12.085; PMID: 16029802
- Mellman I, Coukos G, Dranoff G. Cancer immunotherapy comes of age. Nature 2011; 480:480 - 9; http://dx.doi.org/10.1038/nature10673; PMID: 22193102
- Fry TJ, Mackall CL. Interleukin-7: from bench to clinic. Blood 2002; 99:3892 - 904; http://dx.doi.org/10.1182/blood.V99.11.3892; PMID: 12010786
- Sportès C, Babb RR, Krumlauf MC, Hakim FT, Steinberg SM, Chow CK, Brown MR, Fleisher TA, Noel P, Maric I, et al. Phase I study of recombinant human interleukin-7 administration in subjects with refractory malignancy. Clin Cancer Res 2010; 16:727 - 35; http://dx.doi.org/10.1158/1078-0432.CCR-09-1303; PMID: 20068111
- Rosenberg SA, Sportès C, Ahmadzadeh M, Fry TJ, Ngo LT, Schwarz SL, Stetler-Stevenson M, Morton KE, Mavroukakis SA, Morre M, et al. IL-7 administration to humans leads to expansion of CD8+ and CD4+ cells but a relative decrease of CD4+ T-regulatory cells. J Immunother 2006; 29:313 - 9; http://dx.doi.org/10.1097/01.cji.0000210386.55951.c2; PMID: 16699374
- Fehniger TA, Caligiuri MA. Interleukin 15: biology and relevance to human disease. Blood 2001; 97:14 - 32; http://dx.doi.org/10.1182/blood.V97.1.14; PMID: 11133738
- Pardoll DM, Topalian SL. The role of CD4+ T cell responses in antitumor immunity. Curr Opin Immunol 1998; 10:588 - 94; http://dx.doi.org/10.1016/S0952-7915(98)80228-8; PMID: 9794842
- Curiel TJ. Regulatory T cells and treatment of cancer. Curr Opin Immunol 2008; 20:241 - 6; http://dx.doi.org/10.1016/j.coi.2008.04.008; PMID: 18508251
- Zitvogel L, Apetoh L, Ghiringhelli F, André F, Tesniere A, Kroemer G. The anticancer immune response: indispensable for therapeutic success?. J Clin Invest 2008; 118:1991 - 2001; http://dx.doi.org/10.1172/JCI35180; PMID: 18523649
- Mackall CL, Fry TJ, Gress RE. Harnessing the biology of IL-7 for therapeutic application. Nat Rev Immunol 2011; 11:330 - 42; http://dx.doi.org/10.1038/nri2970; PMID: 21508983
- Darcissac EC, Vidal V, De La Tribonniere X, Mouton Y, Bahr GM. Variations in serum IL-7 and 90K/Mac-2 binding protein (Mac-2 BP) levels analysed in cohorts of HIV-1 patients and correlated with clinical changes following antiretroviral therapy. Clin Exp Immunol 2001; 126:287 - 94; http://dx.doi.org/10.1046/j.1365-2249.2001.01670.x; PMID: 11703373
- Dudley ME, Yang JC, Sherry R, Hughes MS, Royal R, Kammula U, Robbins PF, Huang J, Citrin DE, Leitman SF, et al. Adoptive cell therapy for patients with metastatic melanoma: evaluation of intensive myeloablative chemoradiation preparative regimens. J Clin Oncol 2008; 26:5233 - 9; http://dx.doi.org/10.1200/JCO.2008.16.5449; PMID: 18809613
- Bolotin E, Annett G, Parkman R, Weinberg K. Serum levels of IL-7 in bone marrow transplant recipients: relationship to clinical characteristics and lymphocyte count. Bone Marrow Transplant 1999; 23:783 - 8; http://dx.doi.org/10.1038/sj.bmt.1701655; PMID: 10231140
- Yovino S, Grossman SA. Severity, etiology and possible consequences of treatment-related lymphopenia in patients with newly diagnosed high-grade gliomas. CNS Oncol 2012; 1:149 - 54; http://dx.doi.org/10.2217/cns.12.14; PMID: 23828734
