Abstract
Inhibition of mTOR signaling enhances antitumor memory T lymphocytes while increasing the frequency of immunosuppressive regulatory T cells (Tregs). We report here a strategy to further improve immunologic memory by controlling CD4+ T cells with CD4-depleting monoclonal antibody therapy thereby improving CD8+ memory T cell quality and function. We report that removal of Tregs is the mechanism underlying immunological memory formation in response to this combination immunotherapy.
The key immune signals needed for a primary adaptive immune response has long been well understood. However, the trigger for converting a CD8+ effector cell to a memory cell has only recently been elucidated.Citation1,Citation2 Inhibition of the mTOR pathway modulates cellular metabolism and enhances CD8+ memory T cell formation. Previously, we applied this concept to cancer immunology in preclinical models using multiple syngeneic murine tumors.Citation3 mTOR inhibition produced a multitude of immune effects culminating in the suppression of tumor growth. Although in our system, mTOR inhibition enhanced the quality of the antitumor memory cells, we also observed immunosuppressive effects as well. This was not surprising since the canonical mTOR inhibitor, rapamycin, is routinely used to suppress the immune system following solid organ transplantation. In our preclinical models, mTOR inhibition decreased the proliferation of activated CD4+ and CD8+ T cell in vitro and in vivo, and increased the relative abundance of regulatory T cells (Tregs). Therefore, we asked if a strategy to control Tregs could potentially further enhance the formation of memory T cells in response to mTOR inhibition.
Tregs are arguably the most important negative regulator of cellular immunity. However, there is no translatable strategy to specifically remove or inhibit Tregs. Therefore, we tested an approach to deplete all CD4-expressing T cells using an anti-CD4 monoclonal IgG2b antibody. CD4 depletion was timed to allow the presence of CD4+ T helper cells during primary immune activation. We observed that CD8+ T cells primed in the absence of CD4+ cell failed to express eomesodermin (Eomes) an early memory cell marker. Others have also reported that CD4+ T cells are needed for effective CD8+ memory cell formation.Citation4,Citation5 For example, CD8+ memory cells formed in the absence of CD4+ cells exhibited an exhausted phenotype and increased expression of programmed cell death 1 (PD-1).Citation4,Citation5 However, once CD8+ T effector cells have activated and expanded, CD4+ T cell help is dispensible. Subsequent tumor control is dependent upon CD8+ memory cells that possess the hallmark ability to activate by simply engaging the T-cell receptor with soluble antigens. In other words, antigen presenting cells and CD4+ T helper cells are no longer required. In fact, after the initial stages of immune priming, CD4+ T cell activity is dominated by regulatory function. We, and others, have shown that CD4+ T cell depletion specifically at this point in the immunological cascade leads to enhanced formation of both central and effector CD8+ memory cells and enhanced control of tumor growth.
Prior studies examining CD4+ T-cell depletion as a means to control tumor growth have assumed that the effects of ablating CD4+ immune cells were a direct consequence of diminished Treg levels. However, to firmly establish this as the mechanism-of-action we performed 2 separate experiments. We used a transgenic mouse that carries a transgene for the diphtheria toxin receptor under the control of the forkhead box P3 (Foxp3) promoter. Instead of depleting all CD4 cells, the Foxp3-expressing subset was specifically depleted by virtue of the diphtheria toxin transgene. Similar to CD4 depletion, Foxp3 depletion enhanced CD8 memory formation in the presence of mTOR inhibition. In a reciprocal experiment, activated and GFP-tagged Tregs were replaced following CD4+ T-cell depletion. If Treg depletion is the primary causal factor, we anticipated that replacing Tregs should neutralize any benefits derived from CD4 depletion and this is precisely what we observed. Together, these experiments empirically identified Treg depletion as the cellular mechanism underlying the enhanced immunity following CD4+ T-cell depletion. Interestingly, intratumoral Treg depletion has been identified at the primary mode of action for ipilimumab.Citation6 Based on a positive Phase III trial, ipilimumab was FDA approved for the treatment of advanced melanoma. Therefore, it is intriguing to consider the possibility that targeted CD4+ T-cell depletion may produce a similar immune effect in patients but with a different, potentially less risky, safety profile.
There have been other strategies for targeting Tregs for depletion in cancer patients. Another potential Treg marker is CD25, which is expressed by a majority of Tregs. CD25 is the α chain component of the IL-2 receptor and IL2 signaling is critical for CD4+ and CD8+ T cell stimulation. Therefore, strategies to target CD25 will affect a broad range of immune cells, thereby inducing non-desirable effects on other subsets of immune cells. For example, an anti-CD25 depleting antibody will deplete activated CD8+ lymphocytes also expressing CD25. Another limitation of this strategy is that some Tregs are actually CD25 negative, meaning that they would escape. In murine models, CD25 antibody-targeted cell depletion has been found to effectively prevent tumor initiation, but was ineffective in treating established tumorsCitation7 and has been shown to restrict adoptive immunotherapy.Citation8 Another strategy uses an engineered protein chimeric of interleukin-2 and diphtheria toxin (denileukin diftitox, trade name Ontax) to target CD25 expressing cells. The approach has been tested in renal cell carcinoma (RCC) and melanoma patients,Citation9,Citation10 however clinical effectiveness was limited, possibly due to depletion of CD8+ T effector cells. Although our antibody-based method of CD4+ T-cell depletion is not exclusive to Tregs, it leaves the critical population of CD8 cells intact. Another potential, and yet untested benefit is that anti-CD4 antibody targeted depletion removes the pool of CD4+Foxp3- cells that can go on to express Foxp3 and become future Tregs.
