Abstract
Harnessing the host immune system to eradicate cancer has a high therapeutic potential. One paradigm of anticancer immunotherapy is represented by allogeneic stem cell transplantation. In this setting, the host must be conditioned prior to transplantation, allowing for engraftment and subsequent graft-vs.-tumor reactivity. Conditioning may also be a prerequisite for the efficacy of other immunotherapeutic regimens. In particular, tumor debulking followed by conditioning (aimed at blocking endogenous inhibitory stimuli, for instance upon the depletion of regulatory T cells or the inhibition of immune checkpoints) and subsequent immunization (for instance by means of patient-tailored vaccines) based on innovative adjuvants (such as RIG-I ligands) may allow for the elicitation of superior antitumor immune responses. Repetitive boosting might then maintain immunosurveillance. An intense wave of investigation on the optimal timing of immunostimulatory interventions with respect to the administration of immunogenic chemotherapeutics and on the use of small drugs that promote efficient antitumor immune responses will end up in the generation of highly effective immunotherapeutic anticancer regimens.
Background
When Thierry Boon and colleagues first discovered tumor-associated antigens (TAAs) in the early 1990s, cancer immunotherapy was envisioned as a magic bullet allowing for the specific elimination of malignant cells.Citation1 The following decades clarified that this promise would not hold true. One of the major obstacles against the development of efficient immunotherapeutic regimens was the generalized inability of investigators to translate successful preclinical studies into clinical medicine. Even though tumor-associated antigen (TAA)-specific T cells could be successfully elicited in vitro, the initial enthusiasm about designing cancer vaccines based on peptide-pulsed monocyte-derived dendritic cells (DCs) was rapidly disappointed.Citation2,Citation3 Of note, a consistent finding in most clinical studies testing immunotherapeutic interventions was the long-term clinical benefit obtained by a small subgroup of patients,Citation4 pointing out to the therapeutic potential of this approach. However, only a few factors predicting the propensity of cancer patients to obtain long-term clinical benefits from immunotherapy are currently available.Citation5 Irrespective of this issue, the first clinical studies testing immunotherapy demonstrated that this approach is mainly effective in patients with low tumor burden, early-stage disease, or an indolent disease course. Another obstacle against the development of efficient immunotherapeutic regimens was the lack of a standardized phenotypic and functional monitoring of therapy-elicited immune cells, an issue that has just begun to be addressed by multi-institutional initiatives.Citation6 Indeed, the response criteria applied in other setting, such as the well-known Response Evaluation Criteria in Solid Tumors (RECIST) may not be suitable for analyzing the clinical efficacy of immunotherapy and might have to be redefined and harmonized. Finally, the most relevant clinical endpoint for cancer patients treated with immunotherapy should be overall survival (OS) and not progression-free survival (PFS) or response rate (RR), both of which are prone to be biased by transient inflammatory responses as induced by multiple immunotherapeutic approaches. From a scientific perspective, the recent years have revealed various mechanisms whereby malignant cells can escape immune recognition, which also limit the efficacy of anticancer immunotherapy.Citation7 Additional investigation on this topic based on novel technologies (e.g., 2-photon microscopy), as exemplified by recent studies dissecting the dynamic of immune-cell mediated cancer recognition and elimination,Citation8 will provide novel insights into the major hurdles faced by immune cells within the cancer microenvironment. Integrating all these novel pieces of information from preclinical and clinical research generates an increasingly more holistic picture of anticancer immunotherapy.
Here, we give a concise overview on the current status of cancer vaccine design, with a focus on optimization strategies. We particularly emphasize the development of novel conditioning regimens that attempt to revert the ability of cancer cells to escape immune destruction.
Clinical Status of Immunotherapy—Melanoma and Prostate Cancer
Melanoma
Due to its susceptibility to cytokines as well as to central involvement of leading dermatologists in DC research, melanoma served as a major setting for the establishment of DC-based vaccination in the clinical routine. Various Phase II and III clinical trials tested DC-based vaccination vs. placebo or chemotherapy in melanoma patients, but none of them demonstrated substantial clinical benefits,Citation9 thus far preventing the approval of this immunotherapeutic approach. More recently, melanoma successfully served as a paradigm for immunostimulatory strategies based on the blockage of endogenous immunosuppressive signals. An exemplary compound in this setting is the cytotoxic T lymphocyte-associated protein 4 (CTLA4)-blocking monoclonal antibody (mAb) ipilimumab, which forces the interaction of B7 molecules (on antigen-presenting cells, APCs) with CD28 (on T cells) leading to T-cell activation. Two large Phase III clinical trials enrolling metastatic melanoma patients showed that ipilimumab prolongs OS.Citation10,Citation11 Importantly, the activation of the immune system can induce long-term disease stabilization in some patients. Robust immune activation is often reflected by autoimmune symptoms, including diarrhea, hepatitis, skin toxicity, and hypophysitis. Another promising mAb is the programmed cell death 1 (PDCD1, best known as PD-1)-targeting agent lambrolizumab, which has recently been tested in patients with advanced melanoma, including individuals who progressed on ipilimumab. Lambrolizumab resulted in a high rate of sustained tumor regressions, while toxic side effects were generally mild.Citation12 Moreover, the combination of ipilimumab and another mAb targeting PD-1 (i.e., nivolumab) turned out to have a manageable safety profile and to induces rapid and consistent tumor regression in a substantial proportion of patients.Citation13 Nowadays, the most promising immunotherapeutic approach against melanoma appears to be the combination of vaccines with systemic immunostimulatory agents, such as toptimized adjuvants or immunostimulatory mAbs (see below).
Prostate cancer
Prostate-specific antigen (PSA) and prostatic acid phosphatase (PAP) are TAAs expressed by prostate cancer cells. PROSTVAC is a poxvirus-based vaccine encoding PSA and a combination of co-stimulatory molecules (TRICOM), notably CD80, intercellular adhesion molecule 1 (ICAM1) and CD58 (also known as LFA-3). This concept has first been evaluated using a carcinoembryonic antigen (CEA)-targeting vaccine to promote therapeutically relevant antigen-specific immune responses in colorectal cancer patients.Citation14 Recently published data from a randomized Phase II clinical trial suggests that PROSTVAC significantly improves the 3-y OS rate (30% vs. 17%).Citation15 A Phase III clinical trial has recently been initiated to analyze the efficacy of this vaccine in men with asymptomatic or minimally symptomatic metastatic castrate-resistant prostate cancer (NCT01322490). To date, the only FDA-approved anticancer vaccine is Sipuleucel-T (Provenge®), which is licensed for use in patients with advanced prostate cancer.Citation16 Sipuleucel-T is generated by harvesting the patient’s monocytes, and exposing them ex vivo to a fusion protein including PAP and granulocyte macrophage colony-stimulating factor (GM-CSF). The Phase III IMPACT (IMmunotherapy Prostate AdenoCarcinoma Treatment) trial included 512 individuals with metastatic castration-resistant prostate cancer. In this setting, sipuleucel-T improved OS by approximately 4 mo and reduced the risk of patients to die by 22%.Citation17 Thus, Sipuleucel-T represents a successful paradigm of translation of anticancer vaccines from bench to bedside.
