Abstract
Despite their elevated immunogenicity, melanoma lesions often escape immunosurveillance. We have recently demonstrated that plasmacytoid dendritic cells (pDCs) accumulating within melanomas are prompted to express tumor necrosis factor (ligand) superfamily, member 4 (TNFSF4, best known as OX40L) and inducible T-cell co-stimulator ligand (ICOSL), hence becoming able to trigger TH2 and regulatory immune responses. Such a hijacking of pDCs is associated with early disease relapse. Thus, by actively harnessing the plasticity of pDCs, melanomas promote their own progression.
Even though melanoma is considered as one of the most immunogenic solid tumors, controlling its development and progression remains a challenge.Citation1 The cellular and molecular factors underlying the ability of melanoma cells to escape immunosurveillance have not yet been fully characterized. However, this knowledge is essential to develop novel therapeutic approaches that improve the poor outcome associated with metastatic disease. Plasmacytoid dendritic cells (pDCs) have recently emerged as a pivotal but somewhat enigmatic cancer-associated cell type.Citation2 These cells infiltrate many solid tumors and have a robust capacity to drive effective antitumor immune responses.Citation3,Citation4However, the tumor microenvironment often induce functional alterations in the immune infiltrate leading to tolerance and disease progression.Citation2,Citation5-Citation7 The role of melanoma-infiltrating pDCs in tumor progression and response to therapy remains little explored. pDCs are recruited to primary neoplastic lesions and to sentinel lymph nodes,Citation8 but this is associated with poor prognosis.Citation9 Nonetheless, pDCs have been shown not only to exert a direct cytotoxic activity against tumor cells, but also—once activated via Toll-like receptor 7 (TLR7) or TLR9, to effectively control melanoma growth through the priming of efficient antitumor immune responses.Citation2,Citation4
This controversy prompted us to investigate the pathophysiological role of pDCs in melanoma.Citation10 Our study combined the analysis of a large cohort of Stage I-IV melanoma patients (61 blood samples, 54 tumor samples including primary tumors and cutaneous as well as lymph node metastases) with the use of an innovative melanoma-bearing humanized mouse model (OncoHumice). In this model, human CD34+ hematopoietic progenitor cells (HPCs) were transplanted to reconstitute a human immune system in immunodeficient mice, which then were grafted with human melanoma cells.
In melanoma patients, the robust infiltration of cutaneous lesions and lymph node metastases by pDCs has been associated with early relapse, suggesting that these cells play a role in disease progression. In melanoma-bearing humanized mice, human pDCs rapidly and massively migrated to neoplastic lesions and to tumor-draining lymph nodes. pDCs accumulated to larger degree than their myeloid counterparts, underlining a predominant role of this DC subset in melanoma. To understand the link between tumor-infiltrating pDCs and early relapse, we first examined the migratory profile and activation status of these cells. CD62L-expressing pDCs were found in greater proportions in the blood of melanoma patients than in that of healthy subjects, suggesting that pDCs are mobilized by melanoma. Interestingly, pDCs expressed high levels of co-stimulatory molecules at cutaneous tumor sites, but were poorly activated in tumor-draining lymph nodes. Thus, the functional status of pDCs appears to be modulated by their passage through neoplastic lesions. We further evaluated the fitness of tumor-associated pDCs by analyzing their ability to respond to TLR ligands, which normally are potent pDC activators. The pDCs isolated from the neoplastic lesions and blood of melanoma patients, like those obtained from healthy donors, expressed increased levels of co-stimulatory molecules and secreted interferon α (IFNα) in response to TLR7 and TLR9 ligands. Thus, in contrast with what occurs in breast and ovarian tumors, melanoma-infiltrating pDCs are fully responsive to TLR stimulation.
We next wondered how the functional alterations of pDCs triggered by the tumor microenvironment would affect the elicitation of adaptive immune responses. Strikingly, pDCs isolated from the tumor microenvironment of OncoHumice and melanoma patients stimulated naïve CD4+ T cells to differentiate into pro-inflammatory TH2 cells and regulatory T cells (Tregs). The relative abundance of interleukin (IL)-5-, IL-10- and/or IL-13-producing CD4+ and CD8+ T cells as well as that of FOXP3+CD25hi Tregs was increased within tumor metastases as well as in the blood of melanoma patients. Our findings confirm the clinical relevance of this immunological profile and indicate that pDC-driven adaptive immune responses can been hijacked by melanoma cells.
