Abstract
Paraneoplastic neurological disorders (PNDs) are syndromes that develop in cancer patients when an efficient antitumor immune response, directed against antigens expressed by both malignant cells and healthy neurons, damages the nervous system. Herein, we analyze existing data on the mechanisms of loss of self tolerance and nervous tissue damage that underpin one of the most frequent PNDs, the anti-Hu syndrome. In addition, we discuss future directions and propose potential strategies aimed at blocking deleterious encephalitogenic immune responses while preserving the antineoplastic potential of treatment.
Introduction
Paraneoplastic neurological disorders (PNDs) are neurological manifestations associated with cancer that do not originate from tumor growth or the metabolic, infectious and toxic complications of malignancy and/or its treatment. This group of syndromes is highly diverse in terms of tissue damage and clinical signs. PNDs develop in the frame of naturally occurring antitumor immune responses. The targets of such an immunological response against malignant cells are self antigens physiologically expressed by the normal nervous system and ectopically expressed by cancer cells. One of the most frequent PND is the paraneoplastic encephalomyelopathy/paraneoplastic sensory neuronopathy (PEM/PSN). PEM/PSN has also been named anti-Hu antibody-associated (or simply anti-Hu) syndrome, after its linkage with high circulating levels of anti-Hu antibodies has been appreciated.
Clinical Aspects of the Anti-Hu Syndrome
In 85% of the cases, the anti-Hu syndrome is associated with lung cancer, mostly small-cell lung cancer (SCLC). More rarely, it develops in association with extrathoracic neoplasms such as neuroblastoma or intestinal, prostate, breast, bladder, and ovary carcinomas. Some patients affected by the anti-Hu syndrome present more than one primary cancer. Neurological manifestations compatible with the diagnosis of PEM/PSN have been observed in 1.4% of 432 SCLC patients and much more rarely in individuals bearing other types of cancer.Citation1,Citation2
A polyclonal antibody response against nuclear antigens expressed in the central nervous system (CNS), enteric neurons and dorsal root ganglion neurons, but not in other adult normal tissues was first identified in 1985 in 2 patients presenting SCLC associated with subacute sensory neuropathy.Citation3 The expression “anti-Hu antibodies” was coined based on the first 2 letters of the surname of these patients. Anti-Hu antibodies were soon detected in multiple patients manifesting a rapid development of diverse neurological manifestations, including sensory neuropathy, cerebellar ataxia, limbic encephalitis, brainstem encephalitis, myelitis, or intestinal pseudo-obstruction. Anti-Hu antibodies are in fact frequently detected in multiple of the above-mentioned syndromes occurring in a paraneoplastic context. Interestingly, high titers of anti-Hu antibodies are absent in SCLC patients who do not exhibit neurological disorders.
Of major clinical importance, these neurological manifestations precede the diagnosis of the cancer in more than 80% of the cases. Therefore, the detection of high circulating titers of anti-Hu antibodies in patients with a neurological condition indicates a paraneoplastic syndrome and should lead to a very thorough search of the underlying neoplasm. In some instances, the tumor is discovered only post-mortem or never, indicating either a complete disease regressionCitation4 or the occurrence of such autoimmune neurological manifestations independent of neoplasia. Indeed, cases of limbic encephalitis associated with anti-Hu antibodies but not with cancer have been described in children.Citation5 A spontaneously regressing or occult neuroblastoma cannot be formally excluded in these rare cases.
Interestingly, tumors exhibit a significantly less severe course and a lower propensity to generate metastases in patients with PEM/PSN than in patients who do not develop PNDs.Citation6,Citation7 The effect on tumor spread and overall survival is however moderate, and only 30% of patients survive for more than 2 y.Citation7,Citation8 In as much as 60% of the cases, the prognosis of the patients is determined by the outcome of the neurological disorders rather than by tumor progression.Citation9 Of note, SCLC patients who do not manifest PNDs but harbor low titers of anti-Hu antibodies experience tumors of low stage, frequently respond to therapy, and live longer than cancer patients not producing anti-Hu antibodies.Citation10,Citation11 These findings reinforce the hypothesis that effective antitumor immune responses targeting Hu antigens may be dissociated from autoimmune manifestations.
As anti-Hu antibodies bind to antigens expressed both in malignant cells and healthy neurons, it was tempting to hypothesize that the anti-Hu syndrome could be an autoimmune disease triggered by cancer. This is reminiscent of vitiligo, developing in certain cases of melanoma,Citation12 although in the case of PNDs, the cells targeted by the autoimmune process belong to a different tissue than their malignant counterparts, i.e., the immunoprivileged CNS.
Hu Antigens
Hu antigens in healthy neurons
The search for the molecular targets of anti-Hu antibodies led to the identification of 3 proteins of approximate molecular weight of 35–38 KDa expressed in the CNS. In malignant cells, the 38 KDa band is not detected. Screening a cDNA expression library from the human cerebellum with the sera of patients affected by the anti-Hu syndrome allowed for the cloning of the highly homologous genes HuD (official name: ELAV like neuron-specific RNA binding protein 4, ELAVL4), HuC, (official name: ELAV like neuron-specific RNA binding protein 3, ELAVL3) and HuB (official name; ELAV like neuron-specific RNA binding protein 2, ELAVL2; also known as Hel-N1). The Hu family actually consists of 4 members: HuA (official name: ELAV like RNA binding protein 1, ELAVL1; also known as HuR), HuB, HuC, and HuD. These proteins share extensive homology and are highly-conserved across species.Citation13 HuA, the most divergent, is ubiquitously expressedCitation13,Citation14 and is not recognized by paraneoplastic antibodies. In contrast, HuB, HuC, and HuD expression is restricted to neurons, which all express one or more of these proteins.Citation13
The Hu proteins are highly homologous to the Drosophila melanogaster nuclear protein ELAV (embryonic lethal abnormal visual), which plays a key role in neuronal development.Citation15,Citation16 HuB, HuC, and HuD operate as neuron-specific RNA-binding proteins. They contain 3 distinct RNA recognition motifs that bind to AU-rich sequences, which often characterize the 3′ untranslated region of mRNAs and control their expression.Citation17 Indeed, the Hu proteins have been implicated in mRNA stabilization and RNA turnover.Citation18,Citation19 Among various target mRNAs, HuD has recently been shown to bind, hence stabilizing, the brain-derived neurotrophic factor (BDNF)-coding mRNA.Citation20 Moreover, neuronal Hu proteins have been shown to bind pre-mRNA species, thus regulating their alternative splicing.Citation21 HuD is highly expressed in the nucleus, but also translocates to the cytoplasm, where it localizes within small granules.Citation22 The cytoplasmic localization of HuD appears to be essential for neuronal differentiation.Citation23 In addition, HuD has been shown to interact with AKT1 to trigger the outgrowth or neurites.Citation24 Thus, Hu proteins, in particular HuD, have a major impact on multiple key neuronal processes.
Hu antigens in malignant cells
Whereas HuB, HuC, and HuD are all expressed in CNS neurons, HuD is Hu antigen most frequently expressed by SCLC cells. This is a consistent observation in SCLC patients, irrespective of whether they develop PEM/PSN or not.Citation6,Citation25 The HuD-coding mRNA has also been detected in SCLC cell lines.Citation26 No somatic mutations or deletions/insertions affecting HuD have been observed in SCLC cell lines or tumors, including those from patients with PEM/PSN.Citation27-Citation29 However, a fine analysis of the post-translational modifications of HuD in cancer cells from patients developing or not PNDs has not been performed to date. Indeed, malignant cell-specific post-translational modifications of HuD may favor the development of an immune response that cross-reacts with neuronal Hu proteins. Moreover, the possible function(s) of HuD in the tumor biology, if any, has not been uncovered so far. SCLC and many cancers associated with PEM/PSN exhibit a neuroectodermal origin. The neuroectoderm includes the neural crest (melanocytes, dorsal root ganglia, ganglia of the autonomous nervous system, adrenal glands, Schwann cells, …) and the neural tube (brain, spinal cord, retina, neurohypophysis). As a result, most SCLCs are composed of cells with a neuroendocrine phenotype, characterized by elevated expression levels of L-dopa decarboxylase, neuron-specific enolase, and neuropeptides.Citation30 These features resemble those of neural crest-derived endocrine cells disseminated in the normal bronchial mucosa. Such a shared embryonic origin could explain the expression of HuD by SCLC cells.
Autoimmune responses against Hu proteins
Humoral responses
The serum of patients with the anti-Hu syndrome reacts with HuB, HuC, and HuD. Since HuD is the only Hu protein consistently expressed by SCLCs,Citation25 it is the most likely initiator of autoimmune response against Hu antigens.
