Abstract
Tumor-infiltrating lymphocytes (TILs) are crucial for effective antitumor responses. However, hypoxia can skew T-cell differentiation and function, thereby perturbing TILs. We have demonstrated that TILs and their immune function are associated with tumor vascularization. These features are prognostic for improved disease-specific survival in ovarian cancer. Thus, new immunotherapies should consider how hypoxia impacts antitumor immunity.
In ovarian cancer, it has become increasingly clear that tumor-infiltrating lymphocytes (TILs) confer a survival benefit to patients.Citation1 It is tempting to strive to further define the key TIL subsets driving antitumor immunity, as one can envision developing T cell-based therapy armed with this knowledge. Indeed, refinements in adoptive T-cell therapy seeking to identify antigen-specific populations are currently under intense investigation in melanoma studies. Given the immunosuppressive tumor environment, one must question how TILs perform their anticancer role under these hostile conditions. Generally, hypoxia is a common feature of tumors and low oxygen can attenuate immune responses due to the corresponding signaling events and metabolic changes initiated by T cells in hypoxic environments.Citation2 In ovarian cancer, hypoxia has been observed to promote regulatory T cell (Treg) recruitment and alter CD4+ T cell differentiation through degradation of the T-cell fate regulatory transcription factor FoxP3 and resultant promotion of T helper type-17 (Th17) development.Citation3,Citation4 Conversely, other studies have shown that hypoxia can promote the expression of FoxP3 in CD4+ T cells.Citation5 Regardless, metabolically, it is well established that low oxygen in tumors stabilizes the hypoxia inducible factor-1α (HIF-1α), which in turn reprograms the entire metabolic network of tumors.Citation6 In response, hypoxic tumors increase the production of lactate that promotes the recruitment of myeloid-derived suppressor cells (MDSCs) which have a variety of immunosuppressive properties. For example, a high concentration of lactate has been observed to inhibit cytotoxic T-cell activation and causes a shift toward a Th17/23 cell phenotype.Citation7 Our recent study has shown that T-cell infiltration and antitumor function is dependent upon the degree of tumor vasculature and corresponding oxygenation.Citation8
Using 2 established markers of tissue vascularization and oxygenation, CD31, to mark blood vessel endothelial cells and vascular endothelial growth factor (VEGF), a hypoxia-inducible gene, we assessed the influence of hypoxia on TILs.Citation8 Our initial analysis revealed that, compared with CD31 negative tumors (hypoxic), CD31 positive tumors (vascularized and normoxic) correlate with better disease-specific patient survival. Albeit counterintuitive, we posited that the increase in survival was related to lymphocyte infiltration into the tumor. When a series of tumor sections from the tissue array were histologically assessed for the presence of T cell markers (CD8, CD4, and FoxP3), we found that the expression of each marker had a strong positive correlation with CD31 expression. Further characterization using cytotoxicity markers (TIA-1 and granzyme B) showed that TILs within vascularized tumors were also activated and functional ().
Figure 1. Tissue vascularization influences T-cell infiltration and function. In high-grade serous ovarian tumors, the presence of tumor-infiltrating lymphocytes (TILs) and markers of TIL function are strongly associated with tumor vasculature. Together, these factors are prognostic for improved survival in high-grade serous ovarian cancer. The presence of TILs is dictated by the tumor oxygen gradient, measured using two proxy markers of vascularization (CD31 and VEGF). In tumor regions furthest from the vasculature, T cells have reduced cytolytic function and activate autophagy to survive.
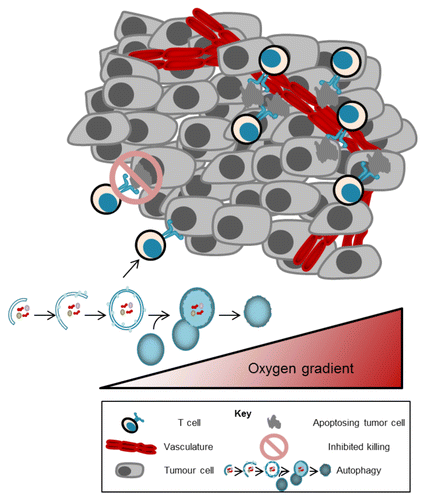
We next performed a series of in vitro assays using murine OTI T cells to determine the direct effect of hypoxia on T-cell function.Citation8 OVA-specific CD8+ T cells were isolated from OTI mice and stimulated under normoxic or hypoxic conditions with the cognate SIINFEKL peptide. As predicted, cells cultured under low oxygen produced lower levels of the cytotoxicity factors tumor necrosis factor α (TNFα) as well as interferon-γ (IFNγ). The decreased ability of hypoxia-exposed T cells to mount an effective cytotoxic response was confirmed using a cell lysis assay. Under hypoxia, T cells exhibited a lower capacity to kill target cells compared with those cultured in normoxia. In addition the suppression of T-cell effector functions under hypoxic conditions, oxygen-starved T cells were found to activate autophagy as a survival mechanism. Although a common adaptation for tumor cells under hypoxia, autophagy had thus far been undocumented for hypoxic responses in T cells.