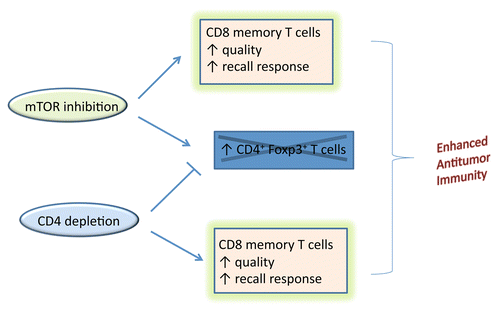
We propose combining mTOR inhibition and CD4+ T-cell depletion to enhance antitumor immunity (). CD4 depletion removes regulatory T cells and the combination enhances the quality and function of antitumor CD8+ memory T cells even when the tumor itself is the only source of antigen and a separate cancer vaccine is not administered. A clearer understanding of the mechanism-of-action of the anti-CD4 antibody immunotherapy described here bolsters the rationale for future clinical investigation.
Figure 1. Enhanced antitumor immunity arises from combining mTOR inhibition with CD4+ T cell depletion. Co-administering both mTOR inhibitors and anti-CD4 antibodies to deplete CD4 lymphocytes enhance the formation of CD8+ memory T lymphocytes. The combination is rational because CD4+ T-cell depletion counters the potentially negative impact of mTOR inhibitors that can enhance immunosuppressive Foxp3-expressing regulatory T cells.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Keywords: CD4, regulatory T cells, mTOR, memory T cell, tumor immunology
References
- Araki K, Turner AP, Shaffer VO, Gangappa S, Keller SA, Bachmann MF, Larsen CP, Ahmed R. mTOR regulates memory CD8 T-cell differentiation. Nature 2009; 460:108 - 12; http://dx.doi.org/10.1038/nature08155; PMID: 19543266
- Pearce EL, Walsh MC, Cejas PJ, Harms GM, Shen H, Wang LS, Jones RG, Choi Y. Enhancing CD8 T-cell memory by modulating fatty acid metabolism. Nature 2009; 460:103 - 7; http://dx.doi.org/10.1038/nature08097; PMID: 19494812
- Wang Y, Wang XY, Subjeck JR, Shrikant PA, Kim HL. Temsirolimus, an mTOR inhibitor, enhances anti-tumour effects of heat shock protein cancer vaccines. Br J Cancer 2011; 104:643 - 52; http://dx.doi.org/10.1038/bjc.2011.15; PMID: 21285988
- Fuse S, Tsai CY, Molloy MJ, Allie SR, Zhang W, Yagita H, Usherwood EJ. Recall responses by helpless memory CD8+ T cells are restricted by the up-regulation of PD-1. J Immunol 2009; 182:4244 - 54; http://dx.doi.org/10.4049/jimmunol.0802041; PMID: 19299723
- Jing W, Yan X, Hallett WH, Gershan JA, Johnson BD. Depletion of CD25⁺ T cells from hematopoietic stem cell grafts increases posttransplantation vaccine-induced immunity to neuroblastoma. Blood 2011; 117:6952 - 62; http://dx.doi.org/10.1182/blood-2010-12-326108; PMID: 21521781
- Simpson TR, Li F, Montalvo-Ortiz W, Sepulveda MA, Bergerhoff K, Arce F, Roddie C, Henry JY, Yagita H, Wolchok JD, et al. Fc-dependent depletion of tumor-infiltrating regulatory T cells co-defines the efficacy of anti-CTLA-4 therapy against melanoma. J Exp Med 2013; 210:1695 - 710; http://dx.doi.org/10.1084/jem.20130579; PMID: 23897981
- Onizuka S, Tawara I, Shimizu J, Sakaguchi S, Fujita T, Nakayama E. Tumor rejection by in vivo administration of anti-CD25 (interleukin-2 receptor alpha) monoclonal antibody. Cancer Res 1999; 59:3128 - 33; PMID: 10397255
- Côté AL, Byrne KT, Steinberg SM, Zhang P, Turk MJ. Protective CD8 memory T cell responses to mouse melanoma are generated in the absence of CD4 T cell help. PLoS One 2011; 6:e26491; http://dx.doi.org/10.1371/journal.pone.0026491; PMID: 22046294
- Dannull J, Su Z, Rizzieri D, Yang BK, Coleman D, Yancey D, Zhang A, Dahm P, Chao N, Gilboa E, et al. Enhancement of vaccine-mediated antitumor immunity in cancer patients after depletion of regulatory T cells. J Clin Invest 2005; 115:3623 - 33; http://dx.doi.org/10.1172/JCI25947; PMID: 16308572
- Mahnke K, Schönfeld K, Fondel S, Ring S, Karakhanova S, Wiedemeyer K, Bedke T, Johnson TS, Storn V, Schallenberg S, et al. Depletion of CD4+CD25+ human regulatory T cells in vivo: kinetics of Treg depletion and alterations in immune functions in vivo and in vitro. Int J Cancer 2007; 120:2723 - 33; http://dx.doi.org/10.1002/ijc.22617; PMID: 17315189