The presence of anti-HuD antibodies can be revealed using a large variety of techniques. The initial screening is generally performed by indirect immunohistochemistry on frozen sections of healthy human or rodent cerebral cortex, which results in a strong nuclear and weak cytoplasmic staining in the presence of anti-HuD antibodies (). To firmly identify the antigenic targets, immunoblotting is performed as a complementary test (), using either neuronal nuclear extracts or recombinant HuD.Citation31-Citation33 ELISA can also be used, but its sensitivity appears to be lower than that than of immunoblotting on purified HuD.Citation33
Figure 1. Detection of autoantibodies associated with the anti-Hu syndrome. (A) Patient sera containing Hu-specific autoantibodies specifically recognize neurons in the central nervous system (CNS) (magnification 200 ×). Briefly, hippocampal (left panels) and cerebellar (right panels) rat slices (EuroImmun) were incubated with serum from either an individual not affected by paraneoplastic neurological disorders (PNDs) (negative control), either a patient with a confirmed anti-Hu syndrome (positive control), or a subject suspected to suffer from the anti-Hu syndrome, followed by the detection of bound IgG using biotinylated goat anti-human IgG, ABC and VIP kits (Eurobio). The test serum turned out to contain anti-neuron antibodies exhibiting a staining pattern compatible with that of anti-Hu antibodies. (B) The molecular identification of the specificity of such neuron-specific IgGs is made by immunoblotting-based diagnostic tests. Patient-derived serum (1/100 dilution) and cerebrospinal fluid (when available) are incubated on a membrane stripped (right panel) with paraneoplastic neurological syndrome-associated antigens, including amphiphysin, CV2, PNMA2 (Ma2/Ta), Ri, Yo, and Hu proteins (EuroImmun). Upon washing, bound IgGs are detected using alkaline phosphatase-conjugated anti-human IgG antibodies and their position is confronted to a reference scheme.
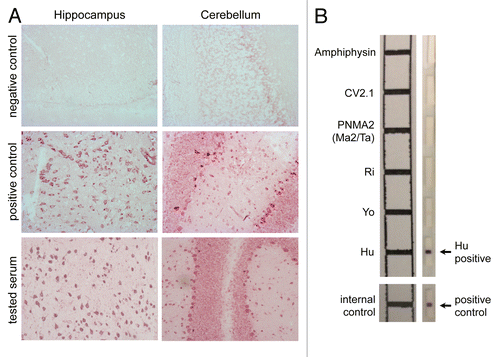
The titers of circulating anti-Hu antibodies usually ranges from 2000 to 64 000 fold dilution in patients with PEM/PSN.Citation32,Citation34 Elevated serum titers of anti-Hu antibodies provide a highly valuable diagnostic biomarker. Conversely, low levels of anti-Hu antibodies can be detected in the absence of PNDs in 16 to 22.5% of SCLC patients and in less than 1% of patients with other malignancies.Citation10,Citation33,Citation35
The anti-HuD IgG response is polyclonal and subclass distribution analyses revealed that anti-Hu IgGs belong predominantly to the IgG1 and IgG3 isotypes.Citation34,Citation36,Citation37 The HuD domains encompassing residues 90–101 and 171–206 are recognized by all anti-Hu serum samples. Conversely, the 223–234, 235–252, and 354–373 epitopes only react with sera of some patients with the anti-Hu syndrome.Citation38
The titers of anti-Hu antibodies in the cerebrospinal fluid (CSF) vary from 100 to 4000 fold dilution, pointing to intrathecal synthesis.Citation32,Citation39,Citation40 Indeed, when adjusted to the same IgG concentration, the CSF/serum anti-Hu antibody index is > 1 in 86% of patients with paraneoplastic encephalomyelopathy.Citation39 In patients exhibiting a CSF/serum antibody index > 1.5, a strong indication of the oligoclonal intrathecal synthesis of HuD-specific IgGs was obtained using isoelectric focusing followed by immunoblotting on HuD-loaded nitrocellulose membranes.Citation41 Collectively, these findings indicate that anti-HuD autoantibodies are produced intrathecally in patients with robust CNS involvement. However, patients presenting with isolated PSNs do not exhibit intrathecal anti-Hu antibody synthesis.Citation32 Interestingly, plasmapheresis succeeds in reducing the levels of anti-Hu autoantibodies in the serum but not in the CSF.Citation39
Anti-Hu antibodies persist over time in the serum of SCLC patients with PEM/PSN while SCLC patients who do not develop PND remain seronegative.Citation42 Importantly, 8–40% of the patients with PEM/PSN and anti-Hu antibodies also harbor autoantibodies targeting other neural antigens.Citation35,Citation43,Citation44
Functional properties of anti-Hu antibodies
Since anti-Hu antibodies target intracellular antigens, their pathogenic and antitumor potential is questionable. Interestingly, a study documented the expression of Hu-related antigens on the surface of SCLC and neuroblastoma cell lines.Citation45 Whether neurons exhibit a similar expression pattern of Hu proteins is currently undetermined.
Experimentally, the contribution of anti-Hu antibodies to the development of PEM/PSN has been investigated in vitro, in cell cultures, and in vivo, upon injection of human antibodies to laboratory animals. In vitro, monoclonal antibodies specific for HuD have been shown to promote the apoptotic demise of neuroblastoma cell lines and primary myenteric neurons.Citation46 The serum or IgG preparations from patients with the anti-Hu syndrome could also kill human HuD-expressing cell lines and primary rodent neurons, a phenomenon that was accelerated in the presence of the complement system.Citation46-Citation48 However, this observation is not universal, and the lack of cytotoxic effects of anti-Hu positive sera on HuD+ cancer cells or primary murine neurons has also been reported.Citation49,Citation50 Moreover, even when neuronal cytotoxicity was observed, the specificity of the pathogenic autoantibodies was questioned, as the patients’ IgGs, but not an anti-HuD monoclonal antibody tested alongside, mediated such an effect.Citation48 As discussed above, the serum of patients with PND often contain multiple neuron-targeting autoantibodies, some of which recognize surface receptors. Therefore, unless proper specificity assays are conducted, for instance upon pre-absorbing IgG preparations on recombinant HuD, formal conclusions regarding the impact of anti-Hu antibodies on neuronal viability are difficult to reach.
The intravenous administration of IgGs from patients with PEM/PSN and high titers of anti-Hu antibody to mice did not result in any CNS lesion.Citation51,Citation52 In addition, all attempts to generate rodent models of the anti-Hu syndrome upon immunization with recombinant HuD failed to induce any signs of disease, CNS inflammation or neuronal degeneration, despite the development of high circulating titers of anti-HuD antibodies.Citation51,Citation53
Thus, although the presence of high anti-Hu titers in PND patients represents a highly valuable diagnostic tool, their role in disease pathogenesis appears at best dubious. Nevertheless, these autoantibodies reflect a strong autoimmune/antitumor immune response in which autoreactive T cells, specific for HuD or other self antigens, are likely to be involved.
T cell responses
The peripheral blood mononuclear cells (PBMCs) of SCLC patients with the anti-Hu syndrome exhibit an accumulation of activated HLA-DR+CD4+ T cells and memory CD45RO+CD4+ helper T cells as compared with anti-Hu antibody-negative SCLC patients.Citation54,Citation55 An increase in activated CD8+ T cells in these patients has also been reported.Citation55 CD25brightCD4+ FOXP3+ T cells of untested immunosuppressive potential are elevated in SCLC patients regardless of the development of PEM/PSN. However, a reduced expression of FOXP3-, transforming growth factor β1 (TGFβ1)- and cytotoxic T lymphocyte-associated protein 4 (CTLA4)-coding mRNAs has been detected in regulatory T cells from SCLC patients with PEM/PSN, suggesting a defect in their immunosuppressive activity.Citation56 Consistent with the pleiocytosis observed in diagnostic CSF analyses,Citation57 increased amounts of T cells, mostly memory CD4+ and CD8+ T cells, characterize the CSF of patients with the anti-Hu syndrome.Citation58
The involvement of (most often circulating) autoreactive T cells in the anti-Hu syndrome has been investigated using a variety of methodological approaches. The PBMCs of SCLC patients with high anti-Hu antibody titers proliferate more vigorously in response to HuD and secrete abundant interferon γ (IFNγ), but not IL-4, as compared with PBMCs from SCLC patients without anti-Hu antibodies.Citation54 HuD is therefore a likely antigenic target for primed autoreactive CD4+ T cells, presumably of the TH1 subtype, implicating cell-mediated immunity in the anti-Hu syndrome. However, CD4+ T-cell depletion, purification or blockade experiments using anti-HLA class II antibodies have not yet been performed, and would be needed to unambiguously identify the responding cell type.
