Abstract
Immune evasion is a hallmark of cancer. We recently identified the adhesion molecule L1CAM as biomarker of pancreatic ductal adenocarcinoma (PDAC) associated with poor prognosis. During inflammation-associated carcinogenesis, L1CAM drives the enrichment of highly immunosuppressive CD4+CD25-CD69+ T cells. Thus, L1CAM may serve as a target in immunomodulatory therapy for PDAC.
Data from genetically engineered mouse models as well as from epidemiological studies indicate that the immune system with active T-effector cells (T-effs) serves as a fundamental barrier to prevent the onset and progression of neoplastic disease. However, the fact that tumors nonetheless develop, despite intact immunity and the enrichment of immune cells in the tumoral microenvironment, suggests that malignant cells are capable to evade attack by the immune system. Tumor immune escape is therefore regarded as a hallmark of cancer.Citation1 One important mechanism of immune evasion is the accumulation of tumor-infiltrating regulatory T cells (Tregs) creating an immunosuppressive microenvironment. Tregs are regarded as the most potent inhibitors of antitumor immunity due to their ability to dampen the activity of CD4+ and CD8+ T cells as well as natural killer (NK) cells mainly by the release of transforming growth factor β (TGFβ1) and IL-10.Citation2 Accordingly, many tumors are characterized by large numbers of CD4+CD25+ or CD4+FoxP3+ regulatory T cells (T-regs) that correlated with poor patient prognosis.Citation3 During progression of highly malignant pancreatic ductal adenocarcinoma (PDAC), Tregs have also been observed to accumulate in the tumor microenvironment, an immunologic manifestation also associated with shortened patient survival.Citation4
Emerging questions include how are Tregs enriched in PDAC, when does this lymphocytic infiltration occur, and how may these cells be characterized? Cancer and stromal cells release of chemoattractants, such as the (C-C) chemokine CCL5, an altered expression profile of adhesion molecules on tumor-associated endothelial cells, as well as the TGFβ1-induced conversion of conventional T cells into Tregs, are potential contributing factors that have been already described for PDAC.Citation5-Citation7 We recently demonstrated that the adhesion molecule L1CAM (CD171), which is upregulated in the pancreatic ductal epithelium during PDAC progression, is associated with poor prognosisCitation8 and is an essential determinant for the accumulation of immunosuppressive T cells.Citation9 The latter was shown by using CD4+CD25+CD127-CD49d- Tregs and effector T cells (Teffs) freshly isolated by magnetic bead separation from blood of healthy donors and directly cocultured with human premalignant (H6c7) or malignant (Panc1) pancreatic ductal epithelial cells, either lacking or exhibiting L1CAM expression, respectively. We observed that L1CAM specifically enhanced Treg cell migration and predominantly dampens the proliferation of Teffs, providing an explanation for the enrichment of CD4+CD25+CD127-CD49d- Tregs in PDAC tissues. However, flow cytometry based characterization of T cells derived from blood and tumor tissues from PDAC patients revealed that tumor tissues were particularly characterized by the abundance of CD4+ T cells lacking CD25 expression but exhibiting high levels of CD69. Albeit primarily regarded as an activation marker in T cells, increasing studies have demonstrated an immunoregulatory function for CD69 also, thus pointing to a novel subset of CD4+CD25-CD69+ immunoregulatory T cells.Citation10 Accordingly, the occurrence of these CD4+CD25-CD69+ T cells could correlate with nodal invasion and higher tumor grading in PDAC patients, underscoring the view that this T-cell subset may be tumor-promoting, akin to Tregs, rather than tumor-suppressing.Citation9 In support of this notion, coculture experiments revealed that high L1CAM expression may decrease CD25 in association with a concomitant increase in CD69 expression in these unique T cells. Most importantly, these CD4+CD25-CD69+ T cells exhibited a regulatory phenotype, as they efficiently inhibited proliferation of autologous T cells comparable to CD4+CD25+CD127-CD49d- Tregs. Besides their potent immunosuppressive role, T cells might contribute to tumor development also by directly impacting epithelial/tumor cells. Tregs as well as Teffs release a plethora of inflammatory cytokines (e.g., TGFβ1, TNFα, IL-6), which are known to account for many cancer hallmarks such as sustained proliferation, apoptosis resistance, invasion and metastasis.Citation1 In line with this idea, preliminary results indicate that CD4+CD25- T cells are potent inducers of epithelial-mesenchymal transition (EMT) in H6c7 cells, a process also involving upregulation of L1CAM expression.
