Abstract
A dual immunotherapy approach employing small-molecule inhibitors of apoptosis (IAP) protein antagonists in combination with innate immune stimuli has proven to be highly synergistic and effective in animal tumor models. This strategy overcomes many of the limitations of either single agent therapy and our results suggest that the combination could be easily and effectively translated to the clinic.
Chemical mimetics of the pro-apoptotic protein, DIABLO, better known as second mitochondria-derived activator of caspases (Smac), are small molecule antagonists that repress key inhibitors of apoptosis (IAPs) proteins – cellular IAP1 (cIAP1) and cellular IAP2 (cIAP2) – by binding and targeting them for degradation. Depending on the compound affinity, specificity, and dosage, Smac mimetics also antagonize or lead to the degradation of X-linked IAP (XIAP), another apoptotic suppressor protein. Several of these Smac mimetics are currently in early phase clinical trials as anticancer agents. The Smac mimetic-induced loss of cIAP1/cIAP2, two critical regulators of tumor necrosis factor (TNF) receptor superfamily and nuclear factor-κB (NF-κB) signaling, sensitizes cancer cells to TNFα- or TNF-related apoptosis-inducing TNF ligand (i.e., TRAIL)-mediated death.Citation1 Importantly, Smac mimetics require the presence of these pro-death cytokine ligands for maximal efficacy. However, to date, methods to safely and effectively provide an exogenous source of these cytokines to cancer patients undergoing treatment with Smac mimetics, an action that, conceptually, could boost antitumor efficacy, have yet to be developed.
We recently discovered that infection of tumor-bearing mice with an oncolytic virus, or alternatively, treatment with a synthetic immune mimetic, can give rise to a cytokine storm (including TNFα and TRAIL) of sufficient intensity to kill tumor cells co-treated with various monovalent or bivalent Smac mimetics (containing one or two IAP binding motifs, respectively).Citation2 We demonstrated synergistic killing of tumor cells in multiple treatment-refractory cancer models in vivo, such as breast and colorectal cancer, leading to increased survival and, in some cases, in durable cures. In addition, treatment of many other types of malignancy (e.g., renal, glioblastoma, and multiple myeloma) demonstrated synergistic combinatorial activities in vitro. This published study also demonstrated the following key points:
1. The Smac mimetic synergy with an oncolytic virus was highly potentiated specifically within the class of vesiculoviruses, typified in our study by two examples of oncolytic rhabdoviruses, Vesicular stomatitis virus (VSV) and Maraba in studies in vitro. The attenuated oncolytic rhabdoviruses are negative sense RNA viruses that replicate quickly and produce a strong interferon (IFN) response. This immune response limits viral spread, thereby suppressing the cancer-killing efficacy of the oncolytic virus but protects the host from viremia.Citation3 However, viral infection, lysis, and the release of tumor antigens and damage-associated molecular patterns (DAMPs) ultimately triggers the immune response to aid in the eradication of tumors.Citation4
2. The combination effect did not exclusively require the local production of cytokines at the site of the tumor, such that we observed systemic production of cytokines to be highly efficacious. In addition, the combination was well tolerated by the animals with acceptable and transient losses in body weight.
3. The cancer cytotoxic effects were mediated by various cytokines, notably Type I or Type II IFNs, as well as TNFα or TRAIL. This was the first demonstration of Smac mimetic synergy with IFNs. Our findings raise the possibility of combining existing immunotherapies comprising recombinant IFN with Smac mimetics in the treatment of cancer.
4. The anticancer effect from the combinatorial treatment was primarily mediated by the innate immune response. However, we could not completely exclude the involvement of the adaptive response, as this arm of the immune system is broadly known to contribute to long-term remission or cure. In a separate report, Dougan and colleagues show that Smac mimetics enhance T-cell antitumor immunity in a cancer vaccine mouse model,Citation5 suggesting that Smac mimetics can exert a multitude of beneficial antitumor immune effects via distinct mechanisms. In fact, the IAPs regulate many aspects of immunity (For a review see ref. Citation6), and IAP antagonism with Smac mimetics in cancer patients is expected to have numerous immune-mediated anticancer effects.