Identification of optimal targets: Tumor-associated antigens
The idea that the immune system might eliminate malignant cells was first developed in the 19th century by Wiliam Coley. He observed that injections of bacterial toxins can induce clinical remissions in some cancer patients, suggesting a potential role for microbial products as adjuvants (see below). However, this assumption as well as the possible role of TAAs that are recognized by specific lymphocytes in the context of MHC molecules (immunosurveillance) was demonstrated in humans only when the group of Thierry Boon characterized the first human TAA, nowadays referred to as MAGE-A1.Citation18 This discovery paved the way to the identification of novel TAAs, which can be subdivided in different categories.Citation19 TAAs can stimulate cellular or humoral immune responses in patients and efficiently drive the elimination of malignant cells. Peptides derived from TAAs are presented in the context of MHC class I or II molecules, where they can be recognized by CD8+ or CD4+ T cells, respectively. The strict dichotomy between the generation of MHC class I and II peptides was challenged by the demonstration that peptides generated from exogenous proteins that are usually presented on MHC class II molecules can also be presented on MHC class I molecules, a phenomenon called cross-priming.Citation20,Citation21 In addition, antigens derived from cytosolic proteins may gain access to MHC class II presentation. Several critical pathways such as the protein degradation by the proteasome, the translocation of epitopes from cytoplasm into intracellular membranes, and autophagy have been involved in this process.Citation22,Citation23
Hans-Georg Rammensee and colleagues could show that specific residues within T-cell epitopes are presented in the context of individual MHC class I molecules and are responsible for the peptide/MHC interaction (anchor positions), while other residues mediate T-cell recognition.Citation24 Based on this knowledge, various algorithms have been developed for predicting the propensity of TAA-derived sequences to bind specific MHC molecules.Citation25 Later on, this approach has been combined with the SEREX technology, allowing the identification of additional important TAAs. Various Phase I/II clinical studies have demonstrated that vaccines designed by these technologies can be successfully employed against several types of malignancies including renal cell carcinoma (RCC), melanoma, prostate, and breast cancer.Citation26 However, the application of these immunotherapeutic strategies is still restricted by the limited number of known TAAs and T-cell epitopes, by the patient’s HLA type, and/or by the availability of tumor tissue.
The approaches used to identify TAA-derived T-cell epitopes are generally laborious and time consuming. Thus, in order to accelerate the development of (ever more personalized) anticancer vaccines, new technologies are necessary. DNA microarray-based and sequencing studies of tumor samples in comparison with the corresponding non-malignant autologous tissues provides a promising new approach to determine antigens that are specifically mutated or aberrantly expressed in malignant cells. Combining genetic data with mass spectrometry studies allows for the identification of MHC ligands in a relatively short time. Using this procedure, several MHC class I- binding epitopes were identified and used to vaccinate patients with metastatic RCC that has previously been treated or not with cyclophosphamide in the context of a randomized Phase II clinical trial.Citation27 The regression of established tumor lesions was infrequent and PFS was comparable in the 2 study arms. However, a prospectively planned analysis demonstrated that patients pretreated with cyclophosphamide who developed an immune response upon the administration of the multipeptide vaccine (IMA901) had a prolonged survival. Interestingly, a single infusion of low dose cyclophosphamide reduced the number of circulating regulatory T cells (Tregs). Moreover, among 6 analyzed populations of myeloid-derived suppressor cells (MDSCs), 2 had a prognostic value for OS, and the levels of apolipoprotein A-I (APOA1) as well as chemokine (C-C motif) ligand 17 (CCL17) were predictive for the induction of immune responses and OS. The results of a randomized Phase III study to determine the clinical benefits of IMA901, which has recently finished recruitment, are eagerly awaited.
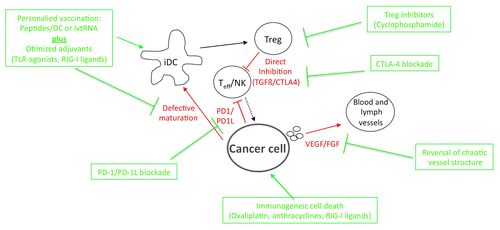
The use of antigenic peptides for vaccination is associated with several obstacles, including the existence of a limited set of antigens that are restricted to a well-defined MHC molecule and the patient-specific expression pattern of MHC-coding alleles. In addition, these vaccines often do not contain MHC class II-binding epitopes capable of stimulating CD4+ T cell responses, which are important for the induction and maintenance of vaccine-induced memory cytotoxic T lymphocytes (CTLs). The introduction of helper T cell epitopes in addition to CTL epitopes or vaccines that contain both CD4- and CD8-restricted antigens like full-length proteins, long peptides,Citation28 tumor lysates or tumor-derived RNA may overcome this limitation (). We have recently demonstrated that the intradermal administration of an in vitro transcribed RNA (ivtRNA) encoding for several TAAs to metastatic RCC patients induces antigen specific CTLs capable of recognizing multiple TAA-derived epitopes that are presented on different MHC molecules.Citation29,Citation30 Furthermore, this approach elicits antigen-specific CD4+ T lymphocytes reacting with MHC class II-binding peptides derived from cytosolic TAAs as a result of autophagy.Citation22 The induction of TAA-specific cytotoxic and helper T lymphocytes in vivo improves OS. Of note, the administration of defined peptides may also generate T lymphocytes that recognize antigenic epitopes not employed for vaccination.Citation31,Citation32 This phenomenon of antigen spreading, which can be detected several months after vaccination mostly among responding patients, was observed in several clinical studies testing peptide vaccines, adoptively transferred T cells or mAbs against CTLA4.Citation33,Citation34 These observations suggest that antigen spreading represents an important mechanism mediating tumor rejection.
Figure 1. General strategies for improving of antitumor immune. The multifaceted strategies set in place by malignant cells to evade the immune system are depicted in red. Potential interventions that may improve the efficacy of vaccination are shown in green. Please refer to the main text for further details.
Which adjuvant to take?