CD8+ T cells are considered to be the killer cell by excellence and are suspected to play a key pathogenic role in paraneoplastic cerebellar degeneration, another type of PND.Citation59,Citation60 In addition, solid tumors can express high level of MHC class I molecules and accumulating data indicate that MHC class I molecules can be upregulated on neurons, in particular during inflammatory conditions.Citation61 Thus, both cancer cells and neurons could be the targets of CD8+ T cells. However, in the past decade, efforts to identify HuD-specific CD8+ T cells in SCLC patients with the anti-Hu syndrome, using a combination of cytokine release assays and flow cytometry based on HLA:HuD peptide tetramers, have yielded conflicting observations. Indeed, some investigators have reported negative results,Citation62,Citation63 while others have found consistent evidence for such HuD-specific CD8+ T cells.Citation64-Citation66 This discrepancy may relate to the methodological approaches employed by these groups. Alternatively, it could be connected to timing, as the PBMCs of patients assessed within the first 3 mo of PEM/PSN onset exhibited more robust IFNγ ELISPOT responses to HuD-derived peptides than the PMBCs of patients tested later.Citation65 Finally, the abovementioned discrepancy may reflect the assay used for the detection of HuD-specific CD8+ T cells. Indeed, in a study involving 4 HLA-A03:01-expressing patients with the anti-Hu syndrome, the PBMCs of only one were found to exhibit a classical cytotoxic profile with IFNγ secretion in response to the HuD140–148 peptide. Surprisingly, the PBMCs of 2 patients displayed a robust production of interleukin (IL)-5 and IL-13 in response to HuD164–172 stimulation, but almost undetectable cytotoxicity or IFNγ production.Citation67 Of note, the PBMCs of none of the control subjects exhibited any type of response to HuD-derived peptides. These data suggest that anti-HuD CD8+ T-cell responses could be skewed toward a type 2, non-cytotoxic, profile in some patients with the anti-Hu syndrome, a pattern that could be easily missed using usual functional assays. However, whether the type of CD8+ T-cell response observed in PBMCs is reflective of what occurs within neoplastic lesions and in the nervous system is unknown. If so, how the effective antitumor response and neurological damage could be mechanistically explained?
Finally, it is possible that autoreactive T cells in the anti-Hu syndrome recognize additional or alternative neuronal antigens. Indeed, although antigens recognized by autoantibodies and autoreactive T cells largely overlap in most organ-specific autoimmune diseases, this is not necessarily the case.Citation68 This aspect has not yet received adequate attention.
How is immune tolerance to neuronal Hu antigens broken in patients with PEM/PSN?
Studies performed in mice revealed that HuD-specific CD8+ T cells can be elicited upon immunization with recombinant adenoviruses expressing full-length HuD.Citation69 However, the need for adjuvants and for further in vitro stimulation to detect HuD-specific CD8+ T cells point to a reduced frequency of HuD-specific T-cell precursors, their limited functional avidity, and/or to the existence of robust immunosuppressive network that operate in vivo to maintain these cells under control. The robust ex vivo CD8+ T-cell response generated in HuD-deficient, but not in wild-type, mice provided direct evidence for partial induction of tolerance to HuD under physiological conditions.Citation69 Interestingly, HuD is expressed, although at low levels, by MHCIIhigh thymic medullary epithelial cells and thymic CD8+ dendritic cells (http://immgen.org/). Importantly, patients with the anti-Hu syndrome do not manifest autoimmune reactions in tissues other than the nervous system, underlining the selective breakdown of tolerance to HuD, but not other Hu antigens such as HuA. However, up to 40% of patients with the anti-Hu syndrome harbor autoantibodies specific for other neuronal proteins, such as the P/Q type voltage-gated calcium channel (associated with Lambert–Eaton myasthenic syndrome), indicating that self sensitization is not exquisitely directed to Hu antigens.Citation43
Given the constant expression of HuD by SCLC cells, it is currently unclear why some patients develop PEM/PSN whereas others do not. Parameters intrinsic to the tumor and/or to the host may contribute to this range of variability. In patients with SCLC and the anti-Hu syndrome, MHC class I molecules were detected by immunohistochemistry at moderate or high levels in the neoplastic lesions of 13 out of 15 cases, whereas such levels were observed in none of 11 SCLC patients who did not develop PEM/PSN. In addition, the focal expression of HLA-DR was more frequent in tumors from SLCL patients with the anti-Hu syndrome than in patients without (6/15 vs 0/11).Citation42 As a cause or consequence of this HLA expression pattern, tumors from PND patients may be heavily infiltrated with inflammatory cells.Citation70 However, a thorough comparison of the cellular and molecular immune signature of neoplastic lesions from patients who developed or not PNDs has not yet been performed. This is highly relevant since the type, density, and location of tumor-infiltrating immune cells as well as the expression of genes related to immunological functions carry prognostic and predictive value.Citation71 As mentioned earlier, either cancer cell-specific post-translational modifications of HuD and an unusual antigen processing within neoplastic lesions could favor the development of autoimmune responses targeting neuronal Hu. Such mechanisms have been implicated in tissue-specific autoimmune diseases in non-paraneoplastic contexts.Citation68,Citation72,Citation73 As for host factors, the HLA-DR3 and HLA-DQ2 have been associated with the anti-Hu syndrome in one study,Citation74 but not in a second one assessing only DR alleles.Citation75 To date, no other genetic polymorphisms have been associated with PEM/PSN.
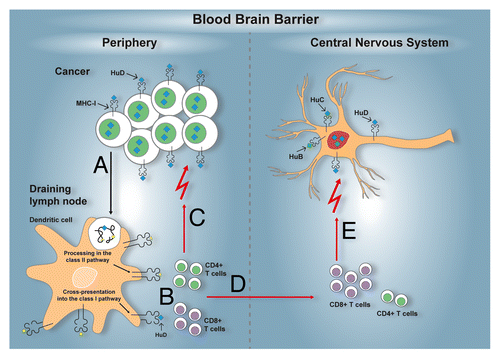
Here is a plausible scenario for the development of an autoimmune reaction against Hu proteins in patients with cancer of neuroectodermal origin. Although all SCLCs express HuD, the cross-presentation of HuD by dendritic cells and the activation of an efficient anti-Hu T-cell response is expected to occur only in a subset of patients, based on the available T-cell repertoire and genetic polymorphisms impacting on the magnitude and quality of the immune response, such as those within the HLA locus. The priming of a T-cell response specific for HuD (and possibly other onconeural antigens) may be sustained within the neoplastic lesion itself, owing to the ability of malignant cells to express MHC molecules, possibly resulting in the inhibition of tumor growth. Since 15% of SCLC patients harbor low circulating titers of anti-Hu antibodies in the absence of neurological manifestations, HuD-specific immune responses might need to exceed a minimal threshold to overcome the relative immune privilege of the CNS. Above such a threshold, the HuD-specific immune response is expected to involve the CNS, as documented by the intrathecal synthesis of HuD-specific IgGs,Citation41 as well as by the detection of HuD-specific CD8+ T cells in the CSF.Citation67 This chronic autoimmune reaction ultimately leads to neuronal destruction and fixed neurological disability (). The stabilization of neurological signs as well as the disappearance of anti-Hu antibodies has been associated with complete tumor response to therapy.Citation7,Citation76 These data suggest that malignant cells fuel the autoimmune process through the release of tumor-associated antigens.
Figure 2. Immunopathogenesis of the anti-Hu syndrome. An anti-Hu syndrome occurs when a vigorous immune response develops against the normally neuron-restricted antigen HuD ectopically expressed by an underlying tumor, most often small-cell lung carcinoma (SCLC). Tissue-resident dendritic cells capture antigens derived from malignant cells, including HuD (A). After the homing of these dendritic cells to tumor-draining lymph nodes, tumor-associated antigens, including HuD, are processed and presented to activate antigen-specific CD4+ and CD8+ T cells (B). The activation of tumor-specific T cells may also occur within neoplastic lesions. In either case, tumor-specific T cells can reach neoplastic lesions. As cancer cells sometimes express MHC class I molecules and likely present HuD-derived peptides, they become attractive targets for HuD-specific CD8+ T cell killing. Partial (or in rare cases complete) control of the tumor growth is therefore afforded (C). In parallel, the HuD-specific T cells activated in tumor-draining lymph nodes and/or within neoplastic lesions, circulate and acquire the capacity to cross the blood-brain-barrier (D). In the central nervous system (CNS), neurons can also express MHC class I molecules, in particular under inflammatory conditions, and can therefore present peptides derived from the highly homologous HuD, HuB, HuC proteins. Thus, neurons become additional targets for HuD-specific CD8+ T cells, resulting in neuronal tissue damage and the related paraneoplastic neurological manifestations (E).
