Abstract
In gastrointestinal stromal tumor (GIST), the most common human sarcoma, tumor-associated macrophages (TAMs) have been found to be abundant and surprisingly M1-like, exhibiting antitumoral activities. However, TAMs switch to M2-like during the course of imatinib therapy, but upon drug resistance TAMs revert to M1-like. Therefore, the oncologic efficacy of TAM depletion may depend on tumor type and treatment status.
TAMs comprise a substantial portion of intratumoral leukocytes, which interact with malignant cells and other tumor-associated cells, such as those of stromal origin or even other immune cells. While macrophages are classically characterized as M1- (i.e., tumor-inhibitory) or M2- (i.e., tumor promoting) polarized,Citation1 most published studies of TAMs in mouse and human cancers have shown that tumor-infiltrating macrophages are predominantly M2-like, and thus support tumor growth. However, these reports are typically based on a few immunohistochemical or cytofluorometric markers, and less frequently on functional assays or depletion studies.Citation2 Thus, while investigators are currently pursuing TAM depletion as a clinical therapy for cancer, a detailed understanding of the complexities of TAM biology in humans remains to be well defined.
We have found that TAMs are present in high numbers in both mouse and human GISTs.Citation3 In genetically engineered GIST mouse strains, TAMs comprise a median of ~40% of intratumoral CD45+ cells, whereas in untreated human GISTs TAMs comprise ~25% of CD45+ lymphoctyes. Mouse models and naturally occurring human GISTs are typically driven by an activating mutation in the KIT proto-oncogene.Citation4 As the constitutively active KIT oncoprotein is initially highly sensitive to the tyrosine kinase inhibitor imatinib mesylate, treatment with this drug leads to either partial response or stable disease in ~80% of patients with metastatic disease.Citation4 Despite these early beneficial responses, unfortunately, the median time to progression is only ~2 y, a pathogenesis usually attributable to acquired resistance to imatinib through additional KIT mutations.Citation5
In the course of our studies, we initially hypothesized that TAM depletion would provide an additional therapeutic option. However, in studies using GIST mice, TAM depletion via colony stimulating factor 1 receptor (CSF1R) inhibition actually increased tumor burden.Citation3 Further investigation of mouse TAMs showed that they were highly inflammatory phenotypically and functionally. For example, they expressed high levels of the surface markers CD11c and MHC II, the co-stimulatory molecules CD80 and CD86, as well as Fc receptors CD64 and CD16/32. Furthermore, TAMs from untreated GIST mice were phagocytic, were capable of presenting antigen, and produced substantial levels of inflammatory cytokines. Also, in vitro characterization demonstrated that mouse TAMs directly inhibited the growth of tumor cells and stimulated T cell proliferation and production of interferon-γ (IFNγ). Thus, they were M1-like. Parallel studies of human GIST primary tumors obtained freshly from consenting patients undergoing surgical resection showed that TAMs from untreated human GISTs were also M1-like in appearance and function, the most important of which is their production of inflammatory cytokines and stimulation of T cell proliferation. These findings were surprising, given that TAMs had previously been thought to be generally M2-like. To our knowledge, freshly isolated human TAMs had not been previously subject to such functional characterization.
Plasticity has long been described as a hallmark of macrophage biology.Citation1 Whereas TAMs in untreated GIST mice and human patients were M1-like, GIST-associated macrophages undergo a remarkable phenotype shift in response to imatinib therapy.Citation3 Following 2 wk of imatinib therapy in mice, TAMs exhibited reduced expression of the M1-markers CD11c, MHCII, CD80 and CD86, as well as lower production of inflammatory cytokines. Concurrently, expression of multiple M2 markers was increased (). TAMs from human tumors responding to imatinib therapy (for a median treatment duration of 6 mo) were subject to a gene expression array, which showed a profound M2-like shift compared with untreated tumors, with 689 genes differentially expressed. Among these were downregulation of MHC class II related transcripts and upregulation of M2 markers, including mRNA’s encoding scavenger receptors, cathepsins, arginase, chitinase, as well as those promoting angiogenesis, neutrophil recruitment, and extracellular matrix remodeling. In tumors that eventually acquired imatinib resistance, there were no longer genes differentially expressed relative to untreated tumors, indicating that the dramatic M2 shift was extinct. In mice, the M2-like shift was at least in part controlled by upregulation of CCAAT/enhancer binding protein (C/EBP) β (Cebpb, best known as C/EBPβ), a transcription factor that has previously been shown to regulate the M2 program.Citation6 In human GISTs, Ingenuity Pathway Analysis of the gene expression array also implicated the C/EBP family of transcription factors as important mediators in the phenotypic and functional alterations apparent between TAMs from untreated vs. sensitive tumors. Functionally, TAMs from untreated and resistant human tumors uniformly stimulated T cell proliferation, while TAMs from some sensitive tumors suppressed T cell proliferation, consistent with their shift toward an M2-like gene expression profile. Overall, TAMs were found to be M1-like in untreated tumors, shifting to M2-like in tumors responding to imatinib, and ultimately reverting to M1-like in GIST tumors acquiring resistance.