Usually, when TAAs, TAA-derived peptides, or tumor-derived RNA are given alone as a vaccine they induce only weak immune responses, calling for the co-administration immunological adjuvants. Adjuvants should improve the delivery and presentation of antigens and increase the stimulatory capacity of APCs. Some of these molecules such as Toll-like receptor (TLR) ligands or aluminum salts boost adaptive and innate immune responses, thereby mediating pleiotropic effects on APCs as well as T, B and natural killer (NK) cells. MF59, a non-toxic derivative of Salmonella (monophosphoryl lipid A, MPL), Montanide, saponins (AS01, AS02, QS21, ISCOM), imiquimod (a TLR7/8 agonists) and CpG oligodeoxynucleotides are the adjuvants most frequently employed in clinical trials.Citation35
However, there is an unmet need to improve these adjuvants to enhance desired TAA-directed immune responses (). Mechanistically, the biology and origin of the adjuvant regulates the type of the immune response it elicits (humoral vs. cellular, TH1 vs. TH2, etc.). Receptors such as pattern-recognition receptors (PRRs), which recognize microbial infections, play a central role in the elicitation of robust immune responses and may have a huge potential as target for adjuvants. PRRs are expressed on the cell surface, at endosomal membranes and in the cytoplasm. Membrane-bound TLRs recognize conserved microbial structures and signal via adaptor molecules like myeloid differentiation primary response 88 (MYD88) or TLR adaptor molecule 1 (TICAM1, best known as TRIF).Citation36 Cytosolic PRRs include the so-called NOD-like receptors (NLRs), such as NOD1 and NOD2 (which bind to bacterial peptidoglycans), as well as receptors sensing microbial nucleic acids, such as DEAD (Asp-Glu-Ala-Asp) box polypeptide 58 (DDX58, best known as RIG-I), interferon (IFN)-induced with helicase C domain 1 (IFIH1, best known as MDA5), absent in melanoma 2 (AIM2) receptors and transmembrane protein 173 (TMEM173, best known as STING). These PRRs generally stimulate the production of Type I IFN and activate the inflammasome, a multi-protein complex leading to the caspase-1 mediated release of interleukin (IL)-1β and IL-18.Citation37 The engagement of cytosolic nucleic acid receptors not only promotes the release of antiviral and pro-inflammatory mediators but may also result in the induction of mitochondrial apoptosis in tumor cells.Citation38 Most forms of apoptosis are considered “immunologically silent” or tolerogenic. In contrast, RIG-I agonist-induced cell death is highly immunogenic,Citation39 making RIG-I an ideal target for the synergistic activation of cell-autonomous and immunological antitumor effects. In line with this idea, RIG-I agonists stimulate ovarian cancer cells to up-regulate MHC class I molecules and to secrete the pro-inflammatory cytokines such as chemokine (C-X-C motif) ligand 10 (CXCL10), chemokine (C-X-C motif) ligand 1 (CCL5), IL-6, tumor necrosis factor α (TNFα), and IFNβ.Citation40 In addition, ovarian cancer cells responding to RIG-I agonists undergo apoptosis and are subsequently taken up by APCs, which in turn express increased levels of MHC class I and II as well as co-stimulatory molecules and secrete CXCL10 and IFNα. Of note, combining a small-interfering RNA (siRNA) specific for the endogenous immunosuppressive cytokine transforming growth factor β1 (TGFβ1) and a RIG-I agonist was shown to induce potent antitumor responses in a preclinical pancreatic cancer model.Citation41 Thus, mimicking viral infection induces an immunogenic variant of cancer cell death that activates innate and antigen-specific immune responses, representing a promising combination partner for anticancer vaccines.
Upcoming Partner(s): Antibodies, Chemotherapy, and Small Drugs
Despite the fact that some cancers such as melanoma or colorectal carcinoma (CRC) can elicit strong immune responses characterized by the recruitment of tumor-infiltrating lymphocytes (TILs), which in some cases are also relevant for prognosis (e.g., CD45RO+ cells at the margin of CRCs),Citation42 malignant cells are able to escape recognition (and hence destruction) by the immune system. This is probably due to the ability of malignant cells to promote immunological tolerance by modulating the tumor microenvironment through the release of soluble factors, cytokines, and chemokines as well as by the activation of immunosuppressive cells (i.e., Tregs and MDSC), eventually leading to immunoediting.Citation43 Other immune escape mechanisms including the loss of MHC and/or TAA expression and the up-regulation of immunosuppressive molecules (e.g,, CD274, best known as PD-L1 and osteoactivin) have been ascribed clinical relevance in various settings.Citation44,Citation45
In the last years, several mAbs specific for molecules that inhibit host immune responses were developed and introduced into the clinical routine (). The first proof-of-concept studies in this settings involved the CTLA4 targeting mAb ipilimumab.Citation10,Citation11 Ipilimumab has been approved in 2011 for the treatment of metastatic melanoma, and is currently under investigation as an adjuvant intervention against high-risk Stage III melanoma (NCT00636168). In brief, proper T-cell activation requires the recognition of cognate antigenic peptides (in the context of MHC molecules) through the T-cell receptor (Signal 1) as well as the delivery of co-stimulatory signals via members of the CD28 receptor family (Signal 2). CD28 is constitutively expressed on T cells and binds to CD80 and CD86 on the surface of APCs. Upon activation, T cells transiently up-regulate CTLA4, which binds to CD80 and CD86 with higher affinity than CD28, hence operating as an immune checkpoint and inhibiting cell cycle progression as well as IL-2 production.Citation46 Under homeostatic conditions CTLA4 restricts the risk of autoimmune disorders,Citation47 but it may also limit the expansion of tumor-specific effector T cells.Citation48 In line with this notion, blocking CTLA4 signaling on T cells restores antitumor immune responses. The clinical relevance of this paradigm has been further substantiated by the promising activity of another CTLA4-blocking antibody, tremelimumab, in patients with advanced stage malignant melanoma.Citation49 Both ipilimumab and tremelimumab could be used for the development of novel immunotherapeutic approaches, and various pre-clinical reports provides a compelling rationale for combining CTLA4-blocking agents with vaccination. Cancer vaccines enhance indeed the capacity of professional APCs (e.g., DCs) to capture and process TAAs, empowering them with the ability to efficiently stimulate tumor-specific T cells. Thus, coupling vaccines with CTLA4-blocking interventions intensifies tumor immunity, resulting in synergistic anti-neoplastic effects in many models.
Allogeneic prostate cancer cell lines engineered to secrete GM-CSF (GVAX) as well as PROSTVAC (see above) combined with recombinant GM-CSF have demonstrated immunological and clinical activity in cancer patients. The results from a Phase II randomized clinical trial indicated that PROSTVAC vaccination might improve OS, leading to the initiation of a large Phase III study. Two clinical trials reported by van den Eertwegh and colleagues and Madan and co-workers tested the concurrent application of ipilimumab together with the above mentioned vaccination therapies.Citation50,Citation51 Interestingly, high levels of CTLA4-expressing CD4+ T cells were shown to predict the response to GVAX.Citation52 Side effects were rare and the major toxic effects were immunological, including endocrinopathies, colitis, hepatitis, pneumonitis, and dermatitis. Objective clinical outcomes including decreases in circulating PSA levels and tumor regression were noted in some patients. The addition of ipilimumab to vaccination favored APC activation and both cellular and humoral antitumor responses. These promising studies should set the stage for further testing the combination of anticancer vaccines with CTLA4-blocking mAbs. However, the optimal dosing and schedule of such a combinatorial immunotherapeutic approach needs to be precisely determined.