To further investigate the mechanisms through which tumor-polarized pDCs promote such a dramatic functional alteration in adaptive immunity, we analyzed a panel of molecules that are known to polarize immune responses toward a TH2 or regulatory profile. We observed a significantly increased proportion of pDCs expressing tumor necrosis factor (ligand) superfamily, member 4 (TNFSF4, best known as OX40L) and inducible T-cell co-stimulator ligand (ICOSL) within the tumor microenvironment, correlating with the frequency of IL-13-producing CD4+ and CD8+ T cells or IL-10-secreting CD4+ T cells, respectively. Strikingly, blocking OX40L in pDC/T cell co-cultures dramatically reduced IL-5, IL-13 and TNFα production, whereas ICOSL neutralization specifically inhibited the secretion of IL-10. Thus, melanoma cells instruct pDCs to establish a pro-inflammatory TH2 and regulatory immunological profile via OX40L and ICOSL. We next investigated which tumor-derived factors would trigger such a functional subversion of pDCs. In both humanized mice and humans, chemokine (C-C motif) ligand 17 (CCL17, also known as TARC), CCL22 (also known as MDC) and matrix metalloproteinase 2 (MMP2) were detected within the tumor microenvironment. CCL17 and CCL22 have previously been involved in the recruitment of TH2 cells and Tregs, as well as in skewing antitumor immune responses toward a pro-inflammatory TH2 profile by modulating myeloid DCs.Citation7 Strikingly, these chemokines promoted the upregulation of OX40L and ICOSL on the surface of pDCs, and high levels of CCL17, CCL22, and MMP2 within neoplastic lesions correlated with early disease relapse in patients.
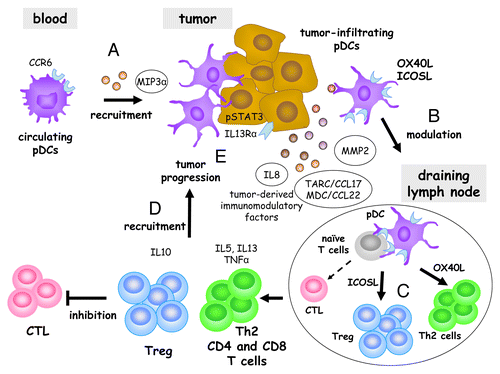
Altogether, our findings provide novel insights into the mechanisms by which melanoma cells hijack the immune system. This appears to involve a novel pDC-dependent pathway that eventually favor disease progression and early relapse (). We have previously shown that circulating pDCs expressing chemokine (C-C motif) receptor 6 (CCR6) are recruited to melanomas by CCL20.Citation8 Tumor-infiltrating pDCs are then prompted to express OX40L and ICOSL which, under MMP2 conditioning, can drive the differentiation of IL-5- and IL.13-producing TH2 CD4+ and CD8+ T cells, as well as the accumulation of IL-10-producing Tregs. These cells can migrate to neoplastic lesions in response to CCL17 and CCL22, ultimately promoting tumor progression and disease relapse.
Figure 1. Mechanisms whereby tumor-infiltrating plasmacytoid dendritic cells are subverted by melanoma. Circulating plasmacytoid dendritic cells expressing chemokine (C-C motif) receptor 6 (CCR6) are recruited to the tumor site by chemokine (C-C motif) ligand 20 (CCL20, also known as MIP3α (A). CCL17 (also known as TARC), CCL22 (also known as MDC) and matrix metalloproteinase 2 (MMP2) produced in the tumor microenvironment prompt pDCs to express tumor necrosis factor (ligand) superfamily, member 4 (TNFSF4, best known as OX40L) and inducible T-cell co-stimulator ligand (ICOSL) (B). After reaching tumor-draining lymph nodes, OX40L-expressing pDCs drive the differentiation of pro-inflammatory TH2 CD4+ and CD8+ T cells that secrete interleukin (IL)-5, IL-13 and tumor necrosis factor α (TNFα), while ICOSL-expressing pDCs induce the emergence of regulatory T cells that release IL-10 (C). CCL17 and CCL22 may recruit pDC-primed TH2 cells and Tregs to the tumor microenvironment (D), where they allow malignant cells to escape from immunosurveillance, hence promoting tumor progression and disease relapse (E). Such a functional hijacking of pDCs by melanoma represents a promising target for the development of novel anticancer (immune) therapies.