- De Ruysscher D, Waer M, Vandeputte M, Aerts R, Vantongelen K, van der Schueren E. Changes of lymphocyte subsets after local irradiation for early stage breast cancer and seminoma testis: long-term increase of activated (HLA-DR+) T cells and decrease of “naive” (CD4-CD45R) T lymphocytes. Eur J Cancer 1992; 28A:1729 - 34; http://dx.doi.org/10.1016/0959-8049(92)90079-H; PMID: 1389495
- Rohowsky-Kochan C, Molinaro D, Devereux C, Troiano R, Bansil S, Zito G, Wolansky L, Jotkowitz A, Denny T, Oleske J, et al. The effect of total lymphoid irradiation and low-dose steroids on T lymphocyte populations in multiple sclerosis: correlation with clinical and MRI status. J Neurol Sci 1997; 152:182 - 92; http://dx.doi.org/10.1016/S0022-510X(97)00156-1; PMID: 9415540
- Tanay A, Field EH, Hoppe RT, Strober S. Long-term followup of rheumatoid arthritis patients treated with total lymphoid irradiation. Arthritis Rheum 1987; 30:1 - 10; http://dx.doi.org/10.1002/art.1780300101; PMID: 2949753
- Guidelines for the use of antiretroviral agents in HIV-infected adults and adolescents. http://aidsinfo.nih.gov/guidelines/html/1/adult-and-adolescent-arv-guidelines/0.
- Nakamura N, Kusunoki Y, Akiyama M. Radiosensitivity of CD4 or CD8 positive human T-lymphocytes by an in vitro colony formation assay.. Radiat Res 1990; 123:224 - 7; http://dx.doi.org/10.2307/3577549; PMID: 2117766
- Gea-Banacloche JC. Rituximab-associated infections. Semin Hematol 2010; 47:187 - 98; http://dx.doi.org/10.1053/j.seminhematol.2010.01.002; PMID: 20350666
- McLaughlin P, Grillo-López AJ, Link BK, Levy R, Czuczman MS, Williams ME, Heyman MR, Bence-Bruckler I, White CA, Cabanillas F, et al. Rituximab chimeric anti-CD20 monoclonal antibody therapy for relapsed indolent lymphoma: half of patients respond to a four-dose treatment program.. J Clin Oncol 1998; 16:2825 - 33; PMID: 9704735
- Mahindra AK, Grossman SA. Pneumocystis carinii pneumonia in HIV negative patients with primary brain tumors.. J Neurooncol 2003; 63:263 - 70; http://dx.doi.org/10.1023/A:1024217527650; PMID: 12892232
- Schiff D. Pneumocystis pneumonia in brain tumor patients: risk factors and clinical features.. J Neurooncol 1996; 27:235 - 40; http://dx.doi.org/10.1007/BF00165480; PMID: 8847557
- Balmanoukian A, Ye X, Herman J, Laheru D, Grossman SA. The association between treatment-related lymphopenia and survival in newly diagnosed patients with resected adenocarcinoma of the pancreas.. Cancer Invest 2012; 30:571 - 6; http://dx.doi.org/10.3109/07357907.2012.700987; PMID: 22812722
- Wild AT, Ye X, Ellsworth SG, Smith JA, Narang AK, Garg T, Campian J, Laheru DA, Zheng L, Wolfgang CL, et al. The association between chemoradiation-related lymphopenia and clinical outcomes in patients with locally advanced pancreatic adenocarcinoma.. Am J Clin Oncol 2013; Forthcoming http://dx.doi.org/10.1097/COC.0b013e3182940ff9; PMID: 23648440
- Campian JL, Ye X, Brock M, Grossman SA. Treatment-related lymphopenia in patients with stage III non-small-cell lung cancer. Cancer Invest 2013; 31:183 - 8; http://dx.doi.org/10.3109/07357907.2013.767342; PMID: 23432821
- Puel A, Leonard WJ. Mutations in the gene for the IL-7 receptor result in T(-)B(+)NK(+) severe combined immunodeficiency disease.. Curr Opin Immunol 2000; 12:468 - 73; http://dx.doi.org/10.1016/S0952-7915(00)00122-9; PMID: 10899029
