Abstract
DNA vaccination consists of administering an antigen-coding nucleotide sequence. In order to improve the efficacy of DNA vaccines, electroporation is one of the most commonly used methods to enhance DNA uptake. Here, we discuss additional immunological effects of electroporation that are key aspects for inducing immunity in response to DNA vaccines.
DNA vaccination consists of the administration of a construct engineered to produce an antigen of interest designed to solicit immunity against pathogens or cancer cells exposing this antigen.Citation1,Citation2 Eventually, the encoded immunogen will be responsible for the generation of a pool of antigen-specific T cells, some of which will remain as memory cells for long-term protection. This technology has been used for a wide range of applications, from laboratory tools to licensed veterinary vaccines. Also relevant to the treatment of human disease, the increasing number of clinical trials predicts the tremendous therapeutic potential for this approach against cancer. Moreover, DNA vaccines present several advantages over other vaccination strategies, among which are the relatively higher stability of DNA that avoids cold chain disruption issues, as well as the low price and the ease in producing good manufacturing practices (GMP) grade DNA. Electroporation via the delivery of electric pulses (EPs) is being increasingly used in DNA vaccination protocols to improve immunization efficacy.Citation3,Citation4 EPs are meant to reversibly permeabilize the target cells and thus enhance the uptake and expression of the gene of interest.Citation5
Several mechanisms are involved in the generation of an adaptive immune response against the antigen encoded by the administered DNA vaccine.Citation2,Citation6 If antigen-presenting cells (APCs), primarily dendritic cells (DCs), are directly transfected at the site of DNA administration, the expressed antigen is presented in association with major histocompatibility complex (MHC) class I molecules (as for endogenous proteins). This will subsequently stimulate the synthesis of a pool of antigen-specific cytotoxic T lymphocytes (CTL) following APC migration to the tumor-draining lymph nodes. Several authors have demonstrated that the epitope display mediated by MHC class I molecules is prone to induce both a T helper type 1 (Th1) response in conjunction with the generation CTLs,Citation7-Citation9 the most potent cytolytic effectors against cancer cells.Citation10,Citation11 With this in mind, a direct transfection of APCs is desirable for anticancer DNA vaccination purposes. However, to reach maximal efficacy, it has to be noted that transfection of non-immune cells must also occur in parallel. In this immunologic scenario, surrounding APCs may engulf either secreted antigen or apoptotic bodies originating from transfected non-immune cells, and display the epitopes by MHC class II (classical presentation) or MHC class I (cross-presentation) molecules. The MHC class II-mediated epitope presentation is essential for proper and long-term immunization. Indeed, CD4+ T cells primed under these circumstances are required for the generation of the CTL pool and for the maintenance and reactivation of memory T cells.Citation12 In any case, APCs, in particular DCs, are the common denominator mandatory for the elicitation of an adaptive immune response against the desired antigen.Citation13
The 2 main target tissues used in DNA vaccination protocols are the muscle and the skin.Citation2 The muscle is considered to be a protein factory as myocytes can produce extensive quantities of antigens for months. On the contrary, cutaneous keratinocytes express relatively lower quantities of antigens over a shorter period of time. However, skin tissues, in particular the epidermis and the dermis, are enriched in various types of APCs, including DCs, in comparison to muscle.Citation14 Thus, the skin route of administration has consequently garnered the interest of researchers and clinicians aiming to develop DNA-based immunizations, particularly in the context of anticancer vaccines.
An increasing number of studies show that EPs applied to tissues, whether normal or tumoral, induces immunological effects. Thus, EPs are potentially more instrumental than would be anticipated if only the enhancement of DNA uptake is considered.