Another promising approach would involve active immunization coupled to the blockade of PD-1 or its main ligand (PD-L1). High levels of PD-1 and PD-L1 were found to correlate with poor prognosis in patients affected by various malignancies.Citation53,Citation54 PD-1 is an inhibitory receptor belonging to the CD28/CTLA4 family and is expressed on activated T lymphocytes, B cells, monocytes, DCs, and Tregs. PD-L1 and another PD-1 ligand (PD-L2), which are expressed on T cells, APCs, and malignant cells, were shown to suppress self-reactive lymphocytes and to inhibit the effector functions of TAA-specific CTLs. These data strongly indicate that targeting the PD-1/PD-L1 axis represents a clinically valuable strategy to restore the cytotoxic activity of TAA-specific T cells. PD-1-blocking antibodies such as CT-011 and MDX-1106 as well as the anti-PD-L1 antibody MDX-1105 are being developed to modulate antitumor immune responses. Two recent studies published in the New England Journal of Medicine have highlighted the therapeutic potential of PD-1- or PD-L1-blocking agents in advanced cancer patients.Citation55,Citation56 Of note, the expression of the antibody target in this context seems to correlate with clinical outcome. Combinatorial approaches including PD-1/PD-L1-blocking mAbs and ipilimumab have recently been presented, exhibiting promising clinical response rates.Citation13 Moreover, the combination of PD-1/PD-L1-blocking mAbs with therapeutic vaccines or targeted anticancer agents (e.g., the BRAF inhibitor vemurafenib) is being explored in melanoma (NCT01176474 and NCT01176461) and advanced cancer patients (NCT01656642), respectively. The latter approach, however, has recently stopped due to liver toxicities, suggesting that other combination schedules (such as a sequential—as opposed to concomitant—administration), might be more promising. In addition, boosting the efficacy of vaccines by blocking PD-1 is also being tested in patients with hematological malignancies, as exemplified by a study in which CT-011 is administered together with a DC-based vaccine to AML patients (NCT01096602).
Combination with Chemotherapy and Small Molecules
Combinatorial regimens involving cytotoxic chemotherapeutics are a mainstay of cancer treatment. Historically, the integration of several agents with distinct mechanisms of action and non-overlapping toxic effects yielded curative treatments for some solid and hematological malignancies.
Most cytotoxic chemotherapeutics employed to date are thought to exert immunosuppressive properties due to their preferential effect on rapidly proliferating cells. However, this assumption had to be reconsidered following the recent discovery that some conventional cytotoxic agents can mediate robust immunostimulatory effects (). For instance, doxorubicin, mitoxantrone, cyclophosphamide, and oxaliplatin have been shown to induce an immunogenic variant of cell death by promoting the release of ATP and other signals by dying cells, resulting in the activation of the NLRP3 inflammasome in APCs and the elicitation of anti-tumor immune responses.Citation57-Citation59 Furthermore, some conventional chemotherapeutics like gemcitabine and oxaliplatin promote the expression of MHC molecules on malignant cells and the cross-presentation of TAAs to CTLs,Citation60 while taxanes preferentially inhibit immunosuppressive cells like Tregs and MDSCs.Citation61 Similar to doxorubicin, taxanes also enhance the permeability of malignant cells, rendering them more susceptible to granzyme B-mediated lysis. Anticancer vaccines could therefore be applied concurrently and/or after induction chemotherapy, which may improve their efficacy by reducing tumor burden, hence limiting the immunosuppressive effects of tumor-derived factors, and inhibiting Tregs as well as MDSCs.
Targeted therapies are increasingly more employed, also as they are thought to be more specific than conventional cytotoxic drugs. However, they usually inhibit several signaling pathways and are not devoid of unwanted or off-target effects. Several tyrosine kinase inhibitors (TKIs), proteasome inhibitors, mammalian target of rapamycin (mTOR) inhibitors and drugs altering epigenetic DNA modification, including histone deacetylase inhibitors and demethylating agents, were shown to elicit immunomodulatory effects, mostly by affecting T-cell or DC functions.Citation62-Citation64 Among the TKIs that robustly activate the immune system, sunitinib might represent the most promising one as it reduces the abundance of both TregsCitation65,Citation66 and MDSCs,Citation67 while facilitating CD4+ T-cell-mediated immune responses, but it does not affects the biology of APCs.Citation68 A randomized Phase III clinical trial is currently ongoing to test IMA901 in combination with sunitinib in patients with metastatic RCC, and clinical data are eagerly awaited (NCT01265901). In addition, vemurafenib (a BRAF inhibitor approved for treatment of melanoma patients bearing BRAF mutations) has been shown to induce a favorable immune milieu by increasing the expression of melanoma-associated antigens,Citation69 rendering it an interesting candidate for combination with other immunostimulatory strategies (e.g., CTLA4- or PD-1/PD-L1-blocking mAbs). The combination of BRAF inhibitors and CTLA4-blocking agents elicits significant hepatic toxicity, demonstrating that sequential administration schedules might be preferable also in this setting. Finally, emerging data demonstrate that the chaotic vessels generally serving neoplastic lesions contribute to immune dysfunction.Citation70 Thus, the normalization of disorganized vessels by means of anti-angiogenic compounds, such vascular endothelial growth factor (VEGF) receptor inhibitors or VEGF-blocking mAbs, could improve endogenous as well as vaccination-induced antitumor immune responses ().Citation71 This might at least in part explain the immunostimulatory effects mediated by sunitinib, which also contributes to vessel normalization by inhibiting VEGFR and platelet-derived growth factor receptor (PDGFR).Citation72
Main Problems to Overcome Via Immunological Conditioning
Multifaceted evasion strategies set in place by malignant cells still limit the efficacy of anticancer immunotherapy.Citation73 In addition, there are several issues that have not been sufficiently investigated so far. For instance, what are the optimal TAAs to target? Which the best vaccination modalities? Which components of the immune response are of utmost importance? Interestingly, virtually no progress has been made toward answering these critical questions in the context of several diseases.
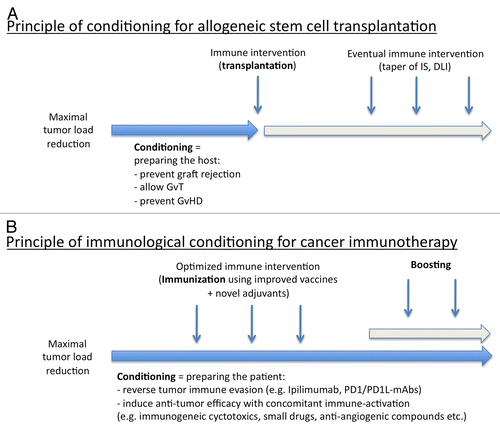
The concept of conditioning before immunotherapy is well established for allogenic stem cell transplantation. In particular, novel reduced-intensity and non-myeloablative conditioning regimens were developed to prevent graft rejection by the immune system and thus represent a paradigm for immunological conditioning. In cancer immunotherapy, conditioning would start already in the course of tumor debulking with conventional chemotherapy or targeted anticancer agents, supporting the generation of endogenous antitumor immune responses via the induction of immunogenic cell death, the inhibition of immunosuppressive cytokines (TGFβ1) and/or the elimination of Tregs and MDSCs. Additional immunosuppressive mechanisms of relevance such as those centered around CTLA4 and the PD-1/PD-L1 axis would be reverted by specific mAbs. Future anticancer vaccination strategies will have to be optimized by selection of optimal TAAs using a personalized approach, depending on disease type and the genetic background of patients (including MHC type). In addition, the development of innovative adjuvants will be critical for vaccines to elicit robust antitumor immune responses.
