Abstract
Pancreatic tumors are rich in immune cell infiltrates that include CD4+ T-cell subsets encompassing both regulatory T cells and TH17 cells. Rather than protecting the organism by exerting an anticancer effect, these T-cell subsets promote tumor formation. Thus, re-activation of antitumor immunity should be investigated for use in pancreatic cancer prevention and therapy.
Pancreatic ductal adenocarcinoma (PDA), the most common form of pancreatic cancer, is one of the deadliest human malignancies.Citation1 In the past 40 y, the 5-y survival rate of pancreatic cancer has not markedly improved pointing to a dire need to develop novel therapeutic approaches. In humans, PDA is characterized by the accumulation of an extensive stroma comprising extracellular matrix components, activated fibroblasts, vascular components, and abundant immune cell infiltrates. Targeting the tumor-associated microenvironment is emerging as a new intervention approach for this deadly disease. However, our understanding of how immune infiltrates regulate pancreatic carcinogenesis is currently incomplete.
Pancreatic cancer in humans is preceded by precursor lesions known as pancreatic intraepithelial neoplasia (PanIN). PanIN is driven by expression of an oncogenic form of the KRAS gene in the pancreatic epitheliumCitation2 and is markedly accelerated by the induction of pancreatitis.Citation3 PanIN formation is accompanied by extensive infiltration of immune cells;Citation4 however, the majority of the infiltrating immune cells are immunosuppressive. Among infiltrating T lymphocytes, CD8+ T cells are rare whereas CD4+ T cells are abundant.Citation4
Here, we describe two recent studies that address the functional role of CD4+ T cells within the pancreatic cancer microenvironment.Citation5,Citation6 Of note, two different genetically engineered mouse models were used: the iKras* mouse modelCitation7 and the KCiMist1 (Mist1CreERT2/+; LSL-KrasG12D) mouse model. In these models, oncogenic Kras is expressed in a tissue-specific and inducible manner using the Tet or CreER system respectively.
In the first study,Citation5 we genetically depleted CD4+ T cells by crossing iKras* mice with CD4−/− mice. The resulting iKras*;CD4−/− mice were found to be susceptible to caerulein-induced pancreatitis. However, unlike iKras* mice, they do not develop PanIN lesions following the induction of pancreatitis. In fact, the low-grade PanIN lesions found in these animals undergo extensive apoptosis and do not persist over time. This is in sharp contrast to iKras* mice, which rapidly develop tissue-wide PanINs after the induction of pancreatitis and show progression to higher grade lesions over time. Thus, this study highlighted the requirement of CD4+ T cells for pancreatic cancer initiation. Of note, CD4+ T cell depletion also led to reduced PanIN formation in the KCiMist1 model.Citation6
Infiltration of CD8+ T cells was increased in iKras*;CD4−/− mice and was often observed in close proximity to epithelial cells in areas of acinar ductal metaplasia and low-grade PanINs. More importantly, CD8+ T cells extracted from iKras*;CD4−/− mice were more active, based on expression of interferon γ (IFNγ) and Granzyme B, and more receptive to further activation upon treatment with anti-CD3 and anti-CD28 antibodies in vitro. When freshly sorted PanIN and CD8+ T cells were co-cultured, CD8+ T cells derived from iKras;CD4−/− mice, but not CD8+ T cells derived from iKras* mice, were activated in response to PanINs. Finally, depletion of CD8+ T cells in iKras*;CD4−/− mice rescued PanIN formation. Taken together, these data show that CD4+ T cells promote PanIN formation by blocking the antitumor immune responses mediated by CD8+ T cells ().
Figure 1. CD4+ T lymphocytes and IL-17 signaling are required for oncogenic Kras-driven pancreatic carcinogenesis. (A) Intrapancreatic CD4+ T cells suppress the antitumor activity of CD8+ T cells during Kras*-driven pancreatic intraepithelial neoplasia (PanIN) formation. T helper 17 (TH17) cells (as well as γδ T cells) secrete IL-17A that signals through IL-17RA in acinar–ductal metaplasia and PanINs, thereby inducing tumor initiation and progression. (B) CD4+ T cell ablation enables effector CD8+ T cell function and induces apoptosis in PanIN cells, thus blocking the onset of pancreatic cancer initiation.