5. Oncolytic virus triggering of the innate immune response could be replaced with non-infectious immunostimulatory molecules, such as the adjuvants poly(I:C) or CpG oligonucleotides. These synthetic pathogen mimetics effectively synergizes with Smac mimetics to significantly induce tumor regression, resulting in durable cures.
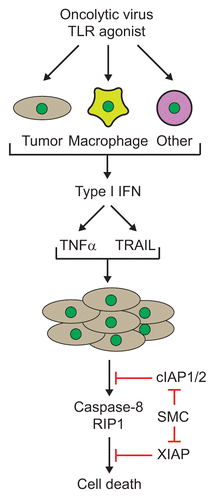
6. The direct infection of all the cancer cells with an oncolytic virus was not required, as non-infected tumor cells could be killed by a bystander mechanism, at least partially due to the induction of diffusible and circulating cytokines (). This cytokine storm produces a cloud of tumor cell death that could be clearly visualized in a virus-spreading assay using an agarose overlay (refer to supplemental data in ref. Citation2).
Figure 1. Cytokine-mediated synergy of Smac mimetics and an oncolytic virus or synthetic Toll-like receptor (TLR) agonist. Infection with oncolytic viruses or treatment with immunostimulatory TLR agonists in various cell types (tumor, macrophages or other cells from the host) leads to the production of interferons, such as IFNβ, which in turn, leads to the production of cytokines such as tumor necrosis factor (TNFα) and TNF-related apoptosis-inducing ligand (TRAIL). Notably, the production of these cytokines is enhanced in the presence of Smac mimetics. Treatment of tumor cells with Smac mimetics leads to the degradation and/or inhibition of the cellular inhibitors of apoptosis (cIAP1/2) and X-linked IAP (XIAP) proteins. Subsequently, the cytokines induce caspase-8- and RIP1-dependent bystander death of Smac mimetic treated tumor cells. RIP1, receptor interacting protein kinase 1; Smac, second mitochondria-derived activator of caspases; SMC, Smac mimetic compound.
7. Smac mimetic treatment did not alter or compromise the host antiviral response to oncolytic VSV infection, contrary to a previous report wherein the loss of cIAP1/2 was shown to lead to an increase in the titer of VSV.Citation7 In fact, two additional studies have also demonstrated that Smac mimetic treatments do not hinder antiviral responses in mice.Citation8,Citation9
A number of the above mentioned immunotherapy agents and derivatives are currently in cancer clinical trials as monotherapies. Our pre-clinical studies indicate that specific combinatorial immunotherapies involving Smac mimetics may be more efficacious than the single-agent approaches, and that these combinatorial approaches are amenable to translation to the clinical setting. Our combination approach provides the essential death triggers (TNFα, TRAIL, and/or IFN) needed for maximal Smac mimetic efficacy in vivo. Even in scenarios where viral spread and oncolysis is limited, widespread bystander killing of uninfected tumor cells could occur as a result of the induction of cytokines through the systemic stimulation of the innate immune response. The idea of combining multiple immunotherapies is one that is likely to flourish with the advent of effective anticancer treatments that are being approved for clinical use, such as blocking antibodies targeting cytotoxic T lymphocyte associated antigen 4 (CTLA-4,) programmed cell death 1 (PDCD1, better known as PD-1) or PD1 ligand (PD-L1).Citation10 Analogous to the use of chemotherapy cocktail approaches to circumvent tumor resistance or to provide drug synergy, the combination of various cancer immunotherapies with Smac mimetics can also be viewed as an effective multipronged approach that could potentially avoid neoplastic or malignant cell resistance to immune attack or apoptotic induction, and would thus be likely to synergistically ablate cancer cells.
