Abstract
An exploration of the immunotherapeutic potential of the pan-Bcl-2 inhibitor GX15–070 (GX15) has revealed that early-activated T cells derived from human peripheral blood are more sensitive to GX15 than are prolonged-activated T cells. Furthermore, non-memory and regulatory T cells also exhibit higher sensitivity to GX15. The implication of these prior findings suggests that GX15 may enhance the efficacy of immunotherapies in clinical settings.
Introduction
There is general agreement that the most successful immune-based cancer therapies combine immunotherapy with other treatment platforms. Such combinatorial approaches are designed to overcome: (1) the host’s inherent tolerance of self-originating tumor-specific T cells and/or (2) the immunosuppressive nature of the tumor microenvironment. Investigators continually seek the optimal combination of immunotherapeutic platforms and complementary regimens to achieve the greatest clinical benefit for their patients.
Our laboratory has explored the synergistic potential of targeted therapy in combination with recombinant poxviral-based vaccines.Citation1 One of the small molecule inhibitors we investigated was GX15–070 (GX15; obatoclax), a pan-Bcl-2 inhibitor that has been extensively tested in clinical trials.Citation2 In a preclinical mouse model, we found that the sensitivity of murine lymphocytes to GX15 was dependent on their activation status: mature T lymphocytes were more resistant to GX15 than early-activated T lymphocytes in vitro.Citation3 In addition, the suppressive function of regulatory T cells (Tregs) isolated from mice treated with GX15 was markedly reduced.Citation3 Based on these observations, we determined that sequential treatment with recombinant vaccine followed by GX15 resulted in the most effective reduction of orthotopic pulmonary tumors.Citation3
To further support the rationale for the combination of GX15 and immunotherapy in the clinical setting, we evaluated the effect of GX15 on subsets of human T lymphocytes in vitro and found that the sensitivity of human T lymphocytes to GX15 was dependent on their activation, memory, and suppression status.Citation4 This was consistent with our preclinical determination that the timing of GX15 administration in relation to an immunotherapeutic agent would be an important factor in clinical trials.Citation4
Effect of GX15 on T-Cell Subsets in Human PBMCs
To our knowledge, our study is the first published work aiming to characterize the effect of GX15 on subsets of human T lymphocytes.Citation4 We initially hypothesized that T lymphocytes differentially respond to GX15 based on temporal differences in their Bcl-2 family protein expression levels upon activation; i.e., GX15 binds to Mcl-1, while other Bcl-2 inhibitors such as ABT-737 and ABT-263 do not. In early and prolonged activation, CD4+ and CD8+ T cells that express the early-activation marker CD69+ were particularly sensitive to GX15 (). We believe that because early-activated T cells express a high level of Mcl-1, which can be upregulated within 10 h after T-cell receptor ligation,Citation5 they are more sensitive to the cytotoxicity of GX15. This reasoning is in line with a published report in which breast cancer cell lines that expressed higher levels of Bcl-2 family members responded better to GX15.Citation6
Figure 1. The effect of the pan-Bcl-2 inhibitor GX15 on subsets of human T cells. (A) T-cell subsets in peripheral blood showed differential sensitivity to GX15 based on their activation, memory, and proliferative status. (B) GX15 preferentially reduced the proportion of Tregs in peripheral blood and altered their phenotype and function, potentially tipping the balance of immune cells toward effector memory T cells in the human tumor microenvironment.
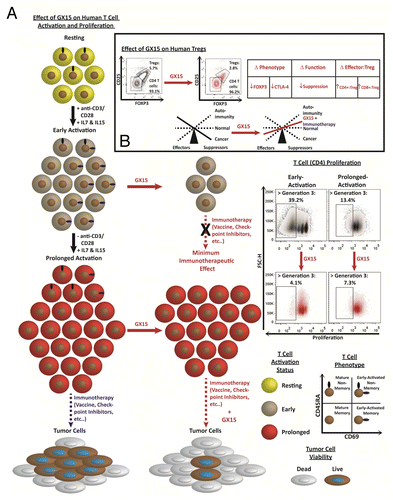
Since Bcl-2 has been shown to play a dynamic role in T-cell differentiation, memory formation, and survival, we investigated the extent to which T-cell sensitivity to GX15 is affected by T-cell memory status in early and prolonged activation. Because memory T cells express high, stable levels of Bcl-2, especially in the presence of IL-7 and IL-15, we hypothesized that GX15 would have a greater effect on memory (CD45RA–) T cells than on non-memory (CD45RA+) T cells. Instead, treatment with GX15 resulted in a significant decrease in non-memory T cells, whereas memory T cells were preserved (). Bcl-2 expression in murine memory T cells was shown to promote tolerance for higher expression of the pro-apoptotic molecule Bim.Citation7 In Bcl-2 knockout mice, naïve T cells were significantly decreased, and Bcl-2 was not required for the generation and maintenance of memory T cells.Citation8 We posit that, upon GX15-mediated inhibition of Bcl-2, human memory T cells are better able to tolerate the pro-apoptotic effects of GX15 due to a compensatory survival mechanism(s) not present in non-memory T cells.