Mechanisms of Tissue Damage in the Nervous System
The anti-Hu paraneoplastic syndrome is a heterogeneous disorder with highly diverse clinical and pathological manifestations in terms of lesion sites, inflammatory infiltrates, and neuronal damage. Despite this heterogeneity, a good correlation exists between the location and magnitude of neuronal loss or tissue damage and the type and severity of the clinical signs. Variable regions of the nervous system can be affected, with a preferential targeting of the hippocampus, lower brain stem, spinal cord, and dorsal root ganglia (DRG).Citation9,Citation77,Citation78 However, the cortex, medulla, amygdala, putamen, cingulate gyrus, and pons can also be affected.Citation77,Citation78 Neuropathological lesions are usually more widespread than anticipated based on clinical data. In the affected areas, neuronal loss, axonal damage, reactive gliosis, and microglial nodules are conspicuous.Citation79 In contrast, there is no evidence of astrocyte and oligodendrocyte loss or demyelination.Citation79 Ongoing neuronal apoptosis is either lacking or only occasionally detected, possibly a result of the subacute/chronic course of the disease or the post-mortem origin of the tissue under investigation.Citation77,Citation79
In patients with PEM/PSN, IgG deposits are detected in the nervous tissue, predominantly in the nuclei of CNS and DRG neurons, and to a lesser degree in the cytoplasm.Citation10,Citation37,Citation80 This cellular distribution is highly reminiscent of the staining pattern observed with anti-Hu antibodies. The extraction of IgGs deposited in nervous tissues revealed that at least part of these IgGs are indeed anti-Hu antibodies. In some patients, abundant IgG deposits have been detected on the neuronal surface,Citation37 suggesting the co-existence of additional anti-neuron autoantibodies. The amount of anti-Hu IgGs in specific areas of the nervous system is higher than its serum counterpart.Citation80 However, the major clinical signs do not correlate with the localization of anti-Hu IgGs, as assessed by histology.Citation78,Citation80 Furthermore, in mice transferred with IgG from patients affected by the anti-Hu syndrome, intraneuronal IgG deposits were no longer detected if the animals were perfused before brain tissue processing.Citation51 The question of whether the anti-Hu IgGs deposited in the neuronal tissue of patients with PEM/PSN represents an artifact or a phenomenon of potential pathogenic relevance is therefore raised. Irrespective of this issue, which requires further investigation, the complement system does not seem to play a significant role in the pathogenesis of the anti-Hu syndrome. Indeed, parenchymal C3 immunoreactivity is weak or absent and no C9neo staining (a marker of complement-mediated tissue injury) is observed in affected brain areas.Citation37,Citation77,Citation79,Citation81
Inflammatory infiltrates are commonly found in DRG as well as in the CNS perivascular space and parenchyma. They are mainly composed of T cells, whereas natural killer (NK) cells are absent.Citation37,Citation77,Citation79,Citation81 B cells are occasionally detected in the perivascular cuffs and meninges but they rarely infiltrate the brain parenchyma. In inflamed tissues, macrophages are mostly restricted to the perivascular space, whereas CD68+ microglial cells are found in the parenchyma.Citation37,Citation77,Citation82,Citation83 Among T lymphocytes, CD4+ T cells are mainly located in perivascular areas, whereas CD8+ T cells represent by far the major component of the parenchymal infiltrates.Citation37,Citation77,Citation79,Citation82,Citation83 CD8+ T cells usually form clusters and frequently localize around neurons.Citation77,Citation79 Immunohistochemical analyses revealed a close contact of cytotoxic granule-containing T cells with neurons in the brain tissue or DRG of patients with the anti-Hu syndrome, with, in some instances, indentation of the neuronal surface by the “attacking” CD8+ T cells.Citation83 MHC class I molecules has been shown to be expressed on neurons in the inflamed nervous tissue from patients with PEM/PSN.Citation79,Citation84 In this context, the close apposition of CD8+ T cells with neurons and the polarization of membranous CD107aCitation79 may reflect a cognate, antigen-dependent, in vivo interaction leading to the neuronal demise. In addition, CD8+ T cell-derived IFNγ has recently been demonstrated to promote the loss of dendrites and synapses, underlying the need to assess presence of this cytokine (or phosphorylated signal transduction and activator of transcription 1, STAT1) in brain sections from patients affected by the anti-Hu syndrome.Citation85 In these individuals, the amount of reactive microglia is increased in all affected areas of the CNS. Microglial cells participate in the clusters of inflammatory cells in the gray matter and, together with CD8+ T cells, surround neurons.Citation77
Collectively, these observations strongly suggest that a cytotoxic CD8+ T cell-mediated attack contributes to neuronal death and axonal damage that characterize the anti-Hu syndrome, and therefore to its neurological manifestations. However, it remains to be determined whether these CD8+ T cells are Hu-specific. Studies of the specificity of tissue-derived TCRs should help resolve this question. To date, the analysis of TCR β chain usage by immunohistochemistry has revealed an overrepresentation of certain Vβ families in 3 out of 5 patients with PEM/PSN. Lesion-infiltrating T cells expressing the expanded Vβ families were mostly CD8+ T cells. Interestingly, in some patients, similarly expanded Vβ sequences were detected in the CSF or affected areas of the nervous system as well as within neoplastic lesions.Citation76,Citation86
Future Directions
Future investigations to obtain better insights into the mechanisms leading to antitumor immunity and nervous tissue autoimmunity in patients with PEM/PDN should integrate the in-depth molecular analysis of tissue samples (neoplastic lesions as well as neural tissue) and the development of relevant animal models. In turn, this new knowledge should assist the development of effective immunotherapies, which are dearly needed for patients suffering from the anti-Hu syndrome.
The mechanisms that trigger anti-Hu antibody-associated PNDs are not yet elucidated but appear tightly linked to the development of a partly efficient antitumor immune response. The comparison of the type, density, and location of tumor-infiltrating immune cells in SCLC patients who developed or not an anti-Hu syndrome, complemented by gene-expression profiling studies, may identify signatures that are predictive of improved tumor control and/or PND development. Confronted to data emerging from a wide variety of cancers,Citation71 this information will help investigators to assess whether the magnitude and functional orientation of intratumoral adaptive immune responses correlate with development of the anti-Hu syndrome. Similarly, the immune reaction developing in the nervous tissue of patients with PEM/PDN needs to be dissected at the molecular level using unbiased approaches. Given the dramatic and subacute course of the neurological disease in many patients with the anti-Hu syndrome, post-mortem tissues are available in which neurons are actively targeted by the immune system. A thorough investigation of the antigen specificity of CNS-infiltrating CD8+ T cells using a combination of tissue laser microdissection, TCR cloning and antigen screeningCitation76,Citation87 will inform whether HuD is the key antigenic target for pathogenic T cells or whether alternative/additional autoantigens are involved.
Animal models represent valuable tools to test hypotheses regarding the initiation and the effector mechanisms of human autoimmune diseases, as well as for the development of new therapeutic strategies. A valid animal model for the anti-Hu syndrome should recapitulate the following features: (1) the existence of one or more antigens that are expressed by both malignant cells and normal neurons; (2) the development of a detectable immune response against such shared antigens; (3) the inhibition of tumor growth by this antitumor immune response; (4) the immune infiltration of the nervous system as well as neuronal death and its related clinical consequences as a result of the antitumor/autoimmune response. To date, no animal model of the anti-Hu syndrome has been successfully developed. Attempts to mimic the human disease through the transfer of patient-derived IgGs or the deliberate immunization of experimental animals with HuD have resulted in high titers of circulating anti-HuD antibodies but no neuropathological lesions.Citation51,Citation88 Interestingly, a mouse strain bearing a conditional deletion of both Trp53 and Rb1 in the lung develops Hu-expressing SCLCs. Although about 15% of these mice also develop high titers of anti-Hu antibodies in the serum, no neurological signs become manifest.Citation89 Similarly, the adoptive transfer of rat TH1 cells specific for an onconeural antigen led to meningeal and perivascular CNS inflammation but failed to induce neuronal damage or overt clinical manifestations in recipient animals.Citation90 Therefore, a robust animal model is still needed to pinpoint the immunological mechanisms leading to neurological tissue damage in patients with the anti-Hu syndrome, and to test therapeutic strategies aimed at blocking the encephalitogenic immune response without affecting productive antitumor immunity.