| Abbreviations: | ||
| cIAP | = | cellular inhibitor of apoptosis |
| CTLA-4 | = | cytotoxic T-lymphocyte antigen-4 |
| DAMP | = | damage-associated molecular pattern |
| IFN | = | interferon |
| NF-κB | = | nuclear factor-κB |
| PD-1 | = | programmed cell death 1 |
| PD-L1 | = | programmed cell death ligand-1 |
| poly(I:C) | = | polyinosinic-polycytidylic acid |
| Smac | = | second mitochondria-derived activator of caspases |
| TLR | = | toll-like receptor |
| TNFα | = | tumour necrosis factor α |
| TRAIL | = | TNF-related apoptosis-inducing ligand |
| VSV | = | Vesicular stomatitis virus |
| XIAP | = | X-linked inhibitor of apoptosis |
Disclosure of Potential Conflicts of Interest
R.G.K. is a scientific founder and shareholder of Aegera Therapeutics (Pharmascience Inc.) which has a Smac mimetic under clinical development.
Citation: Beug ST, LaCasse EC, Korneluk RG. Smac mimetics combined with innate immune stimuli create the perfect cytokine storm to kill tumor cells. OncoImmunology 2014; 3:e28541; 10.4161/onci.28541
References
- Fulda S, Vucic D. Targeting IAP proteins for therapeutic intervention in cancer. Nat Rev Drug Discov 2012; 11:109 - 24; http://dx.doi.org/10.1038/nrd3627; PMID: 22293567
- Beug ST, Tang VA, LaCasse EC, Cheung HH, Beauregard CE, Brun J, Nuyens JP, Earl N, St-Jean M, Holbrook J, et al. Smac mimetics and innate immune stimuli synergize to promote tumor death. Nat Biotechnol 2014; 32:182 - 90; http://dx.doi.org/10.1038/nbt.2806; PMID: 24463573
- McFadden G, Mohamed MR, Rahman MM, Bartee E. Cytokine determinants of viral tropism. Nat Rev Immunol 2009; 9:645 - 55; http://dx.doi.org/10.1038/nri2623; PMID: 19696766
- Russell SJ, Peng KW, Bell JC. Oncolytic virotherapy. Nat Biotechnol 2012; 30:658 - 70; http://dx.doi.org/10.1038/nbt.2287; PMID: 22781695
- Dougan M, Dougan S, Slisz J, Firestone B, Vanneman M, Draganov D, Goyal G, Li W, Neuberg D, Blumberg R, et al. IAP inhibitors enhance co-stimulation to promote tumor immunity. J Exp Med 2010; 207:2195 - 206; http://dx.doi.org/10.1084/jem.20101123; PMID: 20837698
- Beug ST, Cheung HH, LaCasse EC, Korneluk RG. Modulation of immune signalling by inhibitors of apoptosis. Trends Immunol 2012; 33:535 - 45; http://dx.doi.org/10.1016/j.it.2012.06.004; PMID: 22836014
- Mao AP, Li S, Zhong B, Li Y, Yan J, Li Q, Teng C, Shu HB. Virus-triggered ubiquitination of TRAF3/6 by cIAP1/2 is essential for induction of interferon-beta (IFN-beta) and cellular antiviral response. J Biol Chem 2010; 285:9470 - 6; http://dx.doi.org/10.1074/jbc.M109.071043; PMID: 20097753
- Rodrigue-Gervais IG, Labbé K, Dagenais M, Dupaul-Chicoine J, Champagne C, Morizot A, Skeldon A, Brincks EL, Vidal SM, Griffith TS, et al. Cellular inhibitor of apoptosis protein cIAP2 protects against pulmonary tissue necrosis during influenza virus infection to promote host survival. Cell Host Microbe 2014; 15:23 - 35; http://dx.doi.org/10.1016/j.chom.2013.12.003; PMID: 24439895
- Liu S, Chen J, Cai X, Wu J, Chen X, Wu YT, Sun L, Chen ZJ. MAVS recruits multiple ubiquitin E3 ligases to activate antiviral signaling cascades. Elife 2013; 2:e00785; http://dx.doi.org/10.7554/eLife.00785; PMID: 23951545
- Vanneman M, Dranoff G. Combining immunotherapy and targeted therapies in cancer treatment. Nat Rev Cancer 2012; 12:237 - 51; http://dx.doi.org/10.1038/nrc3237; PMID: 22437869
