Abstract
We have previously reported that a subset of breast tumors are infiltrated with IL-17A-producing tumor-associated lymphocytes and that IL-17A cytokine is principally associated with estrogen receptor negative (ER−) and triple negative, basal-like tumors. We established that IL-17A producing lymphocytes induced cancer cell proliferation, chemoresistance, and invasion, indicating that IL-17A is a potential therapeutic target for breast malignancies.
Inflammatory signals from the tumor microenvironment influence tumor behavior by promoting the growth of neoplastic lesions and the dissemination of malignant cells to distal sites. Among the interleukin-17 family of inflammatory cytokines, IL-17A has gained popularity in the context of carcinogenesis and accumulating evidence supports its involvement in human cancer. IL-17A is produced by a subset of CD4+ lymphocytes, called Th17 cells, as well as other cells such as natural killer (NK) cells and macrophages.
Beyond their established role in the pathogenesis of rheumatoid arthritis and psoriasis, both IL-17A cytokine and IL-17A-producing immune cells may participate in carcinogenesis, a premise supported by their increased presence in various malignancies, including breast cancers.Citation1-Citation3 When present, IL-17A participates in tumor progression through the activation of oncogenic pathways such as mitogen-activated protein kinase-related (MAPK/ERK), receptor-tyrosine kinase activated signal transducer and activator-3 (STAT3), and the B cell lymphoma-2 (BCL2) family of anti-apoptotic genes, (among others) that together promote tumor cell survival and metastatic dissemination. The functional participation of IL17A in carcinogenesis has been directly assessed in several mouse models by knocking out the IL17A gene, or alternatively, 1 of the 2 genes encoding its cognate receptor (IL17RA and IL17RC). Mice deficient in IL-17A signaling have been observed to be resistant to the development of carcinogen-induced skin lesions as well as colon and prostate cancer and exhibit diminished growth of melanoma, lung, and bladder tumor cell lines in transplantation settings. IL-17A neutralization also resulted in the drastic diminution of colon cancer cell growth and decreased the growth of ovarian and lung cancer cell lines. Of particular interest, IL-17A neutralizing antibodies reduced the tumor growth and metastasis of several mouse models of breast cancer.Citation4,Citation5
The role of IL-17A in breast cancer has been suggested by studies in vitroCitation1 and further substantiated in animal modelsCitation4,Citation5 and in clinical samples.Citation5,Citation6 Furthermore, IL17R knockdown in 4T1 cells decreased cancer growth in vivo and, of particular surprise, induced cancer cell death.Citation6 Thus, we speculated that IL-17A is an important factor in breast cancer progression and influences breast cancer cell survival. Combining IHC studies and analyses of fresh human biopsies, we found that IL-17A producing cells infiltrate ~20% of breast cancer patient tumors,Citation7 thereby confirming previous reports.Citation1,Citation2 One fifth of the malignant lesions exhibited moderate to heavy infiltration by IL-17A positive immune cells, whereas such cells were absent or rare in normal mammary tissues. Most IL-17A+ immune cells were lymphocytes and macrophages, which, again, is in accordance with previous work.Citation1,Citation2 Concomitantly, a large study from Chen et al. involving 207 breast cancer cases reported that ~18% of the lesions were infiltrated with IL-17A producing cells.Citation3 IL-17A positive tumors were significantly associated with estrogen receptor (ER) negative, progesterone receptor (PR) negative, and triple negative status as well as with higher histological grade and reduced disease free survival. By combining our dataCitation7 with those from Chen et al.Citation3 we concluded that IL-17A is mainly involved in ER− and triple negative tumors. Unpublished observations from our laboratory further suggest that IL-17A is notably involved in inflammatory breast cancer, a very rare subtype of breast cancer with poor prognosis.