An early diagnosis of PEM/PSN is possible since the anti-Hu antibody provides a highly specific biomarker. The effective treatment of the underlying tumor represents the main but not the sole facet of PEM/PSN management. Indeed, a wealth of data suggests that immunotherapy should be initiated before neurological symptoms have reached their plateau. To date, various approaches have been used, ranging from high-dose corticosteroids, to intravenous immunoglobulins and cyclophosphamide, but they have provided benefit to a few patients.Citation7,Citation44,Citation91 A study showed no detectable adverse effects of these immunotherapies on tumor control or patient survival, although it was underpowered to detect the negative impact of a given intervention.Citation7 Based on the information detailed above, pointing at CD8+ T cells as key immune effectors of the neurological tissue damage experienced by patients with the anti-Hu syndrome, an early and aggressive targeting of these cells should be envisioned. Approved molecules preventing the entry of T cells into the nervous system, such as anti-α4 integrin antibodies or functional antagonists of the sphingosine-1-phosphate receptor, represent very attractive possibilities in this respect.Citation92-Citation94 In addition, given the deleterious role of IFNγ in CD8+ T cell-mediated neurological disorders,Citation85,Citation95 IFNγ blockade could also constitute a valuable strategy, provided that it does not interfere with anticancer therapy. Conversely, the blockade of immune checkpointsCitation96 should be tested with utmost caution in patients bearing tumors potentially associated with the anti-Hu syndrome, as they may unleash a dramatic neurological autoimmunity, in particular among individuals with detectable anti-Hu antibodies.
| Abbreviations: | ||
| BDNF | = | brain-derived neurotrophic factor |
| CNS | = | central nervous system |
| CSF | = | cerebrospinal fluid |
| DRG | = | dorsal root ganglia |
| ELAV | = | embryonic lethal abnormal visual |
| PBMC | = | peripheral blood mononuclear cell |
| PEM | = | paraneoplastic encephalomyelopathy |
| PND | = | paraneoplastic neurological disorder |
| PSN | = | paraneoplastic sensory neuronopathy |
| SCLC | = | small-cell lung cancer |
| TCR: | = | T cell receptor |
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
We thank Jean-Yves Delattre from ICM, Pitié-Salpetrière, Paris for the Figure 1, Daniel Gonzalez-Dunia for critical reading of the manuscript and ARC, INSERM and Région Midi-Pyrénées for financial support.
Citation: Pignolet BS, Gebauer CM, Liblau RS. Immunopathogenesis of paraneoplastic neurological syndromes associated with anti-Hu antibodies: A beneficial antitumor immune response going awry. OncoImmunology 2013; 2:e27384; 10.4161/onci.27384
References
- Darnell RB, Posner JB. Observing the invisible: successful tumor immunity in humans. Nat Immunol 2003; 4:201; http://dx.doi.org/10.1038/ni0303-201; PMID: 12605223
- Seute T, Leffers P, ten Velde GP, Twijnstra A. Neurologic disorders in 432 consecutive patients with small cell lung carcinoma. Cancer 2004; 100:801 - 6; http://dx.doi.org/10.1002/cncr.20043; PMID: 14770437
- Graus F, Cordon-Cardo C, Posner JB. Neuronal antinuclear antibody in sensory neuronopathy from lung cancer. Neurology 1985; 35:538 - 43; http://dx.doi.org/10.1212/WNL.35.4.538; PMID: 2984600
- Darnell RB, DeAngelis LM. Regression of small-cell lung carcinoma in patients with paraneoplastic neuronal antibodies. Lancet 1993; 341:21 - 2; http://dx.doi.org/10.1016/0140-6736(93)92485-C; PMID: 8093269
- Honnorat J, Didelot A, Karantoni E, Ville D, Ducray F, Lambert L, Deiva K, Garcia M, Pichit P, Cavillon G, et al. Autoimmune limbic encephalopathy and anti-Hu antibodies in children without cancer. Neurology 2013; 80:2226 - 32; http://dx.doi.org/10.1212/WNL.0b013e318296e9c3; PMID: 23658383
- Dalmau J, Graus F, Rosenblum MK, Posner JB. Anti-Hu--associated paraneoplastic encephalomyelitis/sensory neuronopathy. A clinical study of 71 patients. Medicine (Baltimore) 1992; 71:59 - 72; http://dx.doi.org/10.1097/00005792-199203000-00001; PMID: 1312211
- Keime-Guibert F, Graus F, Broët P, Reñé R, Molinuevo JL, Ascaso C, Delattre JY. Clinical outcome of patients with anti-Hu-associated encephalomyelitis after treatment of the tumor. Neurology 1999; 53:1719 - 23; http://dx.doi.org/10.1212/WNL.53.8.1719; PMID: 10563618
- Monstad SE, Drivsholm L, Storstein A, Aarseth JH, Haugen M, Lang B, Vincent A, Vedeler CA. Hu and voltage-gated calcium channel (VGCC) antibodies related to the prognosis of small-cell lung cancer. J Clin Oncol 2004; 22:795 - 800; http://dx.doi.org/10.1200/JCO.2004.01.028; PMID: 14990634
- Graus F, Keime-Guibert F, Reñe R, Benyahia B, Ribalta T, Ascaso C, Escaramis G, Delattre JY. Anti-Hu-associated paraneoplastic encephalomyelitis: analysis of 200 patients. Brain 2001; 124:1138 - 48; http://dx.doi.org/10.1093/brain/124.6.1138; PMID: 11353730
- Dalmau J, Furneaux HM, Gralla RJ, Kris MG, Posner JB. Detection of the anti-Hu antibody in the serum of patients with small cell lung cancer--a quantitative western blot analysis. Ann Neurol 1990; 27:544 - 52; http://dx.doi.org/10.1002/ana.410270515; PMID: 2163235
- Graus F, Dalmou J, Reñé R, Tora M, Malats N, Verschuuren JJ, Cardenal F, Viñolas N, Garcia del Muro J, Vadell C, et al. Anti-Hu antibodies in patients with small-cell lung cancer: association with complete response to therapy and improved survival. J Clin Oncol 1997; 15:2866 - 72; PMID: 9256130
- Byrne KT, Turk MJ. New perspectives on the role of vitiligo in immune responses to melanoma. Oncotarget 2011; 2:684-94;PMID: 21911918
- Okano HJ, Darnell RB. A hierarchy of Hu RNA binding proteins in developing and adult neurons. J Neurosci 1997; 17:3024 - 37; PMID: 9096138
- Ma WJ, Cheng S, Campbell C, Wright A, Furneaux H. Cloning and characterization of HuR, a ubiquitously expressed Elav-like protein. J Biol Chem 1996; 271:8144 - 51; http://dx.doi.org/10.1074/jbc.271.14.8144; PMID: 8626503
- Robinow S, Campos AR, Yao KM, White K. The elav gene product of Drosophila, required in neurons, has three RNP consensus motifs. Science 1988; 242:1570 - 2; http://dx.doi.org/10.1126/science.3144044; PMID: 3144044
- Robinow S, White K. Characterization and spatial distribution of the ELAV protein during Drosophila melanogaster development. J Neurobiol 1991; 22:443 - 61; http://dx.doi.org/10.1002/neu.480220503; PMID: 1716300
- Chung S, Jiang L, Cheng S, Furneaux H. Purification and properties of HuD, a neuronal RNA-binding protein. J Biol Chem 1996; 271:11518 - 24; http://dx.doi.org/10.1074/jbc.271.19.11518; PMID: 8626712
- Bolognani F, Perrone-Bizzozero NI. RNA-protein interactions and control of mRNA stability in neurons. J Neurosci Res 2008; 86:481 - 9; http://dx.doi.org/10.1002/jnr.21473; PMID: 17853436