Indeed, within hours after the application of EPs in skeletal muscles, a visible local inflammation associated with edema is observed,Citation15-Citation17 potentially due to minor deleterious effects on the tissues causing some EP-associated cell death. This latter statement is supported by several studies showing some minor tissue damages in conjunction with immune cell recruitment following EP application,Citation18,Citation19 as well as an upregulation of apoptosis/necrosis genes.Citation16 Local edema was also observed in tumors treated by electrochemotherapy (ECT), that is the use of EPs to enhance non-permeant anticancer drugs uptake by cancer cells,Citation20 after intramuscular administration of the drug, suggesting that the EPs delivered to the tumor site were possibly solely responsible for the swelling observed.Citation21 Evidence that the edema was more severe in immunocompetent than in immunodeficient mice is consistent with EP-mediated effects dependent upon the presence of an intact immune system. Thus, the edema, that increases the vascular permeability, probably paved the way for the local inflammatory infiltrate of macrophages, DCs,Citation15 and polymorphonuclear leukocytesCitation16,Citation18 observed after EP application on muscles. Moreover, our group showed that infiltrating mononuclear leukocytes were already present in the ECT-treated tumors as early as 25 h after the treatment.Citation22 Hence, this immune cell recruitment is de facto dependent on the direct effects of the EPs since the ECT-mediated cell death becomes detectable only after an initial mitosis arrest lasting 1 to 2 cell cycles and requiring at least 24 h after the treatment.Citation22 Our observations were independently reinforced by those of Gernili et al.Citation23 who showed that ECT treatment of human melanoma stimulated the maturation of pre-existent tumor-resident Langerhans cells, an epidermal subset of DCs, and their subsequent migration to the tumor-draining lymph node as early as 24 h after the treatment. Similarly, our group detected an intratumoral recruitment of DCs expressing CD80/CD86 maturation markers 48 h after the ECT treatment of immunogenic murine tumors in immunocompetent mice.Citation24 However, this observation could arise from the combined effects of EPs and the inflammatory reaction triggered by ECT-mediated cell death, which could also lead to the elicitation of an adaptive immune response. It was also found that the expression of classical APCs maturation markers such as F4/80 antigenCitation16 and MHC class IICitation15 were upregulated once APCs were localized in the EP-treated region of the muscles, even in the absence of chemotherapy drugs.Citation16
Our group showed very recently that a vast amount of ATP is released into the extracellular space following EP administration to cells in vitro (reference OncoImmunology in press). This perfectly fits our previous study demonstrating that the intracellular ATP content of myocytes drops after EP application in vivo.Citation17 Therefore, it can be hypothesized that the release of intracellular ATP into extracellular milieu of the area subject to EPs could be largely responsible for the recruitment of immune cells mentioned above.Citation15,Citation16,Citation18,Citation24 Indeed, ATP plays a chemoattractant role (“find me” signal) for DCs and their precursorsCitation25,Citation26 and favors their differentiation and maturation into DCs with antigen-presenting capacity.Citation27 It has also been postulated that the immune cells recruited into the electroporated areas are responding to tumor necrosis factor-α and interleukin-1β 15, or other pro-inflammatory mediators, secreted by the electropermeabilized myocytes.Citation16 Conceptually then, the liberation of electroporated cell metabolites into the extracellular space ultimately creates a local inflammatory environment prone to the recruitment of immune cells. Consistent with these observations, other authors showed that APCs and polymorphonuclear infiltrates in vaccinated muscles occurred only when DNA injection was coupled with electroporation and was associated with improved immunization efficacy in the context of DNA vaccination.Citation28,Citation29 Finally, our study also shows that in vitro EP application leads to calreticulin exposure on the cell surface (reference OncoImmunology in press). As calreticulin acts as an “eat me” signal for DCs upon binding to the phagocytic receptor CD91, it should be emphasized that EPs may also potentiate the engulfment of the transfected and thus antigen-expressing cells by DCs.
It is now well known that APCs, mostly DCs, significantly impact the outcome of vaccination as they ensure effective T cell priming and maintenance.Citation13,Citation14,Citation30 We highlight here that the use of EPs in DNA vaccination protocols is mandatory not only for efficient gene transfer into target cells but also to trigger the release of proinflammatory molecules and large quantities of ATP from target cells, as well as to elicit calreticulin exposure on the target cell surface (). Thus, EPs play a pivotal role in recruiting the desired APCs to the vaccination site and in favoring antigen uptake by these cells. Even though the gene electrotransfer procedure leads to the efficient transfection of tissue-characteristic cells (such as myocytes or keratinocytes), the subsequent recruitment of the APCs to the vaccinated area and the generation of “eat me” signals produced by the electroporated target cells could be primarily responsible for the EP-based DNA vaccination efficacy. In this case, the APCs engulf the antigens produced by the transfected tissue-characteristic cells and present them via both MHC class I and II molecules in order to mount a proper and long-lasting immune response. In the case of anticancer DNA vaccination, cutaneous tissue should be taken into consideration as a target since gene electrotransfer into the skin is also prone to transfect resident APCs that naturally display the epitopes in the context of MHC class I molecules, ultimately generating both anticancer Th1 cytokines and CTL, the most potent effectors against cancer cells.