Concluding Remarks
Many pieces of the puzzle resulting in optimal immunotherapeutic regimens are available but not yet set together. Here, we propose that patients undergoing active anticancer immunotherapy based on TAA-derived peptides, full-length TAAs, tumor-derived RNA, or DC vaccines should undergo an immunological conditioning prior and/or concomitant to immunization. Chemotherapeutic regimens that induce lymphopenia prior to adoptive T-cell therapy, allowing for the efficient expansion of transferred T cells, have provided exciting results in melanoma patients ().Citation74 Similar to the conditioning approaches that are normally undertaken prior to allogeneic stem cell transplantation, immunological conditioning may represent an important step to increase the success rate of anticancer immunotherapy. In the future, properly designed clinical trials will have to verify this hypothesis by combining chemotherapeutics that reduce tumor burden and eliminate endogenous immunosuppressive factors with the induction and boosting of antitumor immune responses ().
Figure 2. Immunological conditioning as a strategy for boosting anticancer immunotherapy. (A) General conditioning approach for allogeneic stem cell transplantation (SCT). In this scenario, conditioning is generally applied prior to the immunological intervention (i.e., SCT). Specific immunomodulatory interventions (i.e., tapering, if no graft-vs.-host disease, GvHD) is detectable) can also be administered after transplantation. (B) Immunological conditioning in anticancer immunotherapy is primarily used to reverse cancer-associated immunosuppression (IS). DLI, donor lymphocyte infusion; GvT, graft-vs.-tumor.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Citation: Wolf D, A Heine, Brossart P. Implementing combinatorial immunotherapeutic regimens against cancer: The concept of immunological conditioning. OncoImmunology 2013; 2:e27588; 10.4161/onci.27588
References
- Boon T, Cerottini JC, Van den Eynde B, van der Bruggen P, Van Pel A. Tumor antigens recognized by T lymphocytes. Annu Rev Immunol 1994; 12:337 - 65; http://dx.doi.org/10.1146/annurev.iy.12.040194.002005; PMID: 8011285
- Dougan M, Dranoff G. Immune therapy for cancer. Annu Rev Immunol 2009; 27:83 - 117; http://dx.doi.org/10.1146/annurev.immunol.021908.132544; PMID: 19007331
- Rosenberg SA, Yang JC, Restifo NP. Cancer immunotherapy: moving beyond current vaccines. Nat Med 2004; 10:909 - 15; http://dx.doi.org/10.1038/nm1100; PMID: 15340416
- Schadendorf D, Ugurel S, Schuler-Thurner B, Nestle FO, Enk A, Bröcker EB, Grabbe S, Rittgen W, Edler L, Sucker A, et al, DC study group of the DeCOG. Dacarbazine (DTIC) versus vaccination with autologous peptide-pulsed dendritic cells (DC) in first-line treatment of patients with metastatic melanoma: a randomized phase III trial of the DC study group of the DeCOG. Ann Oncol 2006; 17:563 - 70; http://dx.doi.org/10.1093/annonc/mdj138; PMID: 16418308
- Weide B, Zelba H, Derhovanessian E, Pflugfelder A, Eigentler TK, Di Giacomo AM, Maio M, Aarntzen EH, de Vries IJ, Sucker A, et al. Functional T cells targeting NY-ESO-1 or Melan-A are predictive for survival of patients with distant melanoma metastasis. J Clin Oncol 2012; 30:1835 - 41; http://dx.doi.org/10.1200/JCO.2011.40.2271; PMID: 22529253
- Britten CM, Janetzki S, Butterfield LH, Ferrari G, Gouttefangeas C, Huber C, Kalos M, Levitsky HI, Maecker HT, Melief CJ, et al. T cell assays and MIATA: the essential minimum for maximum impact. Immunity 2012; 37:1 - 2; http://dx.doi.org/10.1016/j.immuni.2012.07.010; PMID: 22840835
- Rabinovich GA, Gabrilovich D, Sotomayor EM. Immunosuppressive strategies that are mediated by tumor cells. Annu Rev Immunol 2007; 25:267 - 96; http://dx.doi.org/10.1146/annurev.immunol.25.022106.141609; PMID: 17134371
- Breart B, Lemaître F, Celli S, Bousso P. Two-photon imaging of intratumoral CD8+ T cell cytotoxic activity during adoptive T cell therapy in mice. J Clin Invest 2008; 118:1390 - 7; http://dx.doi.org/10.1172/JCI34388; PMID: 18357341
- Eubel J, Enk AH. Dendritic cell vaccination as a treatment modality for melanoma. Expert Rev Anticancer Ther 2009; 9:1631 - 42; http://dx.doi.org/10.1586/era.09.139; PMID: 19895246
- Hodi FS, O’Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, Gonzalez R, Robert C, Schadendorf D, Hassel JC, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med 2010; 363:711 - 23; http://dx.doi.org/10.1056/NEJMoa1003466; PMID: 20525992
- Robert C, Thomas L, Bondarenko I, O’Day S, M D JW, Garbe C, Lebbe C, Baurain JF, Testori A, Grob JJ, et al. Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N Engl J Med 2011; 364:2517 - 26; http://dx.doi.org/10.1056/NEJMoa1104621; PMID: 21639810
- Hamid O, Robert C, Daud A, Hodi FS, Hwu WJ, Kefford R, Wolchok JD, Hersey P, Joseph RW, Weber JS, et al. Safety and tumor responses with lambrolizumab (anti-PD-1) in melanoma. N Engl J Med 2013; 369:134 - 44; http://dx.doi.org/10.1056/NEJMoa1305133; PMID: 23724846
- Wolchok JD, Kluger H, Callahan MK, Postow MA, Rizvi NA, Lesokhin AM, Segal NH, Ariyan CE, Gordon RA, Reed K, et al. Nivolumab plus ipilimumab in advanced melanoma. N Engl J Med 2013; 369:122 - 33; http://dx.doi.org/10.1056/NEJMoa1302369; PMID: 23724867
- von Mehren M, Arlen P, Tsang KY, Rogatko A, Meropol N, Cooper HS, Davey M, McLaughlin S, Schlom J, Weiner LM. Pilot study of a dual gene recombinant avipox vaccine containing both carcinoembryonic antigen (CEA) and B7.1 transgenes in patients with recurrent CEA-expressing adenocarcinomas. Clin Cancer Res 2000; 6:2219 - 28; PMID: 10873071
