Abstract
T-cell infiltrates are robust prognostic biomarkers in the majority of human cancers. However, the mechanisms that shape a protective T-cell response remain unclear. Our recent study implicates tertiary lymphoid structures in the shaping of an efficient and beneficial immune response in lung cancer patients.
The tumor microenvironment constitutes a complex network of interactions between malignant cells, stromal cells and tumor-infiltrating immune cells. The patient-specific characteristics of this heterogeneous “tumor interactome” have a major impact on disease progression. During the course of the last decade, the prognostic value of immune infiltrates has been extensively documented. The current paradigm is that the presence of abundant tumor-infiltrating immune cells, especially cytotoxic T cells, confers a positive prognostic value in the majority of human cancers.Citation1 Despite this recognized fact, the mechanisms that promote a protective T-cell response against the tumor, especially the sites at which the activation of tumor-specific effector cells occurs, remain poorly understood.
A classical viewpoint is that antitumor immunity takes place in the draining lymph nodes.Citation2 In addition, lymphoid neogenesis can occur in chronic inflammatory settings to generate ectopic, organized lymphoid aggregates, termed tertiary lymphoid structures (TLS). TLS were first described in autoimmune diseases, allograft rejections and infections.Citation3 TLS exhibit features of secondary lymphoid organs with an ongoing immune reaction, and exacerbate the local immune responses in these pro-inflammatory microenvironments.Citation3 More recently, the presence of TLS has also been reported in malignant diseases.Citation4 This poses the question of the potential contribution of TLS in the generation of local immune responses in this generally immunosuppressive microenvironment.
Our team has previously reported that lung tumor-associated TLS present a specific chemo-attractant signature associated with T-cell infiltration.Citation5 High endothelial venules selectively co-localize with TLSCitation5,Citation6 suggesting that these lymphoid structures may provide a key hub for the immigration of peripheral blood immune cells into the tumor. In a recent study published in Cancer Research,Citation7 we investigated the impact of TLS on the characteristics of the intra-tumoral immune contexture and the clinical outcome of patients afflicted with non-small-cell lung cancer (NSCLC) without any neo-adjuvant chemotherapy (from early- to advanced-stage of disease). We first demonstrated that a high density of DC-Lamp+ mature dendritic cells (mDCs), a population specifically detected in TLS, correlates with a high frequency of tumor-infiltrating T cells composed primarily of naïve and central-memory T cells homing in TLS, as well as activated and effector-memory CD8+ T cells.Citation7 Moreover, a specific molecular immune signature characterized by an overexpression and co-regulation of genes influencing T-cell activation, T helper type-1 polarization, and cytotoxic orientation was observed in tumors having a high mDC infiltration compared with tumors with low mDC infiltration.Citation7 These results demonstrate that TLS orchestrate a specific and coordinated immune contexture in human NSCLC.
Based on the close link between mDC and T-cell density in the tumor microenvironment, we asked the question of whether features of the TLS contribute to the positive prognostic value of T-cell infiltrates. Using a retrospective cohort of 376 NSCLC samples (all stages without neo-adjuvant chemotherapy), we confirmed that high infiltration of either mDCs or CD8+ T cells correlated with long-term survival of NSCLC patients. Patients were stratified according to the density of both mDCs and CD8+ T cells in tumors (DC-LampHi/CD8Hi, DC-LampHi/CD8Lo, DC-LampLo/CD8Hi, and DC-LampLo/CD8Lo patients). DC-LampLo/CD8Lo patients had the worst prognosis compared with the other groups, suggesting that analysis for this profile may provide a good strategy to identify patients with the highest risk of death. In contrast, both DC-LampHi/CD8Hi and DC-LampHi/CD8Lo patients had the best clinical outcome, indicating that the outcome of NSCLC patients is strongly influenced primarily by the presence of tumor-infiltrating mDCs that home to the TLS. Furthermore, DC-LampLo/CD8Hi patients were at an intermediate risk of death, exhibiting the same median survival as CD8Lo patients.
This key observation indicates that, among CD8Hi patients who are known to have a favorable clinical outcome, it is possible to identify a DC-LampLo/CD8Hi sub-group with a reduced likelihood of survival. These results suggest that the presence of intra-tumoral mDCs is needed to elicit the positive prognostic value of tumor-infiltrating CD8+ T cells. Thus, DC-Lamp/CD8 score and classical TNM staging represent two powerful and independent prognostic factors for overall survival.
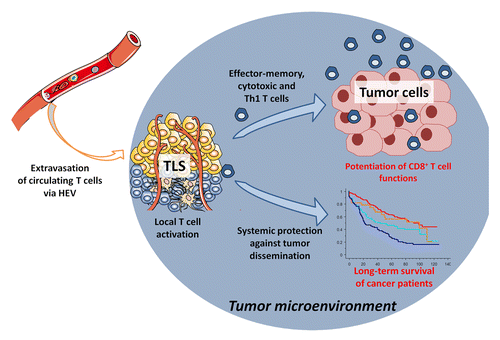
Our data demonstrate that TLS play a major role in the maintenance of a protective cellular-mediated immune response in NSCLC patients. With this in mind, the presence of TLS seems to contradict the current view of the tumor microenvironment as a severely immunosuppressive milieu. We hypothesize that TLS may potentiate the antitumor response by maintaining a chronic restimulation of infiltrating CD8+ T cells, thereby restraining their sensitivity to local anergy. TLS could also play a protective role against tumors by promoting an humoral immune response, as recently shown.Citation8-Citation10 It should be mentioned that the clinical outcome of patients is followed after surgical removal of the tumor. It supports the idea that TLS not only impact the local immune contexture of the tumor, but also may be implicated in the generation of an immunologic memory that mediates immunosurveillance against metastasis ().