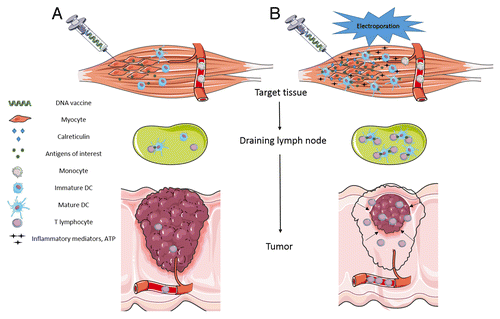
Figure 1. Impact of electroporation on the outcome of anticancer DNA vaccination. (A) DNA vaccination performed without electroporation induces low transgene expression and leads to poor stimulation of APCs. A weak pool of antigen-specific T lymphocytes is generated but is inefficient in driving tumor cell death. (B) DNA vaccination combined with electroporation intensifies transgene expression and stimulates the recruitment of monocytes from the blood stream to the vaccination site of vaccination in response to ATP and other inflammatory mediators released from cells exposed to electric pulses (EPs). Once recruited, DC differentiation and maturation occurs upon antigen engulfment triggered by calreticulin exposure on the surface of cells exposed to the EPs. A significant pool of antigen-specific cytotoxic T lymphocytes (CTL) subsequently forms, potentially leading to tumor shrinkage.
| Abbreviations: | ||
| APCs | = | antigen-presenting cells |
| CTL | = | cytotoxic T lymphocytes |
| DCs | = | dendritic cells |
| ECT | = | electrochemotherapy |
| EPs | = | electric pulses |
| GMP | = | good manufacturing practices |
| MHC | = | major histocompatibility complex |
| Th1 | = | T helper type 1 |
Disclosure of Potential Conflicts of Interest
L.M.M. has a consulting activity for IGEA and holds several patents on electroporation technologies.
Acknowledgments
Our research is performed in the scope of the EBAM European Associated Laboratory and is partly funded by the CNRS, the University of Paris-Sud, Gustave Roussy, the Fondation EDF, and by a grant from the Département du Val-de-Marne through the TELVAC project. Figure 1 was drawn using Servier Medical Art (www.servier.com).
Citation: Calvet CY, André FM, Mir LM. Dual therapeutic benefit of electroporation-mediated DNA vaccination in vivo: Enhanced gene transfer and adjuvant activity. OncoImmunology 2014; 3:e28540; 10.4161/onci.28540
References
- Senovilla L, Vacchelli E, Garcia P, Eggermont A, Fridman WH, Galon J, Zitvogel L, Kroemer G, Galluzzi L. Trial watch: DNA vaccines for cancer therapy. Oncoimmunology 2013; 2:e23803; http://dx.doi.org/10.4161/onci.23803; PMID: 23734328
- Kutzler MA, Weiner DB. DNA vaccines: ready for prime time?. Nat Rev Genet 2008; 9:776 - 88; http://dx.doi.org/10.1038/nrg2432; PMID: 18781156
- Gothelf A, Gehl J. What you always needed to know about electroporation based DNA vaccines. Hum Vaccin Immunother 2012; 8:1694 - 702; http://dx.doi.org/10.4161/hv.22062; PMID: 23111168
- Aurisicchio L, Mancini R, Ciliberto G. Cancer vaccination by electro-gene-transfer. Expert Rev Vaccines 2013; 12:1127 - 37; http://dx.doi.org/10.1586/14760584.2013.836903; PMID: 24066796
- Andre FM, Mir LM. Nucleic acids electrotransfer in vivo: mechanisms and practical aspects. Curr Gene Ther 2010; 10:267 - 80; http://dx.doi.org/10.2174/156652310791823380; PMID: 20557285
- Eschenburg G, Stermann A, Preissner R, Meyer HA, Lode HN. DNA vaccination: using the patient’s immune system to overcome cancer. Clin Dev Immunol 2010; 2010:169484; http://dx.doi.org/10.1155/2010/169484; PMID: 21197271