By combining in vitro and ex vivo experiments assaying lymphocytes isolated from patient biopsies and a global approach to uncover the potential oncogenic pathways activated by IL-17A, we showed that IL-17A exerts several oncogenic effects through direct binding to its receptor on the surface of tumor cells (). ERK kinases were consistently phosphorylated upon IL-17A stimulation in all cell lines tested. ERK kinases, as part of the MAPK-signaling pathway, are integral to the proliferation and survival of many cancer cell types. In support, we demonstrated that IL-17A promotes resistance to docetaxel in breast cell lines in a dose-dependent manner. We also found that IL-17A promotes the proliferation of some breast cancer cell lines. Although this proliferative effect of IL-17A on cancer cells had not been previously reported, IL-17A is known to promote the proliferation of smooth muscle airway cells and human mesenchymal stem cells through the activation of the MAPK-kinase related MEK-ERK pathway. We also confirmed results from Zhu et al.Citation1 and showed that IL-17A stimulates breast cancer cell migration and extracellular matrix invasion in 3D tumor models.
Figure 1. Protumor activities of the inflammatory cytokine IL-17A in breast cancer. Interleukin-17A from the tumor microenvironment binds IL-17R on the surface of breast tumor cells and activates oncogenic ERK and NF-κB pathways, thereby leading to increased proliferation, survival and resistance to chemotherapeutics, and invasiveness. IL-17A also binds IL-17R present on fibroblasts and activates NF-κB and STAT3 pathways leading to production of IL-6 and G-CSF. IL-6, in combination with TGFβ, further activates Th17 cells leading to a chronic inflammatory state and amplification of IL-17A signaling. G-CSF participates to the recruitment of myeloid-derived suppressor cells (MDSCs) that release VEGF leading to tumor angiogenesis and also possess potent immunosuppressive activity further supporting tumor growth. ERK, extracellular signal-regulated kinase; G-CSF, granulocyte-colony stimulating factor; nuclear factor kappa B (NFκB); STAT3, signal transducer and activator-3; TGF, transforming growth factor-β; VEGF, vascular endothelial growth factor.
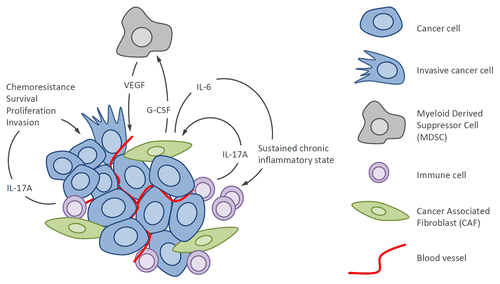
The translational relevance of this work in regards to the therapeutic use of IL-17A antagonists for the treatment of breast cancer relies upon the use of patient biopsy material. We isolated heterogeneous populations of tumor-infiltrating lymphocytes (TILs) from IL-17A+ breast cancer specimens and found that they promoted chemoresistance, proliferation, and migration in an IL-17A-dependent manner, evinced by IL-17A neutralizing monoclonal antibodies that largely abrogated their protumorigenic properties. Taken together with evidence for the critical role of IL-17A in tumor angiogenesis and resistance to vascular endothelial growth factor (VEGF)-blockade,Citation8 these results suggest that IL-17A inhibition could effectively decrease tumor cell dissemination, sensitize cancer cells to chemotherapeutic agents, and prevent resistance to the anti-angiogenic drug Avastin.