Based on these data, the following model can be envisaged on the fatal alliance of T cells and pancreatic ductal epithelial cancer cells, which promotes inflammation-associated PDAC development ():
Figure 1. The fatal alliance of T cells and pancreatic ductal epithelial cancer cells in inflammation-associated PDAC development. Upon pancreatic inflammation CD4+ T cells, most likely T effector cells (Teffs) but also regulatory T cells (Tregs), infiltrate the pancreatic tissue where they come in contact with the pancreatic ductal epithelium. Teffs promote epithelial-mesenchymal transition (EMT)-associated alterations along with enhanced L1CAM expression in pancreatic ductal epithelium by releasing inflammatory cytokines such as TNFα, IL-1β, and IL-6. Tregs add to this scenario via the release of TGFβ1. However, Teffs still efficiently produce IL-2 and IFNγ enabling T helper cell type 1 (Th1)-activity. Still expressed at moderate levels, L1CAM increases tumorigenicity, invasiveness and apoptotic resistance of premalignant epithelial cells in precursor lesions or chronic pancreatitis (CP). However, these effects are even more pronounced in pancreatic ductal adenocarcinoma (PDAC) cells exhibiting strong L1CAM expression. Furthermore, these elevated L1CAM expression levels contribute to the enrichment of immunosuppressive T cells by promoting migration/infiltration of CD4+CD25+CD127-CD49d- Tregs into the pancreas, impairing the proliferation of Teffs and favoring the generation of immunosuppressive CD4+CD25-CD69+ T cells.
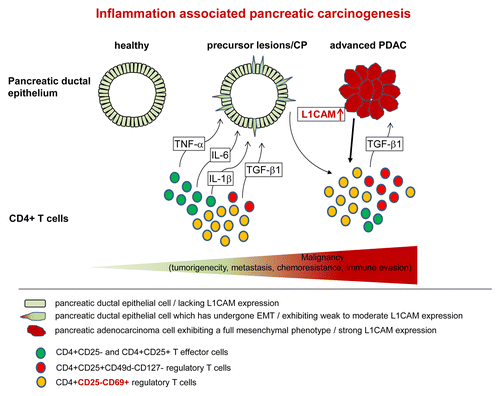
Upon pancreatic inflammation (e.g., during chronic pancreatitis or upon smoldering inflammation induced by smoking, alcohol, or diabetes mellitus), CD4+ T cells (most likely T effector cells) infiltrate the pancreatic tissue where they come in contact with the pancreatic ductal epithelium. By releasing inflammatory cytokines such as TNFα, IL-1β, T cells induce EMT associated alterations along with enhanced L1CAM expression in pancreatic ductal epithelium. Then, on the one hand, L1CAM expression increases the tumorigenecity, invasiveness and apoptotic resistance of normal, or premalignant epithelial cells, and later, cancer cells.Citation8 On the other hand, L1CAM contributes to the enrichment of immunosuppressive T cells by promoting the migration and infiltration of Tregs into the pancreas, impairing the proliferation of Teffs and favoring the generation of immunosuppressive CD4+CD25-CD69+ T cells. In particular, the latter Treg population may contribute to immune evasion quite early during PDAC development considering that these cells are known to be highly abundant already in chronic pancreatitis tissues.Citation9