- Wang Q, Lei C, Wan H, Liu Q. Improved cellular immune response elicited by a ubiquitin-fused DNA vaccine against Mycobacterium tuberculosis. DNA Cell Biol 2012; 31:489 - 95; http://dx.doi.org/10.1089/dna.2011.1309; PMID: 21905875
- Rodriguez F, Zhang J, Whitton JL. DNA immunization: ubiquitination of a viral protein enhances cytotoxic T-lymphocyte induction and antiviral protection but abrogates antibody induction. J Virol 1997; 71:8497 - 503; PMID: 9343207
- Dobaño C, Rogers WO, Gowda K, Doolan DL. Targeting antigen to MHC Class I and Class II antigen presentation pathways for malaria DNA vaccines. Immunol Lett 2007; 111:92 - 102; http://dx.doi.org/10.1016/j.imlet.2007.05.007; PMID: 17604849
- Vesely MD, Kershaw MH, Schreiber RD, Smyth MJ. Natural innate and adaptive immunity to cancer. Annu Rev Immunol 2011; 29:235 - 71; http://dx.doi.org/10.1146/annurev-immunol-031210-101324; PMID: 21219185
- Braumüller H, Wieder T, Brenner E, Aßmann S, Hahn M, Alkhaled M, Schilbach K, Essmann F, Kneilling M, Griessinger C, et al. T-helper-1-cell cytokines drive cancer into senescence. Nature 2013; 494:361 - 5; http://dx.doi.org/10.1038/nature11824; PMID: 23376950
- Castellino F, Germain RN. Cooperation between CD4+ and CD8+ T cells: when, where, and how. Annu Rev Immunol 2006; 24:519 - 40; http://dx.doi.org/10.1146/annurev.immunol.23.021704.115825; PMID: 16551258
- Dudek AM, Martin S, Garg AD, Agostinis P. Immature, Semi-Mature, and Fully Mature Dendritic Cells: Toward a DC-Cancer Cells Interface That Augments Anticancer Immunity. Front Immunol 2013; 4:438; http://dx.doi.org/10.3389/fimmu.2013.00438; PMID: 24376443
- Combadiere B, Liard C. Transcutaneous and intradermal vaccination. Hum Vaccin 2011; 7:811 - 27; http://dx.doi.org/10.4161/hv.7.8.16274; PMID: 21817854
- Chiarella P, Massi E, De Robertis M, Sibilio A, Parrella P, Fazio VM, Signori E. Electroporation of skeletal muscle induces danger signal release and antigen-presenting cell recruitment independently of DNA vaccine administration. Expert Opin Biol Ther 2008; 8:1645 - 57; http://dx.doi.org/10.1517/14712598.8.11.1645; PMID: 18847301
- Peng B, Zhao Y, Xu L, Xu Y. Electric pulses applied prior to intramuscular DNA vaccination greatly improve the vaccine immunogenicity. Vaccine 2007; 25:2064 - 73; http://dx.doi.org/10.1016/j.vaccine.2006.11.042; PMID: 17239494
- Hojman P, Gissel H, Andre FM, Cournil-Henrionnet C, Eriksen J, Gehl J, Mir LM. Physiological effects of high- and low-voltage pulse combinations for gene electrotransfer in muscle. Hum Gene Ther 2008; 19:1249 - 60; http://dx.doi.org/10.1089/hum.2008.059; PMID: 19866489
- Babiuk S, Baca-Estrada ME, Foldvari M, Storms M, Rabussay D, Widera G, Babiuk LA. Electroporation improves the efficacy of DNA vaccines in large animals. Vaccine 2002; 20:3399 - 408; http://dx.doi.org/10.1016/S0264-410X(02)00269-4; PMID: 12213410
- Umeda Y, Marui T, Matsuno Y, Shirahashi K, Iwata H, Takagi H, Matsumoto K, Nakamura T, Kosugi A, Mori Y, et al. Skeletal muscle targeting in vivo electroporation-mediated HGF gene therapy of bleomycin-induced pulmonary fibrosis in mice. Lab Invest 2004; 84:836 - 44; http://dx.doi.org/10.1038/labinvest.3700098; PMID: 15197407
