Abstract
In a mouse model of breast carcinoma, the combination of cyclophosphamide and transforming growth factor β1,2,3 (TGFβ1,2,3)-targeting antibody achieved superior antineoplastic effects. This novel paradigm of synergistic chemoimmunotherapy promises to improve the clinical outcome of cancer patients with micrometastases, and thus deserves further investigation.
Natural and immunotherapy-induced anticancer immune responses rarely lead to tumor rejection owing to the presence of robust immunosuppressive mechanisms. Targeting key molecules that are responsible for such a robust immunosuppression, which (at least in part) is mediated by so-called “immune checkpoints,” constitutes an effective strategy for cancer therapy. This is well exemplified by ipilimumab, a cytotoxic T lymphocyte-associated protein 4 (CTLA4)-specific antibody approved by the US Food and Drug Administration (FDA) for the treatment of melanoma, as well as by programmed cell death 1 (PDCD1, best known as PD-1)- and CD174 (PD-L1)-specific antibodies, which are obtaining promising results in clinical trials.Citation1 Transforming growth factor β (TGFβ) is one of the molecules involved in immune checkpoints. Among other activities, TGFβ promotes the accumulation of CD4+FOXP3+ regulatory T cells (Tregs), which represent a major mediator of cancer-associated immunosuppression.Citation2 We have recently demonstrated that the administration of 1D11, a TGFβ-neutralizing antibody, increases the proportion of FOXP3+ cells among CD4+ tumor-infiltrating lymphocytes (TILs).Citation3 These surprising results reflected the fact that TGFβ potently inhibited the proliferation of Tregs in our model.Citation3 These findings are consistent with the well known anti-proliferative effect of TGFβ on T lymphocytes.Citation4 Thus TGFβ has a dual activity on Tregs: it inhibits the proliferation of naturally-occurring Tregs but promotes the differentiation of naïve CD4+ T cells into FOXP3+ Tregs. Therefore, the overall role of TGFβ in the biology of Tregs needs to be reassessed. Further, the use of TGFβ inhibitors to eliminate tumor-associated Tregs should also be reevaluated.
We used cyclophosphamide to counteract the Treg-stimulatory effects of anti-TGFβ antibody, mostly based on its reported ability to eliminate tumor-infiltrating Tregs.Citation5 The co-administration anti-TGFβ antibody and cyclophosphamide failed to reduce the proportion of Tregs among tumor-infiltrating CD4+ cells.Citation3 Nevertheless, this combination had a potent inhibitory effect on the development of 4T1 tumors in syngeneic BALB/c mice.Citation3 Several lines of evidence indicate that the therapeutic activity of this approach is (at least partially) based on the mobilization of an antitumor immune response in spite of high levels of Tregs. One provocative question can therefore be asked: Do Tregs exert functions other than the inhibition of antitumor immune responses? Indeed, Tregs not always suppress the activity of effector T cells. For example, Tregs and TH17 cells mutually stimulate each other in a tumor necrosis factor α (TNFα)/ TNFα receptor 2 (TNFR2)-dependent manner in vivo.Citation6 TH17 cells are known to mediate antitumor effects that are even superior to those of TH1 cells.Citation7 Interestingly, it has been reported that the antineoplastic effects of cyclophosphamide against colorectal cancers critically relies on TH17 cells, which are induced by intestinal microbiota.Citation8 It is therefore tempting to hypothesize that the expansion of Tregs by anti-TGFβ antibody might promote antitumor TH17 responses induced by cyclophosphamide. This counterintuitive idea needs to be tested in future studies.