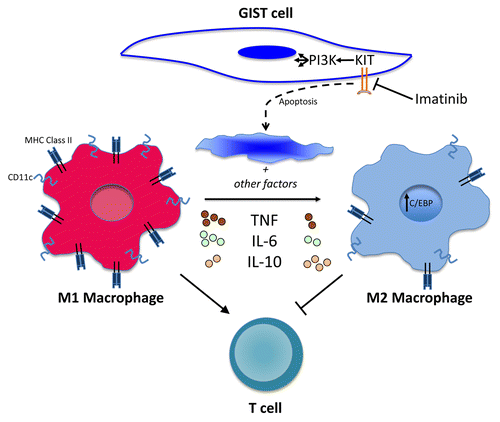
Figure 1. Dynamic changes in tumor-associated macrophages in gastrointestinal stromal tumors in response to imatinib. Tumor-associated macrophages (TAMs) in gastrointestinal stromal tumors (GISTs) are M1-like and antitumoral at baseline. TAMs become M2-like and tumor promoting in response to KIT oncoprotein inhibition by treatment with the tyrosine kinase inhibitor imatinib. Tumor cell apoptosis caused by KIT inhibition and other factors (not shown) lead to the downregulation of M1 markers and cytokines, such as tumor necrosis factor (TNF) and IL-6, and the upregulation of M2 markers and cytokines, such as IL-10. T cells are activated and stimulated to proliferate by M1-like TAMs and inhibited by M2-like TAMs, thereby altering tumor immunity.
There are several agents targeting TAM depletion under investigation in clinical trials, including small molecule inhibitors of CSF1R in breast cancer (NCT01596751) and CCR2 in pancreatic cancer (NCT01413022). Our findings demonstrate that TAM polarity in human cancers is more complex than previously thought, exhibiting dynamic phenotypic and functional stratification that may be dependent upon tumor histology, as well as location and prior therapy. That the outcome of TAM depletion is contingent on their functional polarity, a property that changes during the course of therapy is provocative, yet potentially problematic from a clinical standpoint. Nonetheless, the functional characterization of human TAMs is technically challenging, such that phenotyping TAMs from an individual patient to determine their exact polarity and heterogeneity is not easily performed. However, following the example of our investigations of GIST, it may be possible to study TAMs present in other kinds of tumors at defined phases of care (i.e., before or after treatment) and use this information to determine whether therapeutic TAM depletion may be predicted to be beneficial. We suspect that TAM depletion may be beneficial in the treatment of several human cancers, but in some tumor types, like GIST, targeted TAM depletion may be detrimental. Finally, further study is also essential to determine whether clinically relevant changes in TAM polarization occur during treatment with other, conventional cytotoxic chemotherapeutic agents as well.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Citation: Cavnar MJ, DeMatteo RP. Sarcoma response to targeted therapy dynamically polarizes tumor-associated macrophages. OncoImmunology 2014; 3:e28463; 10.4161/onci.28463
References
- Biswas SK, Mantovani A. Macrophage plasticity and interaction with lymphocyte subsets: cancer as a paradigm. Nat Immunol 2010; 11:889 - 96; http://dx.doi.org/10.1038/ni.1937; PMID: 20856220
- Heusinkveld M, van der Burg SH. Identification and manipulation of tumor associated macrophages in human cancers. J Transl Med 2011; 9:216; http://dx.doi.org/10.1186/1479-5876-9-216; PMID: 22176642
- Cavnar MJ, Zeng S, Kim TS, Sorenson EC, Ocuin LM, Balachandran VP, Seifert AM, Greer JB, Popow R, Crawley MH, et al. KIT oncogene inhibition drives intratumoral macrophage M2 polarization. J Exp Med 2013; 210:2873 - 86; http://dx.doi.org/10.1084/jem.20130875; PMID: 24323358
- Joensuu H, DeMatteo RP. The management of gastrointestinal stromal tumors: a model for targeted and multidisciplinary therapy of malignancy. Annu Rev Med 2012; 63:247 - 58; http://dx.doi.org/10.1146/annurev-med-043010-091813; PMID: 22017446
- Antonescu CR, Besmer P, Guo T, Arkun K, Hom G, Koryotowski B, Leversha MA, Jeffrey PD, Desantis D, Singer S, et al. Acquired resistance to imatinib in gastrointestinal stromal tumor occurs through secondary gene mutation. Clin Cancer Res 2005; 11:4182 - 90; http://dx.doi.org/10.1158/1078-0432.CCR-04-2245; PMID: 15930355
- Ruffell D, Mourkioti F, Gambardella A, Kirstetter P, Lopez RG, Rosenthal N, Nerlov C. A CREB-C/EBPbeta cascade induces M2 macrophage-specific gene expression and promotes muscle injury repair. Proc Natl Acad Sci U S A 2009; 106:17475 - 80; http://dx.doi.org/10.1073/pnas.0908641106; PMID: 19805133