These advances in the understanding of the complex interactions between malignant cells and the immune system may pave the way to the development of novel therapeutic approaches against melanoma. In this setting, TLR agonists represent a promising means to achieve tumor control by reversing the functional impairment of pDCs. Targeting the tumor microenvironment to disrupt the mechanisms that underlie these immunological defects may improve the prognosis of melanoma patients, as illustrated by the recent clinical success of agents that block immune checkpoints programmed cell death 1 (PDCD1)- and cytotoxic T lymphocyte-associated protein 4 (CTLA4)-targeting antibodies.Citation1
| Abbreviations: | ||
| CCL | = | chemokine (C-C motif) ligand 17 |
| IFN | = | interferon |
| pDC | = | plasmacytoid dendritic cell |
| MMP2 | = | matrix metalloproteinase 2 |
| Treg | = | regulatory T cell |
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Citation: Aspord C, Leccia M, Charles J, Plumas J. Melanoma hijacks plasmacytoid dendritic cells to promote its own progression. OncoImmunology 2013; 2:e27402; 10.4161/onci.27402
References
- Eggermont AM, Spatz A, Robert C. Cutaneous melanoma. Lancet 2013; pii: S0140-6736:60802 - 8; PMID: 24054424
- Pinto A, Rega A, Crother TR, Sorrentino R. Plasmacytoid dendritic cells and their therapeutic activity in cancer. Oncoimmunology 2012; 1:726 - 34; http://dx.doi.org/10.4161/onci.20171; PMID: 22934264
- Villadangos JA, Young L. Antigen-presentation properties of plasmacytoid dendritic cells. Immunity 2008; 29:352 - 61; http://dx.doi.org/10.1016/j.immuni.2008.09.002; PMID: 18799143
- Liu C, Lou Y, Lizée G, Qin H, Liu S, Rabinovich B, Kim GJ, Wang YH, Ye Y, Sikora AG, et al. Plasmacytoid dendritic cells induce NK cell-dependent, tumor antigen-specific T cell cross-priming and tumor regression in mice. J Clin Invest 2008; 118:1165 - 75; PMID: 18259609
- Lande R, Gilliet M. Plasmacytoid dendritic cells: key players in the initiation and regulation of immune responses. Ann N Y Acad Sci 2010; 1183:89 - 103; http://dx.doi.org/10.1111/j.1749-6632.2009.05152.x; PMID: 20146710
- Sisirak V, Faget J, Gobert M, Goutagny N, Vey N, Treilleux I, Renaudineau S, Poyet G, Labidi-Galy SI, Goddard-Leon S, et al. Impaired IFN-α production by plasmacytoid dendritic cells favors regulatory T-cell expansion that may contribute to breast cancer progression. Cancer Res 2012; 72:5188 - 97; http://dx.doi.org/10.1158/0008-5472.CAN-11-3468; PMID: 22836755
- Godefroy E, Bhardwaj N. Dysregulation of anti-tumor immunity by the matrix metalloproteinase-2. Oncoimmunology 2012; 1:109 - 11; http://dx.doi.org/10.4161/onci.1.1.17994; PMID: 22720227
- Charles J, Di Domizio J, Salameire D, Bendriss-Vermare N, Aspord C, Muhammad R, Lefebvre C, Plumas J, Leccia MT, Chaperot L. Characterization of circulating dendritic cells in melanoma: role of CCR6 in plasmacytoid dendritic cell recruitment to the tumor. J Invest Dermatol 2010; 130:1646 - 56; http://dx.doi.org/10.1038/jid.2010.24; PMID: 20220766
- Jensen TO, Schmidt H, Møller HJ, Donskov F, Høyer M, Sjoegren P, Christensen IJ, Steiniche T. Intratumoral neutrophils and plasmacytoid dendritic cells indicate poor prognosis and are associated with pSTAT3 expression in AJCC stage I/II melanoma. Cancer 2012; 118:2476 - 85; http://dx.doi.org/10.1002/cncr.26511; PMID: 21953023
- Aspord C, Leccia M-T, Charles J, Plumas J. Plasmacytoid dendritic cells support melanoma progression by promoting Th2 and regulatory immunity through OX40L and ICOSL. Cancer Immunol Research 2013; 1:1 - 14