Figure 1. Cancer-associated tertiary lymphoid structures drive antitumor immune responses. In the lung tumor microenvironment, the presence of tertiary lymphoid structures (TLS) may foster the immigration of immune cells via high endothelial venules (HEVs), thereby promoting a protective cellular immune response that ultimately positively affects the clinical outcome of cancer patients.
Many studies have highlighted the positive impact of intra-tumoral T-cell infiltrates on the clinical outcome of cancer patients, supporting the idea that T-cell density could serve as a contributive biomarker for the staging of cancers.Citation1 Our findings suggest that TLS represent a novel biomarker useful for the optimization of the immune score-based approaches to stage cancer patients. Combination of DC-Lamp and CD8 markers allows the identification of a new group of patients with a high risk of death (DC-LampLo/CD8Hi patients). These patients may be in the greatest need and may benefit the most from anticancer therapy. Indeed, the cellular and molecular mechanisms that provide an optimal milieu for the neogenesis of TLS in cancer patients, as well as the predictive value of TLS after chemotherapy, radiotherapy and/or immunotherapeutic treatments, will be important, clinically relevant questions to address in the near future.
| Abbreviations: | ||
| mDC | = | mature dendritic cell |
| NSCLC | = | non-small-cell lung cancer |
| OS | = | overall survival |
| TLS | = | tertiary lymphoid structure |
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- Fridman WH, Pagès F, Sautès-Fridman C, Galon J. The immune contexture in human tumours: impact on clinical outcome. Nat Rev Cancer 2012; 12:298 - 306; http://dx.doi.org/10.1038/nrc3245; PMID: 22419253
- Mellman I, Coukos G, Dranoff G. Cancer immunotherapy comes of age. Nature 2011; 480:480 - 9; http://dx.doi.org/10.1038/nature10673; PMID: 22193102
- Neyt K, Perros F, GeurtsvanKessel CH, Hammad H, Lambrecht BN. Tertiary lymphoid organs in infection and autoimmunity. Trends Immunol 2012; 33:297 - 305; http://dx.doi.org/10.1016/j.it.2012.04.006; PMID: 22622061
- Goc J, Fridman WH, Sautès-Fridman C, Dieu-Nosjean M-C. Characteristics of tertiary lymphoid structures in primary cancers. Oncoimmunology 2013; 2:e26836; http://dx.doi.org/10.4161/onci.26836; PMID: 24498556
- de Chaisemartin L, Goc J, Damotte D, Validire P, Magdeleinat P, Alifano M, Cremer I, Fridman W-H, Sautès-Fridman C, Dieu-Nosjean M-C. Characterization of chemokines and adhesion molecules associated with T cell presence in tertiary lymphoid structures in human lung cancer. Cancer Res 2011; 71:6391 - 9; http://dx.doi.org/10.1158/0008-5472.CAN-11-0952; PMID: 21900403
- Martinet L, Filleron T, Le Guellec S, Rochaix P, Garrido I, Girard J-P. High endothelial venule blood vessels for tumor-infiltrating lymphocytes are associated with lymphotoxin β-producing dendritic cells in human breast cancer. J Immunol 2013; 191:2001 - 8; http://dx.doi.org/10.4049/jimmunol.1300872; PMID: 23825314
- Goc J, Germain C, Vo-Bourgais TKD, Lupo A, Klein C, Knockaert S, de Chaisemartin L, Ouakrim H, Becht E, Alifano M, et al. Dendritic cells in tumor-associated tertiary lymphoid structures signal a Th1 cytotoxic immune contexture and license the positive prognostic value of infiltrating CD8+ T cells. Cancer Res 2014; 74:705 - 15; http://dx.doi.org/10.1158/0008-5472.CAN-13-1342; PMID: 24366885
- Gu-Trantien C, Loi S, Garaud S, Equeter C, Libin M, de Wind A, Ravoet M, Le Buanec H, Sibille C, Manfouo-Foutsop G, et al. CD4⁺ follicular helper T cell infiltration predicts breast cancer survival. J Clin Invest 2013; 123:2873 - 92; http://dx.doi.org/10.1172/JCI67428; PMID: 23778140
- Germain MC, Gnjatic DS, Tamzalit MF, Knockaert MS, Remark DR, Goc MJ, Lepelley MA, Becht ME, Katsahian MS, Bizouard MG, et al. Presence of B cells in tertiary lymphoid structures is associated with a protective immunity in patients with lung cancer. Am J Respir Crit Care Med 2014; 189:832 - 44; http://dx.doi.org/10.1164/rccm.201309-1611OC; PMID: 24484236
- Cipponi A, Mercier M, Seremet T, Baurain J-F, Théate I, van den Oord J, Stas M, Boon T, Coulie PG, van Baren N. Neogenesis of lymphoid structures and antibody responses occur in human melanoma metastases. Cancer Res 2012; 72:3997 - 4007; http://dx.doi.org/10.1158/0008-5472.CAN-12-1377; PMID: 22850419