- Kantoff PW, Schuetz TJ, Blumenstein BA, Glode LM, Bilhartz DL, Wyand M, Manson K, Panicali DL, Laus R, Schlom J, et al. Overall survival analysis of a phase II randomized controlled trial of a Poxviral-based PSA-targeted immunotherapy in metastatic castration-resistant prostate cancer. J Clin Oncol 2010; 28:1099 - 105; http://dx.doi.org/10.1200/JCO.2009.25.0597; PMID: 20100959
- Small EJ, Schellhammer PF, Higano CS, Redfern CH, Nemunaitis JJ, Valone FH, Verjee SS, Jones LA, Hershberg RM. Placebo-controlled phase III trial of immunologic therapy with sipuleucel-T (APC8015) in patients with metastatic, asymptomatic hormone refractory prostate cancer. J Clin Oncol 2006; 24:3089 - 94; http://dx.doi.org/10.1200/JCO.2005.04.5252; PMID: 16809734
- Kantoff PW, Higano CS, Shore ND, Berger ER, Small EJ, Penson DF, Redfern CH, Ferrari AC, Dreicer R, Sims RB, et al, IMPACT Study Investigators. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N Engl J Med 2010; 363:411 - 22; http://dx.doi.org/10.1056/NEJMoa1001294; PMID: 20818862
- van der Bruggen P, Traversari C, Chomez P, Lurquin C, De Plaen E, Van den Eynde B, Knuth A, Boon T. A gene encoding an antigen recognized by cytolytic T lymphocytes on a human melanoma. Science 1991; 254:1643 - 7; http://dx.doi.org/10.1126/science.1840703; PMID: 1840703
- Novellino L, Castelli C, Parmiani G. A listing of human tumor antigens recognized by T cells: March 2004 update. Cancer Immunol Immunother 2005; 54:187 - 207; http://dx.doi.org/10.1007/s00262-004-0560-6; PMID: 15309328
- Arnold D, Faath S, Rammensee H, Schild H. Cross-priming of minor histocompatibility antigen-specific cytotoxic T cells upon immunization with the heat shock protein gp96. J Exp Med 1995; 182:885 - 9; http://dx.doi.org/10.1084/jem.182.3.885; PMID: 7650492
- Brossart P, Bevan MJ. Presentation of exogenous protein antigens on major histocompatibility complex class I molecules by dendritic cells: pathway of presentation and regulation by cytokines. Blood 1997; 90:1594 - 9; PMID: 9269778
- Dörfel D, Appel S, Grünebach F, Weck MM, Müller MR, Heine A, Brossart P. Processing and presentation of HLA class I and II epitopes by dendritic cells after transfection with in vitro-transcribed MUC1 RNA. Blood 2005; 105:3199 - 205; http://dx.doi.org/10.1182/blood-2004-09-3556; PMID: 15618468
- Joffre OP, Segura E, Savina A, Amigorena S. Cross-presentation by dendritic cells. Nat Rev Immunol 2012; 12:557 - 69; http://dx.doi.org/10.1038/nri3254; PMID: 22790179
- Rammensee HG. Chemistry of peptides associated with MHC class I and class II molecules. Curr Opin Immunol 1995; 7:85 - 96; http://dx.doi.org/10.1016/0952-7915(95)80033-6; PMID: 7772286
- Feldhahn M, Thiel P, Schuler MM, Hillen N, Stevanovic S, Rammensee HG, Kohlbacher O. EpiToolKit--a web server for computational immunomics. Nucleic Acids Res 2008; 36:W519 - 22; http://dx.doi.org/10.1093/nar/gkn229; PMID: 18440979
- Jäger D. Potential target antigens for immunotherapy identified by serological expression cloning (SEREX). Methods Mol Biol 2007; 360:319 - 26; PMID: 17172736
- Walter S, Weinschenk T, Stenzl A, Zdrojowy R, Pluzanska A, Szczylik C, Staehler M, Brugger W, Dietrich PY, Mendrzyk R, et al. Multipeptide immune response to cancer vaccine IMA901 after single-dose cyclophosphamide associates with longer patient survival. Nat Med 2012; 18:1254 - 61; http://dx.doi.org/10.1038/nm.2883; PMID: 22842478
- Melief CJ, van der Burg SH. Immunotherapy of established (pre)malignant disease by synthetic long peptide vaccines. Nat Rev Cancer 2008; 8:351 - 60; http://dx.doi.org/10.1038/nrc2373; PMID: 18418403
- Heine A, Grünebach F, Holderried T, Appel S, Weck MM, Dörfel D, Sinzger C, Brossart P. Transfection of dendritic cells with in vitro-transcribed CMV RNA induces polyclonal CD8+- and CD4+-mediated CMV-specific T cell responses. Mol Ther 2006; 13:280 - 8; http://dx.doi.org/10.1016/j.ymthe.2005.08.019; PMID: 16219490
- Rittig SM, Haentschel M, Weimer KJ, Heine A, Muller MR, Brugger W, Horger MS, Maksimovic O, Stenzl A, Hoerr I, et al. Intradermal vaccinations with RNA coding for TAA generate CD8+ and CD4+ immune responses and induce clinical benefit in vaccinated patients. Mol Ther 2011; 19:990 - 9; http://dx.doi.org/10.1038/mt.2010.289; PMID: 21189474
- Corbière V, Chapiro J, Stroobant V, Ma W, Lurquin C, Lethé B, van Baren N, Van den Eynde BJ, Boon T, Coulie PG. Antigen spreading contributes to MAGE vaccination-induced regression of melanoma metastases. Cancer Res 2011; 71:1253 - 62; http://dx.doi.org/10.1158/0008-5472.CAN-10-2693; PMID: 21216894
- Wierecky J, Müller MR, Wirths S, Halder-Oehler E, Dörfel D, Schmidt SM, Häntschel M, Brugger W, Schröder S, Horger MS, et al. Immunologic and clinical responses after vaccinations with peptide-pulsed dendritic cells in metastatic renal cancer patients. Cancer Res 2006; 66:5910 - 8; http://dx.doi.org/10.1158/0008-5472.CAN-05-3905; PMID: 16740731
- Brossart P, Wirths S, Stuhler G, Reichardt VL, Kanz L, Brugger W. Induction of cytotoxic T-lymphocyte responses in vivo after vaccinations with peptide-pulsed dendritic cells. Blood 2000; 96:3102 - 8; PMID: 11049990
- Ribas A, Glaspy JA, Lee Y, Dissette VB, Seja E, Vu HT, Tchekmedyian NS, Oseguera D, Comin-Anduix B, Wargo JA, et al. Role of dendritic cell phenotype, determinant spreading, and negative costimulatory blockade in dendritic cell-based melanoma immunotherapy. J Immunother 2004; 27:354 - 67; http://dx.doi.org/10.1097/00002371-200409000-00004; PMID: 15314544