- Perrone-Bizzozero N, Bolognani F. Role of HuD and other RNA-binding proteins in neural development and plasticity. J Neurosci Res 2002; 68:121 - 6; http://dx.doi.org/10.1002/jnr.10175; PMID: 11948657
- Allen M, Bird C, Feng W, Liu G, Li W, Perrone-Bizzozero NI, Feng Y. HuD promotes BDNF expression in brain neurons via selective stabilization of the BDNF long 3’UTR mRNA. PLoS ONE 2013; 8:e55718; http://dx.doi.org/10.1371/journal.pone.0055718; PMID: 23383270
- Ince-Dunn G, Okano HJ, Jensen KB, Park WY, Zhong R, Ule J, Mele A, Fak JJ, Yang C, Zhang C, et al. Neuronal Elav-like (Hu) proteins regulate RNA splicing and abundance to control glutamate levels and neuronal excitability. Neuron 2012; 75:1067 - 80; http://dx.doi.org/10.1016/j.neuron.2012.07.009; PMID: 22998874
- Anderson KD, Merhege MA, Morin M, Bolognani F, Perrone-Bizzozero NI. Increased expression and localization of the RNA-binding protein HuD and GAP-43 mRNA to cytoplasmic granules in DRG neurons during nerve regeneration. Exp Neurol 2003; 183:100 - 8; http://dx.doi.org/10.1016/S0014-4886(03)00103-1; PMID: 12957493
- Kasashima K, Terashima K, Yamamoto K, Sakashita E, Sakamoto H. Cytoplasmic localization is required for the mammalian ELAV-like protein HuD to induce neuronal differentiation. Genes Cells 1999; 4:667 - 83; http://dx.doi.org/10.1046/j.1365-2443.1999.00292.x; PMID: 10620013
- Fujiwara T, Fukao A, Sasano Y, Matsuzaki H, Kikkawa U, Imataka H, Inoue K, Endo S, Sonenberg N, Thoma C, et al. Functional and direct interaction between the RNA binding protein HuD and active Akt1. Nucleic Acids Res 2012; 40:1944 - 53; http://dx.doi.org/10.1093/nar/gkr979; PMID: 22075994
- Manley GT, Smitt PS, Dalmau J, Posner JB. Hu antigens: reactivity with Hu antibodies, tumor expression, and major immunogenic sites. Ann Neurol 1995; 38:102 - 10; http://dx.doi.org/10.1002/ana.410380117; PMID: 7541976
- Cho JH, Noguchi M. Expression of HuD (a paraneoplastic encephalomyelitis antigen) mRNA in lung cancer. J Korean Med Sci 1997; 12:305 - 10; PMID: 9288629
- Carpentier AF, Voltz R, DesChamps T, Posner JB, Dalmau J, Rosenfeld MR. Absence of HuD gene mutations in paraneoplastic small cell lung cancer tissue. Neurology 1998; 50:1919; http://dx.doi.org/10.1212/WNL.50.6.1919; PMID: 9633765
- D’Alessandro V, Muscarella LA, la Torre A, Bisceglia M, Parrella P, Scaramuzzi G, Storlazzi CT, Trombetta D, Kok K, De Cata A, et al. Molecular analysis of the HuD gene in neuroendocrine lung cancers. Lung Cancer 2010; 67:69 - 75; http://dx.doi.org/10.1016/j.lungcan.2009.03.022; PMID: 19410329
- Sekido Y, Bader SA, Carbone DP, Johnson BE, Minna JD. Molecular analysis of the HuD gene encoding a paraneoplastic encephalomyelitis antigen in human lung cancer cell lines. Cancer Res 1994; 54:4988 - 92; PMID: 8069866
- Gazdar AF, Carney DN, Becker KL, Deftos LJ, Liang V, Go W, Marangos PJ, Moody TW, Wolfsen AR, Zweig MH. Expression of peptide and other markers in lung cancer cell lines. Recent Results Cancer Res 1985; 99:167 - 74; http://dx.doi.org/10.1007/978-3-642-82533-0_17; PMID: 2999915
- Graus F, Elkon KB, Cordon-Cardo C, Posner JB. Sensory neuronopathy and small cell lung cancer. Antineuronal antibody that also reacts with the tumor. Am J Med 1986; 80:45 - 52; http://dx.doi.org/10.1016/0002-9343(86)90047-1; PMID: 2417479
- Vega F, Graus F, Chen QM, Poisson M, Schuller E, Delattre JY. Intrathecal synthesis of the anti-Hu antibody in patients with paraneoplastic encephalomyelitis or sensory neuronopathy: clinical-immunologic correlation. Neurology 1994; 44:2145 - 7; http://dx.doi.org/10.1212/WNL.44.11.2145; PMID: 7969974
- Verschuuren JJP, Perquin M, ten Velde G, De Baets M, Vriesman PB, Twijnstra A. Anti-Hu antibody titre and brain metastases before and after treatment for small cell lung cancer. J Neurol Neurosurg Psychiatry 1999; 67:353 - 7; http://dx.doi.org/10.1136/jnnp.67.3.353; PMID: 10449558
- Blaes F, Klotz M, Funke D, Strittmatter M, Kraus J, Kaps M. Disturbance in the serum IgG subclass distribution in patients with anti-Hu positive paraneoplastic neurological syndromes. Eur J Neurol 2002; 9:369 - 72; http://dx.doi.org/10.1046/j.1468-1331.2002.00416.x; PMID: 12099920
- Monstad SE, Knudsen A, Salvesen HB, Aarseth JH, Vedeler CA. Onconeural antibodies in sera from patients with various types of tumours. Cancer Immunol Immunother 2009; 58:1795 - 800; http://dx.doi.org/10.1007/s00262-009-0690-y; PMID: 19294382
- Greenlee JE, Boyden JW, Pingree M, Brashear HR, Clawson SA, Keeney PM. Antibody types and IgG subclasses in paraneoplastic neurological syndromes. J Neurol Sci 2001; 184:131 - 7; http://dx.doi.org/10.1016/S0022-510X(01)00442-7; PMID: 11239946
- Jean WC, Dalmau J, Ho A, Posner JB. Analysis of the IgG subclass distribution and inflammatory infiltrates in patients with anti-Hu-associated paraneoplastic encephalomyelitis. Neurology 1994; 44:140 - 7; http://dx.doi.org/10.1212/WNL.44.1.140; PMID: 8290049
- Sodeyama N, Ishida K, Jaeckle KA, Zhang L, Azuma A, Yamada M, Mizusawa H, Wada Y. Pattern of epitopic reactivity of the anti-Hu antibody on HuD with and without paraneoplastic syndrome. J Neurol Neurosurg Psychiatry 1999; 66:97 - 9; http://dx.doi.org/10.1136/jnnp.66.1.97; PMID: 9886463
- Furneaux HF, Reich L, Posner JB. Autoantibody synthesis in the central nervous system of patients with paraneoplastic syndromes. Neurology 1990; 40:1085 - 91; http://dx.doi.org/10.1212/WNL.40.7.1085; PMID: 2356009
- Graus F, Vega F, Delattre JY, Bonaventura I, Reñé R, Arbaiza D, Tolosa E. Plasmapheresis and antineoplastic treatment in CNS paraneoplastic syndromes with antineuronal autoantibodies. Neurology 1992; 42:536 - 40; http://dx.doi.org/10.1212/WNL.42.3.536; PMID: 1312683
- Rauer S, Kaiser R. Demonstration of anti-HuD specific oligoclonal bands in the cerebrospinal fluid from patients with paraneoplastic neurological syndromes. Qualitative evidence of anti-HuD specific IgG-synthesis in the central nervous system. J Neuroimmunol 2000; 111:241 - 4; http://dx.doi.org/10.1016/S0165-5728(00)00391-X; PMID: 11063845
- Dalmau J, Graus F, Cheung NK, Rosenblum MK, Ho A, Cañete A, Delattre JY, Thompson SJ, Posner JB. Major histocompatibility proteins, anti-Hu antibodies, and paraneoplastic encephalomyelitis in neuroblastoma and small cell lung cancer. Cancer 1995; 75:99 - 109; http://dx.doi.org/10.1002/1097-0142(19950101)75:1<99::AID-CNCR2820750117>3.0.CO;2-I; PMID: 7804984
- Pittock SJ, Kryzer TJ, Lennon VA. Paraneoplastic antibodies coexist and predict cancer, not neurological syndrome. Ann Neurol 2004; 56:715 - 9; http://dx.doi.org/10.1002/ana.20269; PMID: 15468074
- Sillevis Smitt P, Grefkens J, de Leeuw B, van den Bent M, van Putten W, Hooijkaas H, Vecht C. Survival and outcome in 73 anti-Hu positive patients with paraneoplastic encephalomyelitis/sensory neuronopathy. J Neurol 2002; 249:745 - 53; http://dx.doi.org/10.1007/s00415-002-0706-4; PMID: 12111309