When administered 3 d after the inoculation of cancer cells, anti-TGFβ antibodies and cyclophosphamide completely inhibited the development of 4T1 tumors, resulting in long-term disease-free survival in up to 80% of mice.Citation3 Therefore, a systemic combinatorial therapy based on TGFβ inhibition and cyclophosphamide is promising for the treatment of patients with micrometastatic or widespread metastatic disease once primary tumors are controlled by surgery and radiation therapy. In support of this notion, this combinatorial immunochemotherapeutic regimen achieved a remarkable inhibition of lung metastasis upon resection of primary 4T1 tumors.Citation3 In our study, we employed a single dose of cyclophosphamide. Thus, dose optimization is likely to yield improved therapeutic effects. Moreover, the addition of other antineoplastic agents, such as other forms of chemotherapy and immunotherapy, as well as radiotherapy, targeted anticancer agents and hormonotherapy, may also enhance the efficacy of cyclophosphamide plus TGFβ inhibitors. This possibility should also be addressed in future studies.
We consider the combination of cyclophosphamide and TGFβ inhibitors as a form of chemoimmunotherapy, a new terminology to indicate treatments including both anticancer chemotherapeutics and immunostimulatory interventions, which can achieve optimal clinical outcomes in cancer patients.Citation9 However, both cyclophosphamide and anti-TGFβ antibodies alone have the capacity to act on immune as well as on cancer cells, implying that their combination is somewhat different from other forms of immunochemotherapy. One may ask whether the ability of these agents to activate immune cells and to exert a direct effect on malignant cells are redundant. Our data indicate that cyclophosphamide plays a major role in the induction of interferon γ (IFNγ), while anti-TGFβ antibody mainly enables tumor infiltration by T cells. Furthermore, cyclophosphamide appears to be mainly responsible for the myeloid-derived suppressor cells (MDSC)-inhibiting effect of our combinatorial regimen. Thus, 2 therapeutic agents have different effects on immune cells.
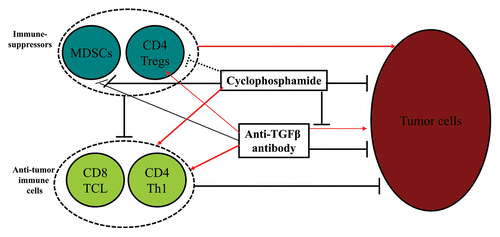
Cyclophosphamide is a well-characterized alkylating agent with cytotoxic effect on neoplastic cells. TGFβ has a dual role in oncogenesis, yet tumor-promoting effects are believed to prevail in advanced disease. Thus anti-TGFβ antibodies can inhibit the survival as well as the invasive/metastatic potential of cancer cells.Citation10 Conceivably, undesirable tumor-promoting effects of anti-TGFβ antibodies may be overcome by cyclophosphamide. Therefore, the roles of cyclophosphamide and anti-TGFβ antibodies are complementary and collaborative, rather than redundant. Our data suggest that cyclophosphamide and TGFβ inhibition synergize and convert tumor-associated immunosuppression into a protective response, at least in part by inducing the death of cancer cells and concomitantly allowing for the elicitation of antitumor immunity ().
Figure 1. Effects of cyclophosphamide and anti-TGFβ antibody on malignant and immune cells. Cyclophosphamide induces the demise of cancer cells, inhibits the tumor-promoting effects of transforming growth factor β (TGFβ)-specific antibodies, reduces the number of CD11b+Gr1+ myeloid-derived suppressor cells (MDSCs) and induces maturation of MDSCs, may transiently limit the number of regulatory T cells (Tregs), and stimulates the differentiation of antitumor TH cells. Anti-TGFβ antibodies normally inhibit the survival as well as the invasive/metastatic potential of cancer cells, but may also promote their growth (by blocking the anti-proliferative activity of TGFβ), at least in some settings, promote the expansion of Tregs, induce the massive infiltration of neoplastic lesions by T cells, and, in concert with cyclophosphamide, reduce the number of MDSCs while promoting the maturation of CD11b+Gr1+ cells. Solid black lines: inhibition/blockade; dashed black lines: uncertain inhibition; red arrows: stimulation/promotion/activation.