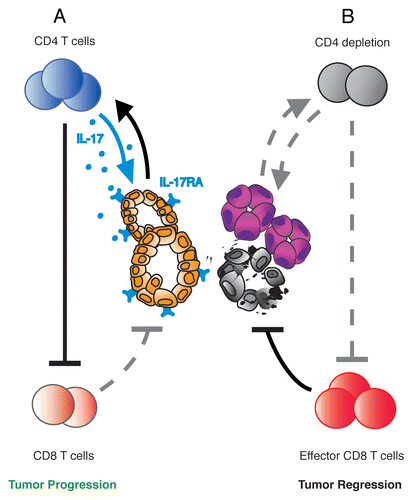
CD4+ T cells are a heterogenous population. High numbers of regulatory T cells and T helper 17 (TH17) cells were observed in the pancreatic microenvironment in a Kras-dependent manner.Citation5 The level of TH17 cells was also found to be elevated in the pancreatic immune-infiltrates of KCiMist1 mice and formed the focus of the second study,Citation6 which demonstrated that these cells were required for initiation and progression of pancreatic tumorigenesis.
Using the KCiMist1 model, McAllister et al. showed that oncogenic Kras and chronic pancreatitis synergistically recruited TH17 and interleukin-17 (IL-17)+/γδT cells to the pancreatic microenvironment.Citation6 Different and complementary approaches were used to address the function of these cells in pancreatic tumorigenesis. Adenoviral-mediated IL-17 overexpression in the pancreas of KCiMist-1 mice markedly accelerated PanIN initiation and progression. In contrast, genetic inhibition of IL-17 signaling by transplanting KCiMist1 mice with bone marrow from IL-17 deficient mice, or alternatively, by a pharmacological approach in which KCiMist1 mice were treated with IL-17 and IL-17RA monoclonal antibodies, prevented PanIN formation ().
Several changes in immune infiltration and inflammatory signaling were observed in both iKras*;CD4−/− mice and KCiMist1 mice upon inactivation of the IL-17 axis. In both studies, a decrease in myeloid-derived suppressor cells (MDSC), typically abundant within the pancreatic cancer microenvironmentCitation4 and required for pancreatic cancer development, was observed. Moreover, in both investigations, a reduction in the activation of the IL-6/p-Stat3 signaling axis, was detected, a key pathway required for PanIN formation and progression.Citation8,Citation9 Expression of granulocyte macrophage colony stimulating factor (GM-CSF, also known as Csf2), another important factor for pancreatic cancer progression,Citation10,Citation11 was also reduced, possibly explaining the diminished MDSC recruitment. The interactions between individual immune subsets, as well as the role of other components of the stroma in regulating the inflammatory microenvironment of pancreatic cancer, are areas of active investigation.
Taken together, these studies highlight an important role for CD4+ T cells, specifically the Th17 cell subset, during the pancreatic cancer onset and disease progression, and argue for immune modulation as a valid therapeutic approach for this dreaded disease.