- Torà M, Graus F, de Bolòs C, Real FX. Cell surface expression of paraneoplastic encephalomyelitis/sensory neuronopathy-associated Hu antigens in small-cell lung cancers and neuroblastomas. Neurology 1997; 48:735 - 41; http://dx.doi.org/10.1212/WNL.48.3.735; PMID: 9065557
- De Giorgio R, Bovara M, Barbara G, Canossa M, Sarnelli G, De Ponti F, Stanghellini V, Tonini M, Cappello S, Pagnotta E, et al. Anti-HuD-induced neuronal apoptosis underlying paraneoplastic gut dysmotility. Gastroenterology 2003; 125:70 - 9; http://dx.doi.org/10.1016/S0016-5085(03)00664-4; PMID: 12851872
- Greenlee JE, Parks TN, Jaeckle KA. Type IIa (‘anti-Hu’) antineuronal antibodies produce destruction of rat cerebellar granule neurons in vitro. Neurology 1993; 43:2049 - 54; http://dx.doi.org/10.1212/WNL.43.10.2049; PMID: 8413965
- Schäfer KH, Klotz M, Mergner D, Mestres P, Schimrigk K, Blaes F. IgG-mediated cytotoxicity to myenteric plexus cultures in patients with paraneoplastic neurological syndromes. J Autoimmun 2000; 15:479 - 84; http://dx.doi.org/10.1006/jaut.2000.0454; PMID: 11090247
- Hormigo A, Lieberman F. Nuclear localization of anti-Hu antibody is not associated with in vitro cytotoxicity. J Neuroimmunol 1994; 55:205 - 12; http://dx.doi.org/10.1016/0165-5728(94)90011-6; PMID: 7829670
- Tanaka K, Ding X, Tanaka M. Effects of antineuronal antibodies from patients with paraneoplastic neurological syndrome on primary-cultured neurons. J Neurol Sci 2004; 217:25 - 30; http://dx.doi.org/10.1016/j.jns.2003.08.006; PMID: 14675605
- Sillevis Smitt PA, Manley GT, Posner JB. Immunization with the paraneoplastic encephalomyelitis antigen HuD does not cause neurologic disease in mice. Neurology 1995; 45:1873 - 8; http://dx.doi.org/10.1212/WNL.45.10.1873; PMID: 7477985
- Uchuya M, Graus F, Vega F, Reñé R, Delattre JY. Intravenous immunoglobulin treatment in paraneoplastic neurological syndromes with antineuronal autoantibodies. J Neurol Neurosurg Psychiatry 1996; 60:388 - 92; http://dx.doi.org/10.1136/jnnp.60.4.388; PMID: 8774401
- Sakai K, Gofuku M, Kitagawa Y, Ogasawara T, Hirose G. Induction of anti-Purkinje cell antibodies in vivo by immunizing with a recombinant 52-kDa paraneoplastic cerebellar degeneration-associated protein. J Neuroimmunol 1995; 60:135 - 41; http://dx.doi.org/10.1016/0165-5728(95)00063-8; PMID: 7642741
- Benyahia B, Liblau R, Merle-Béral H, Tourani JM, Dalmau J, Delattre JY. Cell-mediated autoimmunity in paraneoplastic neurological syndromes with anti-Hu antibodies. Ann Neurol 1999; 45:162 - 7; http://dx.doi.org/10.1002/1531-8249(199902)45:2<162::AID-ANA5>3.0.CO;2-R; PMID: 9989617
- de Beukelaar JW, Sillevis Smitt PA, Hop WC, Kraan J, Hooijkaas H, Verjans GM, Gratama JW. Imbalances in circulating lymphocyte subsets in Hu antibody associated paraneoplastic neurological syndromes. Eur J Neurol 2007; 14:1383 - 91; http://dx.doi.org/10.1111/j.1468-1331.2007.01986.x; PMID: 18028190
- Tani T, Tanaka K, Idezuka J, Nishizawa M. Regulatory T cells in paraneoplastic neurological syndromes. J Neuroimmunol 2008; 196:166 - 9; http://dx.doi.org/10.1016/j.jneuroim.2008.03.002; PMID: 18455243
- Psimaras D, Carpentier AF, Rossi C, Euronetwork PNS. Cerebrospinal fluid study in paraneoplastic syndromes. J Neurol Neurosurg Psychiatry 2010; 81:42 - 5; http://dx.doi.org/10.1136/jnnp.2008.159483; PMID: 19324868
- de Jongste AH, de Graaf MT, van den Broek PD, Kraan J, Smitt PA, Gratama JW. Elevated numbers of regulatory T cells, central memory T cells and class-switched B cells in cerebrospinal fluid of patients with anti-Hu antibody associated paraneoplastic neurological syndromes. J Neuroimmunol 2013; 258:85 - 90; http://dx.doi.org/10.1016/j.jneuroim.2013.02.006; PMID: 23566401
- Albert ML, Darnell JC, Bender A, Francisco LM, Bhardwaj N, Darnell RB. Tumor-specific killer cells in paraneoplastic cerebellar degeneration. Nat Med 1998; 4:1321 - 4; http://dx.doi.org/10.1038/3315; PMID: 9809559
- Santomasso BD, Roberts WK, Thomas A, Williams T, Blachère NE, Dudley ME, Houghton AN, Posner JB, Darnell RB. A T-cell receptor associated with naturally occurring human tumor immunity. Proc Natl Acad Sci USA 2007; 104:19073 - 8; http://dx.doi.org/10.1073/pnas.0704336104; PMID: 18045792
- Liblau RS, Gonzalez-Dunia D, Wiendl H, Zipp F. Neurons as targets for T cells in the nervous system. Trends Neurosci 2013; 36:315 - 24; http://dx.doi.org/10.1016/j.tins.2013.01.008; PMID: 23478065
- de Beukelaar JW, Verjans GM, van Norden Y, Milikan JC, Kraan J, Hooijkaas H, Sintnicolaas K, Gratama JW, Sillevis Smitt PA. No evidence for circulating HuD-specific CD8+ T cells in patients with paraneoplastic neurological syndromes and Hu antibodies. Cancer Immunol Immunother 2007; 56:1501 - 6; http://dx.doi.org/10.1007/s00262-007-0295-2; PMID: 17597332
- de Jongste AH, de Graaf MT, Martinuzzi E, van den Broek PD, Kraan J, Lamers CH, Mallone R, Gratama JW, Sillevis Smitt PA. Three sensitive assays do not provide evidence for circulating HuD-specific T cells in the blood of patients with paraneoplastic neurological syndromes with anti-Hu antibodies. Neuro-oncol 2012; 14:841 - 8; http://dx.doi.org/10.1093/neuonc/nos118; PMID: 22591661
- Plonquet A, Garcia-Pons F, Fernandez E, Philippe C, Marquet J, Rouard H, Delfau-Larue MH, Kosmatopoulos K, Lemonnier F, Farcet JP, et al. Peptides derived from the onconeural HuD protein can elicit cytotoxic responses in HHD mouse and human. J Neuroimmunol 2003; 142:93 - 100; http://dx.doi.org/10.1016/S0165-5728(03)00269-8; PMID: 14512168
- Rousseau A, Benyahia B, Dalmau J, Connan F, Guillet JG, Delattre JY, Choppin J. T cell response to Hu-D peptides in patients with anti-Hu syndrome. J Neurooncol 2005; 71:231 - 6; http://dx.doi.org/10.1007/s11060-004-1723-1; PMID: 15735910
- Tanaka M, Tanaka K. Pathogenesis and treatment of paraneoplastic neurologic syndrome. Expert Rev Neurother 2002; 2:901 - 9; http://dx.doi.org/10.1586/14737175.2.6.901; PMID: 19810923
- Roberts WK, Deluca IJ, Thomas A, Fak J, Williams T, Buckley N, Dousmanis AG, Posner JB, Darnell RB. Patients with lung cancer and paraneoplastic Hu syndrome harbor HuD-specific type 2 CD8+ T cells. J Clin Invest 2009; 119:2042 - 51; http://dx.doi.org/10.1172/JCI36131; PMID: 19509467
- Meresse B, Malamut G, Cerf-Bensussan N. Celiac disease: an immunological jigsaw. Immunity 2012; 36:907 - 19; http://dx.doi.org/10.1016/j.immuni.2012.06.006; PMID: 22749351
- DeLuca I, Blachère NE, Santomasso B, Darnell RB. Tolerance to the neuron-specific paraneoplastic HuD antigen. PLoS ONE 2009; 4:e5739; http://dx.doi.org/10.1371/journal.pone.0005739; PMID: 19492067
- Rosenblum MK. Paraneoplasia and autoimmunologic injury of the nervous system: the anti-Hu syndrome. Brain Pathol 1993; 3:199 - 212; http://dx.doi.org/10.1111/j.1750-3639.1993.tb00747.x; PMID: 8293180
- Galon J, Angell HK, Bedognetti D, Marincola FM. The continuum of cancer immunosurveillance: prognostic, predictive, and mechanistic signatures. Immunity 2013; 39:11 - 26; http://dx.doi.org/10.1016/j.immuni.2013.07.008; PMID: 23890060