- Mir LM. Bases and rationale of the electrochemotherapy. Ejc Suppl 2006; 4:38 - 44; http://dx.doi.org/10.1016/j.ejcsup.2006.08.005
- Mir LM, Orlowski S, Belehradek J Jr., Paoletti C. Electrochemotherapy potentiation of antitumour effect of bleomycin by local electric pulses. Eur J Cancer 1991; 27:68 - 72; http://dx.doi.org/10.1016/0277-5379(91)90064-K; PMID: 1707289
- Mekid H, Tounekti O, Spatz A, Cemazar M, El Kebir FZ, Mir LM. In vivo evolution of tumour cells after the generation of double-strand DNA breaks. Br J Cancer 2003; 88:1763 - 71; http://dx.doi.org/10.1038/sj.bjc.6600959; PMID: 12771993
- Gerlini G, Sestini S, Di Gennaro P, Urso C, Pimpinelli N, Borgognoni L. Dendritic cells recruitment in melanoma metastasis treated by electrochemotherapy. Clin Exp Metastasis 2013; 30:37 - 45; http://dx.doi.org/10.1007/s10585-012-9505-1; PMID: 22735940
- Roux S, Bernat C, Al-Sakere B, Ghiringhelli F, Opolon P, Carpentier AF, Zitvogel L, Mir LM, Robert C. Tumor destruction using electrochemotherapy followed by CpG oligodeoxynucleotide injection induces distant tumor responses. Cancer Immunol Immunother 2008; 57:1291 - 300; http://dx.doi.org/10.1007/s00262-008-0462-0; PMID: 18259749
- Elliott MR, Chekeni FB, Trampont PC, Lazarowski ER, Kadl A, Walk SF, Park D, Woodson RI, Ostankovich M, Sharma P, et al. Nucleotides released by apoptotic cells act as a find-me signal to promote phagocytic clearance. Nature 2009; 461:282 - 6; http://dx.doi.org/10.1038/nature08296; PMID: 19741708
- la Sala A, Ferrari D, Di Virgilio F, Idzko M, Norgauer J, Girolomoni G. Alerting and tuning the immune response by extracellular nucleotides. J Leukoc Biol 2003; 73:339 - 43; http://dx.doi.org/10.1189/jlb.0802418; PMID: 12629147
- Ma Y, Adjemian S, Yang H, Catani JP, Hannani D, Martins I, Michaud M, Kepp O, Sukkurwala AQ, Vacchelli E, et al. ATP-dependent recruitment, survival and differentiation of dendritic cell precursors in the tumor bed after anticancer chemotherapy. Oncoimmunology 2013; 2:e24568; http://dx.doi.org/10.4161/onci.24568; PMID: 23894718
- Liu J, Kjeken R, Mathiesen I, Barouch DH. Recruitment of antigen-presenting cells to the site of inoculation and augmentation of human immunodeficiency virus type 1 DNA vaccine immunogenicity by in vivo electroporation. J Virol 2008; 82:5643 - 9; http://dx.doi.org/10.1128/JVI.02564-07; PMID: 18353952
- Babiuk S, Baca-Estrada ME, Foldvari M, Middleton DM, Rabussay D, Widera G, Babiuk LA. Increased gene expression and inflammatory cell infiltration caused by electroporation are both important for improving the efficacy of DNA vaccines. J Biotechnol 2004; 110:1 - 10; http://dx.doi.org/10.1016/j.jbiotec.2004.01.015; PMID: 15099900
- Akbari O, Panjwani N, Garcia S, Tascon R, Lowrie D, Stockinger B. DNA vaccination: transfection and activation of dendritic cells as key events for immunity. J Exp Med 1999; 189:169 - 78; http://dx.doi.org/10.1084/jem.189.1.169; PMID: 9874573