Humanized anti-TGFβ antibodies and other TGFβ inhibitors are currently being tested in clinical trials, while cyclophosphamide is one of the most frequently used chemotherapeutics in the clinic. Thus, it is tempting to consider their combination as an effective way to achieve superior clinical responses in cancer patients, especially those bearing advances neoplasms associated with micrometastases.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
This project has been funded in whole or in part with federal funds from the National Cancer Institute, National Institutes of Health, under contract HHSN26120080001E. The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the US Government. This Research was supported (in part) by the Intramural Research Program of the NIH, National Cancer Institute, Center for Cancer Research.
Citation: Chen X, Wakefield LM, Oppenheim JJ. Synergistic antitumor effects of a TGFβ inhibitor and cyclophosphamide. OncoImmunology 2014; 3:e28247; 10.4161/onci.28247
References
- Kyi C, Postow MA. Checkpoint blocking antibodies in cancer immunotherapy. FEBS Lett 2014; 588:368 - 76; http://dx.doi.org/10.1016/j.febslet.2013.10.015; PMID: 24161671
- Nishikawa H, Sakaguchi S. Regulatory T cells in cancer immunotherapy. Curr Opin Immunol 2014; 27C:1 - 7; http://dx.doi.org/10.1016/j.coi.2013.12.005; PMID: 24413387
- Chen X, Yang Y, Zhou Q, Weiss JM, Howard OZ, McPherson JM, Wakefield LM, Oppenheim JJ. Effective chemoimmunotherapy with anti-TGFβ antibody and cyclophosphamide in a mouse model of breast cancer. PLoS One 2014; 9:e85398; http://dx.doi.org/10.1371/journal.pone.0085398; PMID: 24416401
- Fox FE, Ford HC, Douglas R, Cherian S, Nowell PC. Evidence that TGF-beta can inhibit human T-lymphocyte proliferation through paracrine and autocrine mechanisms. Cell Immunol 1993; 150:45 - 58; http://dx.doi.org/10.1006/cimm.1993.1177; PMID: 8393732
- van der Most RG, Currie AJ, Mahendran S, Prosser A, Darabi A, Robinson BW, Nowak AK, Lake RA. Tumor eradication after cyclophosphamide depends on concurrent depletion of regulatory T cells: a role for cycling TNFR2-expressing effector-suppressor T cells in limiting effective chemotherapy. Cancer Immunol Immunother 2009; 58:1219 - 28; http://dx.doi.org/10.1007/s00262-008-0628-9; PMID: 19052741
- Zhou Q, Hu Y, Howard OM, Oppenheim JJ, Chen X. In vitro generated Th17 cells support the expansion and phenotypic stability of CD4(+)Foxp3(+) regulatory T cells in vivo. Cytokine 2014; 65:56 - 64; http://dx.doi.org/10.1016/j.cyto.2013.09.008; PMID: 24080164
- Muranski P, Borman ZA, Kerkar SP, Klebanoff CA, Ji Y, Sanchez-Perez L, Sukumar M, Reger RN, Yu Z, Kern SJ, et al. Th17 cells are long lived and retain a stem cell-like molecular signature. Immunity 2011; 35:972 - 85; http://dx.doi.org/10.1016/j.immuni.2011.09.019; PMID: 22177921
- Viaud S, Saccheri F, Mignot G, Yamazaki T, Daillère R, Hannani D, Enot DP, Pfirschke C, Engblom C, Pittet MJ, et al. The intestinal microbiota modulates the anticancer immune effects of cyclophosphamide. Science 2013; 342:971 - 6; http://dx.doi.org/10.1126/science.1240537; PMID: 24264990
- Chen G, Emens LA. Chemoimmunotherapy: reengineering tumor immunity. Cancer Immunol Immunother 2013; 62:203 - 16; http://dx.doi.org/10.1007/s00262-012-1388-0; PMID: 23389507
- Pickup M, Novitskiy S, Moses HL. The roles of TGFβ in the tumour microenvironment. Nat Rev Cancer 2013; 13:788 - 99; http://dx.doi.org/10.1038/nrc3603; PMID: 24132110