Finally, GX15 treatment of PBMCs from ovarian cancer patients decreased Tregs in proportion and function () and also diminished the expression levels of FOXP3 and CTLA-4 (). These effects of GX15 on Tregs may be analogous to those of cyclophosphamide, which similarly decreases the number, expression of canonical markers and suppressive capacity of Tregs.Citation9 There is evidence that cyclophosphamide preferentially targets Tregs due to their low level of intracellular ATP.Citation10 We have shown that Tregs express higher levels of CD69Citation4 and thus may express higher levels of Bcl-2 family proteins to overcome their low energy, rendering them more sensitive to GX15. Based on these findings, we believe that GX15 could be used in a context similar to cyclophosphamide in cancer immunotherapy.
Clinical Implications
Our study supports the clinical use of GX15 in combination with an immunotherapeutic platform, such as a vaccine or immune checkpoint inhibitor. In a combination regimen, optimum scheduling of each agent is important since immunity induced by immunotherapy may decrease if GX15 is administered during early T-cell activation, as proliferating early-activated T cells are most sensitive to GX15 than are prolonged-activated T cells (). To achieve optimum immunotherapeutic potential, administration of an immunostimulatory agent directed at T cells should precede treatment with GX15 long enough for T cells to mature and acquire proliferative resistance to GX15 (). In addition, because Tregs are particularly sensitive to GX15 while memory T cells are preserved, the immunoregulatory balance could tip toward effector memory T cells in the human tumor microenvironment ().
This study provides a rationale for the sequential combination of GX15 with an immunotherapeutic platform—a rationale that could be extended to the use of other Bcl-2 inhibitors, such as ABT-737 and ABT-263.
| Abbreviations: | ||
| GX15 | = | GX15-070 |
| Tregs | = | T regulatory cells |
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- Hodge JW, Ardiani A, Farsaci B, Kwilas AR, Gameiro SR. The tipping point for combination therapy: cancer vaccines with radiation, chemotherapy, or targeted small molecule inhibitors. Semin Oncol 2012; 39:323 - 39; http://dx.doi.org/10.1053/j.seminoncol.2012.02.006; PMID: 22595055
- Joudeh J, Claxton D. Obatoclax mesylate : pharmacology and potential for therapy of hematological neoplasms. Expert Opin Investig Drugs 2012; 21:363 - 73; http://dx.doi.org/10.1517/13543784.2012.652302; PMID: 22324354
- Farsaci B, Sabzevari H, Higgins JP, Di Bari MG, Takai S, Schlom J, Hodge JW. Effect of a small molecule BCL-2 inhibitor on immune function and use with a recombinant vaccine. Int J Cancer 2010; 127:1603 - 13; http://dx.doi.org/10.1002/ijc.25177; PMID: 20091862
- Kim PS, Jochems C, Grenga I, Donahue RN, Tsang KY, Gulley JL, Schlom J, Farsaci B. Pan-Bcl-2 inhibitor, GX15-070 (obatoclax), decreases human T regulatory lymphocytes while preserving effector T lymphocytes: a rationale for its use in combination immunotherapy. J Immunol 2014; 192:2622 - 33; http://dx.doi.org/10.4049/jimmunol.1301369; PMID: 24516200
- Wang M, Windgassen D, Papoutsakis ET. A global transcriptional view of apoptosis in human T-cell activation. BMC Med Genomics 2008; 1:53; http://dx.doi.org/10.1186/1755-8794-1-53; PMID: 18947405
- Hudson SG, Halleran DR, Nevaldine B, Shapiro A, Hutchison RE, Hahn PJ. Microarray determination of Bcl-2 family protein inhibition sensitivity in breast cancer cells. Exp Biol Med (Maywood) 2013; 238:248 - 56; http://dx.doi.org/10.1177/1535370212474582; PMID: 23576806
- Kurtulus S, Tripathi P, Moreno-Fernandez ME, Sholl A, Katz JD, Grimes HL, Hildeman DA. Bcl-2 allows effector and memory CD8+ T cells to tolerate higher expression of Bim. J Immunol 2011; 186:5729 - 37; http://dx.doi.org/10.4049/jimmunol.1100102; PMID: 21451108
- Wojciechowski S, Tripathi P, Bourdeau T, Acero L, Grimes HL, Katz JD, Finkelman FD, Hildeman DA. Bim/Bcl-2 balance is critical for maintaining naive and memory T cell homeostasis. J Exp Med 2007; 204:1665 - 75; PMID: 17591857
- Le DT, Jaffee EM. Regulatory T-cell modulation using cyclophosphamide in vaccine approaches: a current perspective. Cancer Res 2012; 72:3439 - 44; http://dx.doi.org/10.1158/0008-5472.CAN-11-3912; PMID: 22761338
- Zhao J, Cao Y, Lei Z, Yang Z, Zhang B, Huang B. Selective depletion of CD4+CD25+Foxp3+ regulatory T cells by low-dose cyclophosphamide is explained by reduced intracellular ATP levels. Cancer Res 2010; 70:4850 - 8; http://dx.doi.org/10.1158/0008-5472.CAN-10-0283; PMID: 20501849