- Berzofsky JA, Terabe M, Wood LV. Strategies to use immune modulators in therapeutic vaccines against cancer. Semin Oncol 2012; 39:348 - 57; http://dx.doi.org/10.1053/j.seminoncol.2012.02.002; PMID: 22595057
- Takeda K, Kaisho T, Akira S. Toll-like receptors. Annu Rev Immunol 2003; 21:335 - 76; http://dx.doi.org/10.1146/annurev.immunol.21.120601.141126; PMID: 12524386
- Franchi L, Eigenbrod T, Muñoz-Planillo R, Nuñez G. The inflammasome: a caspase-1-activation platform that regulates immune responses and disease pathogenesis. Nat Immunol 2009; 10:241 - 7; http://dx.doi.org/10.1038/ni.1703; PMID: 19221555
- Ting JP, Willingham SB, Bergstralh DT. NLRs at the intersection of cell death and immunity. Nat Rev Immunol 2008; 8:372 - 9; http://dx.doi.org/10.1038/nri2296; PMID: 18362948
- Besch R, Poeck H, Hohenauer T, Senft D, Häcker G, Berking C, Hornung V, Endres S, Ruzicka T, Rothenfusser S, et al. Proapoptotic signaling induced by RIG-I and MDA-5 results in type I interferon-independent apoptosis in human melanoma cells. J Clin Invest 2009; 119:2399 - 411; PMID: 19620789
- Kübler K, Gehrke N, Riemann S, Böhnert V, Zillinger T, Hartmann E, Pölcher M, Rudlowski C, Kuhn W, Hartmann G, et al. Targeted activation of RNA helicase retinoic acid-inducible gene-I induces proimmunogenic apoptosis of human ovarian cancer cells. Cancer Res 2010; 70:5293 - 304; http://dx.doi.org/10.1158/0008-5472.CAN-10-0825; PMID: 20551064
- Ellermeier J, Wei J, Duewell P, Hoves S, Stieg MR, Adunka T, Noerenberg D, Anders HJ, Mayr D, Poeck H, et al. Therapeutic efficacy of bifunctional siRNA combining TGF-β1 silencing with RIG-I activation in pancreatic cancer. Cancer Res 2013; 73:1709 - 20; http://dx.doi.org/10.1158/0008-5472.CAN-11-3850; PMID: 23338611
- Pagès F, Berger A, Camus M, Sanchez-Cabo F, Costes A, Molidor R, Mlecnik B, Kirilovsky A, Nilsson M, Damotte D, et al. Effector memory T cells, early metastasis, and survival in colorectal cancer. N Engl J Med 2005; 353:2654 - 66; http://dx.doi.org/10.1056/NEJMoa051424; PMID: 16371631
- Schreiber RD, Old LJ, Smyth MJ. Cancer immunoediting: integrating immunity’s roles in cancer suppression and promotion. Science 2011; 331:1565 - 70; http://dx.doi.org/10.1126/science.1203486; PMID: 21436444
- Gajewski TF. Failure at the effector phase: immune barriers at the level of the melanoma tumor microenvironment. Clin Cancer Res 2007; 13:5256 - 61; http://dx.doi.org/10.1158/1078-0432.CCR-07-0892; PMID: 17875753
- Kerkar SP, Restifo NP. Cellular constituents of immune escape within the tumor microenvironment. Cancer Res 2012; 72:3125 - 30; http://dx.doi.org/10.1158/0008-5472.CAN-11-4094; PMID: 22721837
- Sigal LH. Basic science for the clinician 55: CTLA-4. J Clin Rheumatol 2012; 18:155 - 8; http://dx.doi.org/10.1097/RHU.0b013e31824ea103; PMID: 22456519
- Chambers CA, Sullivan TJ, Allison JP. Lymphoproliferation in CTLA-4-deficient mice is mediated by costimulation-dependent activation of CD4+ T cells. Immunity 1997; 7:885 - 95; http://dx.doi.org/10.1016/S1074-7613(00)80406-9; PMID: 9430233
- Quezada SA, Simpson TR, Peggs KS, Merghoub T, Vider J, Fan X, Blasberg R, Yagita H, Muranski P, Antony PA, et al. Tumor-reactive CD4(+) T cells develop cytotoxic activity and eradicate large established melanoma after transfer into lymphopenic hosts. J Exp Med 2010; 207:637 - 50; http://dx.doi.org/10.1084/jem.20091918; PMID: 20156971
- McArthur GA, Ribas A. Targeting oncogenic drivers and the immune system in melanoma. J Clin Oncol 2013; 31:499 - 506; http://dx.doi.org/10.1200/JCO.2012.45.5568; PMID: 23248252
- Madan RA, Mohebtash M, Arlen PM, Vergati M, Rauckhorst M, Steinberg SM, Tsang KY, Poole DJ, Parnes HL, Wright JJ, et al. Ipilimumab and a poxviral vaccine targeting prostate-specific antigen in metastatic castration-resistant prostate cancer: a phase 1 dose-escalation trial. Lancet Oncol 2012; 13:501 - 8; http://dx.doi.org/10.1016/S1470-2045(12)70006-2; PMID: 22326924
- van den Eertwegh AJ, Versluis J, van den Berg HP, Santegoets SJ, van Moorselaar RJ, van der Sluis TM, Gall HE, Harding TC, Jooss K, Lowy I, et al. Combined immunotherapy with granulocyte-macrophage colony-stimulating factor-transduced allogeneic prostate cancer cells and ipilimumab in patients with metastatic castration-resistant prostate cancer: a phase 1 dose-escalation trial. Lancet Oncol 2012; 13:509 - 17; http://dx.doi.org/10.1016/S1470-2045(12)70007-4; PMID: 22326922
- Santegoets SJ, Stam AG, Lougheed SM, Gall H, Scholten PE, Reijm M, Jooss K, Sacks N, Hege K, Lowy I, et al. T cell profiling reveals high CD4+CTLA-4 + T cell frequency as dominant predictor for survival after prostate GVAX/ipilimumab treatment. Cancer Immunol Immunother 2013; 62:245 - 56; http://dx.doi.org/10.1007/s00262-012-1330-5; PMID: 22878899
- Hino R, Kabashima K, Kato Y, Yagi H, Nakamura M, Honjo T, Okazaki T, Tokura Y. Tumor cell expression of programmed cell death-1 ligand 1 is a prognostic factor for malignant melanoma. Cancer 2010; 116:1757 - 66; http://dx.doi.org/10.1002/cncr.24899; PMID: 20143437
- Sfanos KS, Bruno TC, Meeker AK, De Marzo AM, Isaacs WB, Drake CG. Human prostate-infiltrating CD8+ T lymphocytes are oligoclonal and PD-1+. Prostate 2009; 69:1694 - 703; http://dx.doi.org/10.1002/pros.21020; PMID: 19670224
- Brahmer JR, Tykodi SS, Chow LQ, Hwu WJ, Topalian SL, Hwu P, Drake CG, Camacho LH, Kauh J, Odunsi K, et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med 2012; 366:2455 - 65; http://dx.doi.org/10.1056/NEJMoa1200694; PMID: 22658128
- Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, Powderly JD, Carvajal RD, Sosman JA, Atkins MB, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med 2012; 366:2443 - 54; http://dx.doi.org/10.1056/NEJMoa1200690; PMID: 22658127
- Ghiringhelli F, Apetoh L, Tesniere A, Aymeric L, Ma Y, Ortiz C, Vermaelen K, Panaretakis T, Mignot G, Ullrich E, et al. Activation of the NLRP3 inflammasome in dendritic cells induces IL-1beta-dependent adaptive immunity against tumors. Nat Med 2009; 15:1170 - 8; http://dx.doi.org/10.1038/nm.2028; PMID: 19767732
- Michaud M, Martins I, Sukkurwala AQ, Adjemian S, Ma Y, Pellegatti P, Shen S, Kepp O, Scoazec M, Mignot G, et al. Autophagy-dependent anticancer immune responses induced by chemotherapeutic agents in mice. Science 2011; 334:1573 - 7; http://dx.doi.org/10.1126/science.1208347; PMID: 22174255
- Obeid M, Tesniere A, Ghiringhelli F, Fimia GM, Apetoh L, Perfettini JL, Castedo M, Mignot G, Panaretakis T, Casares N, et al. Calreticulin exposure dictates the immunogenicity of cancer cell death. Nat Med 2007; 13:54 - 61; http://dx.doi.org/10.1038/nm1523; PMID: 17187072
- Nowak AK, Lake RA, Marzo AL, Scott B, Heath WR, Collins EJ, Frelinger JA, Robinson BW. Induction of tumor cell apoptosis in vivo increases tumor antigen cross-presentation, cross-priming rather than cross-tolerizing host tumor-specific CD8 T cells. J Immunol 2003; 170:4905 - 13; PMID: 12734333
- Garnett CT, Schlom J, Hodge JW. Combination of docetaxel and recombinant vaccine enhances T-cell responses and antitumor activity: effects of docetaxel on immune enhancement. Clin Cancer Res 2008; 14:3536 - 44; http://dx.doi.org/10.1158/1078-0432.CCR-07-4025; PMID: 18519787
- Nencioni A, Beck J, Werth D, Grünebach F, Patrone F, Ballestrero A, Brossart P. Histone deacetylase inhibitors affect dendritic cell differentiation and immunogenicity. Clin Cancer Res 2007; 13:3933 - 41; http://dx.doi.org/10.1158/1078-0432.CCR-06-2903; PMID: 17606727
- Triozzi PL, Aldrich W, Achberger S, Ponnazhagan S, Alcazar O, Saunthararajah Y. Differential effects of low-dose decitabine on immune effector and suppressor responses in melanoma-bearing mice. Cancer Immunol Immunother 2012; 61:1441 - 50; http://dx.doi.org/10.1007/s00262-012-1204-x; PMID: 22310929
- Wolf AM, Wolf D, Rumpold H, Ludwiczek S, Enrich B, Gastl G, Weiss G, Tilg H. The kinase inhibitor imatinib mesylate inhibits TNF-alpha production in vitro and prevents TNF-dependent acute hepatic inflammation. Proc Natl Acad Sci U S A 2005; 102:13622 - 7; http://dx.doi.org/10.1073/pnas.0501758102; PMID: 16174751
- Adotevi O, Pere H, Ravel P, Haicheur N, Badoual C, Merillon N, Medioni J, Peyrard S, Roncelin S, Verkarre V, et al. A decrease of regulatory T cells correlates with overall survival after sunitinib-based antiangiogenic therapy in metastatic renal cancer patients. J Immunother 2010; 33:991 - 8; http://dx.doi.org/10.1097/CJI.0b013e3181f4c208; PMID: 20948437
- Terme M, Pernot S, Marcheteau E, Sandoval F, Benhamouda N, Colussi O, Dubreuil O, Carpentier AF, Tartour E, Taieb J. VEGFA-VEGFR pathway blockade inhibits tumor-induced regulatory T-cell proliferation in colorectal cancer. Cancer Res 2013; 73:539 - 49; http://dx.doi.org/10.1158/0008-5472.CAN-12-2325; PMID: 23108136
- Ko JS, Zea AH, Rini BI, Ireland JL, Elson P, Cohen P, Golshayan A, Rayman PA, Wood L, Garcia J, et al. Sunitinib mediates reversal of myeloid-derived suppressor cell accumulation in renal cell carcinoma patients. Clin Cancer Res 2009; 15:2148 - 57; http://dx.doi.org/10.1158/1078-0432.CCR-08-1332; PMID: 19276286
- Hipp MM, Hilf N, Walter S, Werth D, Brauer KM, Radsak MP, Weinschenk T, Singh-Jasuja H, Brossart P. Sorafenib, but not sunitinib, affects function of dendritic cells and induction of primary immune responses. Blood 2008; 111:5610 - 20; http://dx.doi.org/10.1182/blood-2007-02-075945; PMID: 18310500
- Frederick DT, Piris A, Cogdill AP, Cooper ZA, Lezcano C, Ferrone CR, Mitra D, Boni A, Newton LP, Liu C, et al. BRAF inhibition is associated with enhanced melanoma antigen expression and a more favorable tumor microenvironment in patients with metastatic melanoma. Clin Cancer Res 2013; 19:1225 - 31; http://dx.doi.org/10.1158/1078-0432.CCR-12-1630; PMID: 23307859
- Hamzah J, Jugold M, Kiessling F, Rigby P, Manzur M, Marti HH, Rabie T, Kaden S, Gröne HJ, Hämmerling GJ, et al. Vascular normalization in Rgs5-deficient tumours promotes immune destruction. Nature 2008; 453:410 - 4; http://dx.doi.org/10.1038/nature06868; PMID: 18418378
- Huang Y, Yuan J, Righi E, Kamoun WS, Ancukiewicz M, Nezivar J, Santosuosso M, Martin JD, Martin MR, Vianello F, et al. Vascular normalizing doses of antiangiogenic treatment reprogram the immunosuppressive tumor microenvironment and enhance immunotherapy. Proc Natl Acad Sci U S A 2012; 109:17561 - 6; http://dx.doi.org/10.1073/pnas.1215397109; PMID: 23045683
- Matsumoto S, Batra S, Saito K, Yasui H, Choudhuri R, Gadisetti C, Subramanian S, Devasahayam N, Munasinghe JP, Mitchell JB, et al. Antiangiogenic agent sunitinib transiently increases tumor oxygenation and suppresses cycling hypoxia. Cancer Res 2011; 71:6350 - 9; http://dx.doi.org/10.1158/0008-5472.CAN-11-2025; PMID: 21878530
- Curiel TJ. Tregs and rethinking cancer immunotherapy. J Clin Invest 2007; 117:1167 - 74; http://dx.doi.org/10.1172/JCI31202; PMID: 17476346
- Muranski P, Boni A, Wrzesinski C, Citrin DE, Rosenberg SA, Childs R, Restifo NP. Increased intensity lymphodepletion and adoptive immunotherapy--how far can we go?. Nat Clin Pract Oncol 2006; 3:668 - 81; http://dx.doi.org/10.1038/ncponc0666; PMID: 17139318