These results suggested that positive outcomes associated with TIL depend upon the oxygenation state of the tumor. Therefore, a series of Kaplan–Meier analyses were performed, comparing survival rates in groups of patients stratified according to the presence of each of the vascularity markers.Citation8 These analyses showed that immune infiltrates led to longer disease-specific survival only when tumors were well vascularized and CD31+. Interestingly, in the case of the Treg marker FoxP3, there was a significant difference in these survival rates between patients harboring hypoxic vs. normoxic tumors, suggesting that the presence of T cells expressing FoxP3 are only beneficial to the patient when their tumor is highly vascularized.
Our study supports the concept that modulation of the tumor vasculature may be a viable avenue to improve anticancer immunity (). One such approach aims to stimulate T-cell adhesion to the epithelial cells within the tumor vasculature, and subsequent T-cell tumor infiltration. Considering that vascular growth factors such as VEGF can inhibit adhesion ligand expression in a state known as epithelial cell anergy (thus suppressing T-cell extravasation and tumor infiltration) increasing oxygenation to prevent such signals could be beneficial.Citation9 A specific therapy where this approach is being pursued is the tumor vasculature targeting agent NGR-TNF in which the inflammatory cytokine TNFα is fused with a short peptide sequence targeting tumor-associated blood vessels.Citation10 TNFα activates epithelial cells leading to adhesion molecule expression and immune cell infiltration. If this technique were applied in tumors undergoing angiogenesis, perhaps the infiltration suppression issue could be surpassed.
Because of the altered intratumoral T-cell activity, the hypoxic tumor environment may limit clinical strategies aiming to induce beneficial immune responses. This suggests that novel approaches altering the hypoxic response or the metabolism of T cells could counter the immunosuppressive effects of hypoxia. Overall, these findings demonstrate that the CD8+ T cell response is a crucial component of antitumor immunity and their presence within the malignant lesion requires adequate oxygenation in order for TILs to beneficially impact patient outcomes.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Citation: Neilson D, Macpherson S, Townsend KN, Lum JJ. Tumor vascularity in ovarian cancer: T cells need breathing room. OncoImmunology 2014; 3:e28272; 10.4161/onci.28272
References
- Milne K, Köbel M, Kalloger SE, Barnes RO, Gao D, Gilks CB, Watson PH, Nelson BH. Systematic analysis of immune infiltrates in high-grade serous ovarian cancer reveals CD20, FoxP3 and TIA-1 as positive prognostic factors. PLoS One 2009; 4:e6412; http://dx.doi.org/10.1371/journal.pone.0006412; PMID: 19641607
- Lukashev D, Klebanov B, Kojima H, Grinberg A, Ohta A, Berenfeld L, Wenger RH, Ohta A, Sitkovsky M. Cutting edge: hypoxia-inducible factor 1alpha and its activation-inducible short isoform I.1 negatively regulate functions of CD4+ and CD8+ T lymphocytes. J Immunol 2006; 177:4962 - 5; http://dx.doi.org/10.4049/jimmunol.177.8.4962; PMID: 17015677
- Facciabene A, Peng X, Hagemann IS, Balint K, Barchetti A, Wang LP, Gimotty PA, Gilks CB, Lal P, Zhang L, et al. Tumour hypoxia promotes tolerance and angiogenesis via CCL28 and T(reg) cells. Nature 2011; 475:226 - 30; http://dx.doi.org/10.1038/nature10169; PMID: 21753853
- Dang EV, Barbi J, Yang HY, Jinasena D, Yu H, Zheng Y, Bordman Z, Fu J, Kim Y, Yen HR, et al. Control of T(H)17/T(reg) balance by hypoxia-inducible factor 1. Cell 2011; 146:772 - 84; http://dx.doi.org/10.1016/j.cell.2011.07.033; PMID: 21871655
- Ben-Shoshan J, Maysel-Auslender S, Mor A, Keren G, George J. Hypoxia controls CD4+CD25+ regulatory T-cell homeostasis via hypoxia-inducible factor-1alpha. Eur J Immunol 2008; 38:2412 - 8; http://dx.doi.org/10.1002/eji.200838318; PMID: 18792019
- Semenza GL. HIF-1 mediates metabolic responses to intratumoral hypoxia and oncogenic mutations. J Clin Invest 2013; 123:3664 - 71; http://dx.doi.org/10.1172/JCI67230; PMID: 23999440
- Husain Z, Seth P, Sukhatme VP. Tumor-derived lactate and myeloid-derived suppressor cells: Linking metabolism to cancer immunology. Oncoimmunology 2013; 2:e26383; http://dx.doi.org/10.4161/onci.26383; PMID: 24404426
- Townsend KN, Spowart JE, Huwait H, Eshragh S, West NR, Elrick MA, Kalloger SE, Anglesio M, Watson PH, Huntsman DG, et al. Markers of T cell infiltration and function associate with favorable outcome in vascularized high-grade serous ovarian carcinoma. PLoS One 2013; 8:e82406; http://dx.doi.org/10.1371/journal.pone.0082406; PMID: 24376535
- Piali L, Fichtel A, Terpe HJ, Imhof BA, Gisler RH. Endothelial vascular cell adhesion molecule 1 expression is suppressed by melanoma and carcinoma. J Exp Med 1995; 181:811 - 6; http://dx.doi.org/10.1084/jem.181.2.811; PMID: 7530765
- Curnis F, Arrigoni G, Sacchi A, Fischetti L, Arap W, Pasqualini R, Corti A. Differential binding of drugs containing the NGR motif to CD13 isoforms in tumor vessels, epithelia, and myeloid cells. Cancer Res 2002; 62:867 - 74; PMID: 11830545