Finally, an outstanding question that remains is what roles, if any, do other isoforms of the IL-17 family play in malignant disease? So far, the potential involvement of IL-17C and IL-17D in cancer remains completely unexplored. On the other hand, IL-17B has been recently demonstrated to correlate with mammary cell transformation and to foster the survival of breast cancer cells by activating the nuclear transcription factor kappa B (NF-κB) and BCL-2 pathways.Citation9 Furthermore, results from our group substantiate an important role for IL-17B, and its receptor IL-17RB, in mammary tumorigenesis (manuscript in preparation). In contrast, IL-17E, an alternative ligand for IL-17RB, has been reported to induce breast cancer cell apoptosis, however, this effect may potentially occur via competition with IL-17B.Citation10 Therefore, further work is needed to understand the respective roles of IL-17A, and the other isoforms of this important pro-inflammatory cytokine family, in the tumor microenvironment, findings that may lead to the identification of novel cancer therapeutic targets.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Citation: Bastid J, Bonnefoy N, Eliaou JF, Bensussan A. Lymphocyte-derived interleukin-17A adds another brick in the wall of inflammation-induced breast carcinogenesis. OncoImmunology 2014; 3:e28273; 10.4161/onci.28273
References
- Zhu X, Mulcahy LA, Mohammed RA, Lee AH, Franks HA, Kilpatrick L, Yilmazer A, Paish EC, Ellis IO, Patel PM, et al. IL-17 expression by breast-cancer-associated macrophages: IL-17 promotes invasiveness of breast cancer cell lines. Breast Cancer Res 2008; 10:R95; http://dx.doi.org/10.1186/bcr2195; PMID: 19014637
- Benevides L, Cardoso CR, Tiezzi DG, Marana HR, Andrade JM, Silva JS. Enrichment of regulatory T cells in invasive breast tumor correlates with the upregulation of IL-17A expression and invasiveness of the tumor. Eur J Immunol 2013; 43:1518 - 28; http://dx.doi.org/10.1002/eji.201242951; PMID: 23529839
- Chen WC, Lai YH, Chen HY, Guo HR, Su IJ, Chen HH. Interleukin-17-producing cell infiltration in the breast cancer tumour microenvironment is a poor prognostic factor. Histopathology 2013; 63:225 - 33; http://dx.doi.org/10.1111/his.12156; PMID: 23738752
- Das Roy L, Pathangey LB, Tinder TL, Schettini JL, Gruber HE, Mukherjee P. Breast-cancer-associated metastasis is significantly increased in a model of autoimmune arthritis. Breast Cancer Res 2009; 11:R56; http://dx.doi.org/10.1186/bcr2345; PMID: 19643025
- Roy LD, Ghosh S, Pathangey LB, Tinder TL, Gruber HE, Mukherjee P. Collagen induced arthritis increases secondary metastasis in MMTV-PyV MT mouse model of mammary cancer. BMC Cancer 2011; 11:365; http://dx.doi.org/10.1186/1471-2407-11-365; PMID: 21859454
- Nam JS, Terabe M, Kang MJ, Chae H, Voong N, Yang YA, Laurence A, Michalowska A, Mamura M, Lonning S, et al. Transforming growth factor beta subverts the immune system into directly promoting tumor growth through interleukin-17. Cancer Res 2008; 68:3915 - 23; http://dx.doi.org/10.1158/0008-5472.CAN-08-0206; PMID: 18483277
- Cochaud S, Giustiniani J, Thomas C, Laprevotte E, Garbar C, Savoye AM, Curé H, Mascaux C, Alberici G, Bonnefoy N, et al. IL-17A is produced by breast cancer TILs and promotes chemoresistance and proliferation through ERK1/2. Sci Rep 2013; 3:3456; http://dx.doi.org/10.1038/srep03456; PMID: 24316750
- Chung AS, Wu X, Zhuang G, Ngu H, Kasman I, Zhang J, Vernes JM, Jiang Z, Meng YG, Peale FV, et al. An interleukin-17-mediated paracrine network promotes tumor resistance to anti-angiogenic therapy. Nat Med 2013; 19:1114 - 23; http://dx.doi.org/10.1038/nm.3291; PMID: 23913124
- Huang CK, Yang CY, Jeng YM, Chen CL, Wu HH, Chang YC, Ma C, Kuo WH, Chang KJ, Shew JY, et al. Autocrine/paracrine mechanism of interleukin-17B receptor promotes breast tumorigenesis through NF-κB-mediated antiapoptotic pathway. Oncogene 2013; Forthcoming http://dx.doi.org/10.1038/onc.2013.268; PMID: 23851503
- Furuta S, Jeng YM, Zhou L, Huang L, Kuhn I, Bissell MJ, Lee WH. IL-25 causes apoptosis of IL-25R-expressing breast cancer cells without toxicity to nonmalignant cells. Sci Transl Med 2011; 3:78ra31; http://dx.doi.org/10.1126/scitranslmed.3001374; PMID: 21490275