- Roifman CM, Zhang J, Chitayat D, Sharfe N. A partial deficiency of interleukin-7R alpha is sufficient to abrogate T-cell development and cause severe combined immunodeficiency.. Blood 2000; 96:2803 - 7; PMID: 11023514
- Hakim FT, Gress RE. Reconstitution of the lymphocyte compartment after lymphocyte depletion: a key issue in clinical immunology.. Eur J Immunol 2005; 35:3099 - 102; http://dx.doi.org/10.1002/eji.200535385; PMID: 16231288
- Napolitano LA, Burt TD, Bacchetti P, Barrón Y, French AL, Kovacs A, Anastos K, Young M, McCune JM, Greenblatt RM. Increased circulating interleukin-7 levels in HIV-1-infected women.. J Acquir Immune Defic Syndr 2005; 40:581 - 4; http://dx.doi.org/10.1097/01.qai.0000187442.53708.b4; PMID: 16284535
- Sereti I, Dunham RM, Spritzler J, Aga E, Proschan MA, Medvik K, Battaglia CA, Landay AL, Pahwa S, Fischl MA, et al, ACTG 5214 Study Team. IL-7 administration drives T cell-cycle entry and expansion in HIV-1 infection. Blood 2009; 113:6304 - 14; http://dx.doi.org/10.1182/blood-2008-10-186601; PMID: 19380868
- Budagian V, Bulanova E, Paus R, Bulfone-Paus S. IL-15/IL-15 receptor biology: a guided tour through an expanding universe.. Cytokine Growth Factor Rev 2006; 17:259 - 80; http://dx.doi.org/10.1016/j.cytogfr.2006.05.001; PMID: 16815076
- Mitchell DA, Cui X, Schmittling RJ, Sanchez-Perez L, Snyder DJ, Congdon KL, Archer GE, Desjardins A, Friedman AH, Friedman HS, et al. Monoclonal antibody blockade of IL-2 receptor α during lymphopenia selectively depletes regulatory T cells in mice and humans. Blood 2011; 118:3003 - 12; http://dx.doi.org/10.1182/blood-2011-02-334565; PMID: 21768296
- Miller CN, Hartigan-O’Connor DJ, Lee MS, Laidlaw G, Cornelissen IP, Matloubian M, Coughlin SR, McDonald DM, McCune JM. IL-7 production in murine lymphatic endothelial cells and induction in the setting of peripheral lymphopenia. Int Immunol 2013; 25:471 - 83; http://dx.doi.org/10.1093/intimm/dxt012; PMID: 23657000
- Andarawewa KL, Paupert J, Pal A, Barcellos-Hoff MH. New rationales for using TGFbeta inhibitors in radiotherapy.. Int J Radiat Biol 2007; 83:803 - 11; http://dx.doi.org/10.1080/09553000701711063; PMID: 18058368
- Tang J, Nuccie BL, Ritterman I, Liesveld JL, Abboud CN, Ryan DH. TGF-beta down-regulates stromal IL-7 secretion and inhibits proliferation of human B cell precursors.. J Immunol 1997; 159:117 - 25; PMID: 9200446
- Stupp R, Hegi ME, Mason WP, van den Bent MJ, Taphoorn MJ, Janzer RC, Ludwin SK, Allgeier A, Fisher B, Belanger K, et al, European Organisation for Research and Treatment of Cancer Brain Tumour and Radiation Oncology Groups, National Cancer Institute of Canada Clinical Trials Group. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial.. Lancet Oncol 2009; 10:459 - 66; http://dx.doi.org/10.1016/S1470-2045(09)70025-7; PMID: 19269895
- Mielke S, Rezvani K, Savani BN, Nunes R, Yong AS, Schindler J, Kurlander R, Ghetie V, Read EJ, Solomon SR, et al. Reconstitution of FOXP3+ regulatory T cells (Tregs) after CD25-depleted allotransplantation in elderly patients and association with acute graft-versus-host disease. Blood 2007; 110:1689 - 97; http://dx.doi.org/10.1182/blood-2007-03-079160; PMID: 17478639