Besides demonstrating a novel function of L1CAM in the generation of an immunosuppressive microenvironment, this study provides novel insights into the plasticity of T cells, particularly Tregs, in human PDAC. Furthermore, it suggests that the number and phenotypes of immunosuppressive T cells within tumors might be much higher and more complex than assumed so far. This knowledge is pivotal for the development of therapeutic strategies aiming to efficiently target this potent immunological barrier in highly malignant PDAC. Given the fundamental role of L1CAM in the mediation of chemoresistance, cell invasion and metastatic spread, as well as in immunoregulation, L1CAM has great potential as an immunotherapeutic target for the treatment of pancreatic cancer.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell 2011; 144:646 - 74; http://dx.doi.org/10.1016/j.cell.2011.02.013; PMID: 21376230
- Byrne WL, Mills KH, Lederer JA, O’Sullivan GC. Targeting regulatory T cells in cancer. Cancer Res 2011; 71:6915 - 20; http://dx.doi.org/10.1158/0008-5472.CAN-11-1156; PMID: 22068034
- Zou W. Regulatory T cells, tumour immunity and immunotherapy. Nat Rev Immunol 2006; 6:295 - 307; http://dx.doi.org/10.1038/nri1806; PMID: 16557261
- Hiraoka N, Onozato K, Kosuge T, Hirohashi S. Prevalence of FOXP3+ regulatory T cells increases during the progression of pancreatic ductal adenocarcinoma and its premalignant lesions. Clin Cancer Res 2006; 12:5423 - 34; http://dx.doi.org/10.1158/1078-0432.CCR-06-0369; PMID: 17000676
- Tan MC, Goedegebuure PS, Belt BA, Flaherty B, Sankpal N, Gillanders WE, Eberlein TJ, Hsieh CS, Linehan DC. Disruption of CCR5-dependent homing of regulatory T cells inhibits tumor growth in a murine model of pancreatic cancer. J Immunol 2009; 182:1746 - 55; http://dx.doi.org/10.4049/jimmunol.182.3.1746; PMID: 19155524
- Nummer D, Suri-Payer E, Schmitz-Winnenthal H, Bonertz A, Galindo L, Antolovich D, Koch M, Büchler M, Weitz J, Schirrmacher V, et al. Role of tumor endothelium in CD4+ CD25+ regulatory T cell infiltration of human pancreatic carcinoma. J Natl Cancer Inst 2007; 99:1188 - 99; http://dx.doi.org/10.1093/jnci/djm064; PMID: 17652277
- Moo-Young TA, Larson JW, Belt BA, Tan MC, Hawkins WG, Eberlein TJ, Goedegebuure PS, Linehan DC. Tumor-derived TGF-beta mediates conversion of CD4+Foxp3+ regulatory T cells in a murine model of pancreas cancer. J Immunother 2009; 32:12 - 21; http://dx.doi.org/10.1097/CJI.0b013e318189f13c; PMID: 19307989
- Geismann C, Morscheck M, Koch D, Bergmann F, Ungefroren H, Arlt A, Tsao MS, Bachem MG, Altevogt P, Sipos B, et al. Up-regulation of L1CAM in pancreatic duct cells is transforming growth factor beta1- and slug-dependent: role in malignant transformation of pancreatic cancer. Cancer Res 2009; 69:4517 - 26; http://dx.doi.org/10.1158/0008-5472.CAN-08-3493; PMID: 19435915
- Grage-Griebenow E, Jerg E, Gorys A, Wicklein D, Wesch D, Freitag-Wolf S, Goebel L, Vogel I, Becker T, Ebsen M, et al. L1CAM promotes enrichment of immunosuppressive T cells in human pancreatic cancer correlating with malignant progression. Mol Oncol 2014; Forthcoming http://dx.doi.org/10.1016/j.molonc.2014.03.001; PMID: 24746181
- Sancho D, Gómez M, Sánchez-Madrid F. CD69 is an immunoregulatory molecule induced following activation. Trends Immunol 2005; 26:136 - 40; http://dx.doi.org/10.1016/j.it.2004.12.006; PMID: 15745855