| Abbreviations: | ||
| ADM | = | acinar–ductal metaplasia |
| GM-CSF | = | granulocyte macrophage colony stimulating factor |
| IFNγ | = | interferon-gamma |
| IL-6 | = | interleukin 6 |
| IL-17 | = | interleukin 17 |
| IL-17RA | = | IL-17 receptor A |
| MDSC | = | myeloid-derived suppressor cell |
| PanIN | = | pancreatic intraepithelial neoplasia |
| PDA | = | pancreatic ductal adenocarcinoma |
| TH17 | = | T helper 17 cell |
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- American Cancer Society. Cancer Facts & Figures 2014. Atlanta: American Cancer Society; 2014 [cited 2014 June 5]. Available from: http://www.cancer.org/research/cancerfactsstatistics/cancerfactsfigures2014/index
- Hingorani SR, Petricoin EF, Maitra A, Rajapakse V, King C, Jacobetz MA, Ross S, Conrads TP, Veenstra TD, Hitt BA, et al. Preinvasive and invasive ductal pancreatic cancer and its early detection in the mouse. Cancer Cell 2003; 4:437 - 50; http://dx.doi.org/10.1016/S1535-6108(03)00309-X; PMID: 14706336
- Morris JP 4th, Cano DA, Sekine S, Wang SC, Hebrok M. Beta-catenin blocks Kras-dependent reprogramming of acini into pancreatic cancer precursor lesions in mice. J Clin Invest 2010; 120:508 - 20; http://dx.doi.org/10.1172/JCI40045; PMID: 20071774
- Clark CE, Hingorani SR, Mick R, Combs C, Tuveson DA, Vonderheide RH. Dynamics of the immune reaction to pancreatic cancer from inception to invasion. Cancer Res 2007; 67:9518 - 27; http://dx.doi.org/10.1158/0008-5472.CAN-07-0175; PMID: 17909062
- Zhang Y, Yan W, Mathew E, Bednar F, Wan S, Collins MA, Evans RA, Welling TH, Vonderheide RH, di Magliano MP. CD4+ T lymphocyte ablation prevents pancreatic carcinogenesis in mice. Cancer Immunol Res 2014; 2.
- McAllister F, Bailey JM, Alsina J, Nirschl CJ, Sharma R, Fan H, Rattigan Y, Roeser JC, Lankapalli RH, Zhang H, et al. Oncogenic Kras Activates a Hematopoietic-to-Epithelial IL-17 Signaling Axis in Preinvasive Pancreatic Neoplasia. Cancer Cell 2014; 25:621 - 37; http://dx.doi.org/10.1016/j.ccr.2014.03.014; PMID: 24823639
- Collins MA, Bednar F, Zhang Y, Brisset JC, Galbán S, Galbán CJ, Rakshit S, Flannagan KS, Adsay NV, Pasca di Magliano M. Oncogenic Kras is required for both the initiation and maintenance of pancreatic cancer in mice. J Clin Invest 2012; 122:639 - 53; http://dx.doi.org/10.1172/JCI59227; PMID: 22232209
- Zhang Y, Yan W, Collins MA, Bednar F, Rakshit S, Zetter BR, Stanger BZ, Chung I, Rhim AD, di Magliano MP. Interleukin-6 is required for pancreatic cancer progression by promoting MAPK signaling activation and oxidative stress resistance. Cancer Res 2013; 73:6359 - 74; http://dx.doi.org/10.1158/0008-5472.CAN-13-1558-T; PMID: 24097820
- Lesina M, Kurkowski MU, Ludes K, Rose-John S, Treiber M, Klöppel G, Yoshimura A, Reindl W, Sipos B, Akira S, et al. Stat3/Socs3 activation by IL-6 transsignaling promotes progression of pancreatic intraepithelial neoplasia and development of pancreatic cancer. Cancer Cell 2011; 19:456 - 69; http://dx.doi.org/10.1016/j.ccr.2011.03.009; PMID: 21481788
- Pylayeva-Gupta Y, Lee KE, Hajdu CH, Miller G, Bar-Sagi D. Oncogenic Kras-induced GM-CSF production promotes the development of pancreatic neoplasia. Cancer Cell 2012; 21:836 - 47; http://dx.doi.org/10.1016/j.ccr.2012.04.024; PMID: 22698407
- Bayne LJ, Beatty GL, Jhala N, Clark CE, Rhim AD, Stanger BZ, Vonderheide RH. Tumor-derived granulocyte-macrophage colony-stimulating factor regulates myeloid inflammation and T cell immunity in pancreatic cancer. Cancer Cell 2012; 21:822 - 35; http://dx.doi.org/10.1016/j.ccr.2012.04.025; PMID: 22698406