- Marrack P, Kappler JW. Do MHCII-presented neoantigens drive type 1 diabetes and other autoimmune diseases? Cold Spring Harb Perspect Med 2012; 2:a007765; http://dx.doi.org/10.1101/cshperspect.a007765; PMID: 22951444
- Sollid LM, Jabri B. Celiac disease and transglutaminase 2: a model for posttranslational modification of antigens and HLA association in the pathogenesis of autoimmune disorders. Curr Opin Immunol 2011; 23:732 - 8; http://dx.doi.org/10.1016/j.coi.2011.08.006; PMID: 21917438
- de Graaf MT, de Beukelaar JW, Haasnoot GW, Levering WH, Rogemond V, Didelot A, Honnorat J, Gratama JW, Smitt PA. HLA-DQ2+ individuals are susceptible to Hu-Ab associated paraneoplastic neurological syndromes. J Neuroimmunol 2010; 226:147 - 9; http://dx.doi.org/10.1016/j.jneuroim.2010.05.035; PMID: 20547426
- Uchuya M, Fleury A, Graus F, Costagliola D, Liblau R, Merle-Beral H, Théodorou I, Delattre JY. Lack of association between human leukocyte antigens and the anti-Hu syndrome in patients with small-cell lung cancer. Neurology 1998; 50:565 - 6; http://dx.doi.org/10.1212/WNL.50.2.565; PMID: 9484403
- Pellkofer HL, Voltz R, Goebels N, Hohlfeld R, Dornmair K. Cross-reactive T-cell receptors in tumor and paraneoplastic target tissue. Arch Neurol 2009; 66:655 - 8; http://dx.doi.org/10.1001/archneurol.2009.56; PMID: 19433667
- Bernal F, Graus F, Pifarré A, Saiz A, Benyahia B, Ribalta T. Immunohistochemical analysis of anti-Hu-associated paraneoplastic encephalomyelitis. Acta Neuropathol 2002; 103:509 - 15; http://dx.doi.org/10.1007/s00401-001-0498-0; PMID: 11935268
- Dalmau J, Posner JB. Neurologic paraneoplastic antibodies (anti-Yo; anti-Hu; anti-Ri): the case for a nomenclature based on antibody and antigen specificity. Neurology 1994; 44:2241 - 6; http://dx.doi.org/10.1212/WNL.44.12.2241; PMID: 7991105
- Bien CG, Vincent A, Barnett MH, Becker AJ, Blümcke I, Graus F, Jellinger KA, Reuss DE, Ribalta T, Schlegel J, et al. Immunopathology of autoantibody-associated encephalitides: clues for pathogenesis. Brain 2012; 135:1622 - 38; http://dx.doi.org/10.1093/brain/aws082; PMID: 22539258
- Dalmau J, Furneaux HM, Rosenblum MK, Graus F, Posner JB. Detection of the anti-Hu antibody in specific regions of the nervous system and tumor from patients with paraneoplastic encephalomyelitis/sensory neuronopathy. Neurology 1991; 41:1757 - 64; http://dx.doi.org/10.1212/WNL.41.11.1757; PMID: 1944905
- Graus F, Campo E, Cruz-Sanchez F, Ribalta T, Palacin A. Expression of lymphocyte, macrophage and class I and II major histocompatibility complex antigens in normal human dorsal root ganglia. J Neurol Sci 1990; 98:203 - 11; http://dx.doi.org/10.1016/0022-510X(90)90261-K; PMID: 1700807
- Antoine JC, Mosnier JF, Honnorat J, Convers P, Absi L, Lapras J, Michel D. Paraneoplastic demyelinating neuropathy, subacute sensory neuropathy, and anti-Hu antibodies: clinicopathological study of an autopsy case. Muscle Nerve 1998; 21:850 - 7; http://dx.doi.org/10.1002/(SICI)1097-4598(199807)21:7<850::AID-MUS2>3.0.CO;2-5; PMID: 9626244
- Wanschitz J, Hainfellner JA, Kristoferitsch W, Drlicek M, Budka H. Ganglionitis in paraneoplastic subacute sensory neuronopathy: a morphologic study. Neurology 1997; 49:1156 - 9; http://dx.doi.org/10.1212/WNL.49.4.1156; PMID: 9339709
- Plonquet A, Gherardi RK, Créange A, Antoine JC, Benyahia B, Grisold W, Drlicek M, Dreyfus P, Honnorat J, Khouatra C, et al. Oligoclonal T-cells in blood and target tissues of patients with anti-Hu syndrome. J Neuroimmunol 2002; 122:100 - 5; http://dx.doi.org/10.1016/S0165-5728(01)00452-0; PMID: 11777548
- Kreutzfeldt M, Bergthaler A, Fernandez M, Brück W, Steinbach K, Vorm M, Coras R, Blümcke I, Bonilla WV, Fleige A, et al. Neuroprotective intervention by interferon-γ blockade prevents CD8+ T cell-mediated dendrite and synapse loss. J Exp Med 2013; 210:2087 - 103; http://dx.doi.org/10.1084/jem.20122143; PMID: 23999498
- Voltz R, Dalmau J, Posner JB, Rosenfeld MR. T-cell receptor analysis in anti-Hu associated paraneoplastic encephalomyelitis. Neurology 1998; 51:1146 - 50; http://dx.doi.org/10.1212/WNL.51.4.1146; PMID: 9781545
- Siewert K, Malotka J, Kawakami N, Wekerle H, Hohlfeld R, Dornmair K. Unbiased identification of target antigens of CD8+ T cells with combinatorial libraries coding for short peptides. Nat Med 2012; 18:824 - 8; http://dx.doi.org/10.1038/nm.2720; PMID: 22484809
- Carpentier AF, Rosenfeld MR, Delattre JY, Whalen RG, Posner JB, Dalmau J. DNA vaccination with HuD inhibits growth of a neuroblastoma in mice. Clin Cancer Res 1998; 4:2819 - 24; PMID: 9829748
- Kazarian M, Calbo J, Proost N, Carpenter CL, Berns A, Laird-Offringa IA. Immune response in lung cancer mouse model mimics human anti-Hu reactivity. J Neuroimmunol 2009; 217:38 - 45; http://dx.doi.org/10.1016/j.jneuroim.2009.08.014; PMID: 19765830
- Pellkofer H, Schubart AS, Höftberger R, Schutze N, Pagany M, Schüller M, Lassmann H, Hohlfeld R, Voltz R, Linington C. Modelling paraneoplastic CNS disease: T-cells specific for the onconeuronal antigen PNMA1 mediate autoimmune encephalomyelitis in the rat. Brain 2004; 127:1822 - 30; http://dx.doi.org/10.1093/brain/awh205; PMID: 15201193
- Oh SJ, Dropcho EJ, Claussen GC. Anti-Hu-associated paraneoplastic sensory neuropathy responding to early aggressive immunotherapy: report of two cases and review of literature. Muscle Nerve 1997; 20:1576 - 82; http://dx.doi.org/10.1002/(SICI)1097-4598(199712)20:12<1576::AID-MUS13>3.0.CO;2-Z; PMID: 9390671
- Brinkmann V, Billich A, Baumruker T, Heining P, Schmouder R, Francis G, Aradhye S, Burtin P. Fingolimod (FTY720): discovery and development of an oral drug to treat multiple sclerosis. Nat Rev Drug Discov 2010; 9:883 - 97; http://dx.doi.org/10.1038/nrd3248; PMID: 21031003
- Ifergan I, Kebir H, Alvarez JI, Marceau G, Bernard M, Bourbonnière L, Poirier J, Duquette P, Talbot PJ, Arbour N, et al. Central nervous system recruitment of effector memory CD8+ T lymphocytes during neuroinflammation is dependent on α4 integrin. Brain 2011; 134:3560 - 77; http://dx.doi.org/10.1093/brain/awr268; PMID: 22058139
- Planas R, Jelčić I, Schippling S, Martin R, Sospedra M. Natalizumab treatment perturbs memory- and marginal zone-like B-cell homing in secondary lymphoid organs in multiple sclerosis. Eur J Immunol 2012; 42:790 - 8; http://dx.doi.org/10.1002/eji.201142108; PMID: 22144343
- Scheikl T, Pignolet B, Dalard C, Desbois S, Raison D, Yamazaki M, Saoudi A, Bauer J, Lassmann H, Hardin-Pouzet H, et al. Cutting edge: neuronal recognition by CD8 T cells elicits central diabetes insipidus. J Immunol 2012; 188:4731 - 5; http://dx.doi.org/10.4049/jimmunol.1102998; PMID: 22504649
- Riley JL. Combination checkpoint blockade--taking melanoma immunotherapy to the next level. N Engl J Med 2013; 369:187 - 9; http://dx.doi.org/10.1056/NEJMe1305484; PMID: 23724866