Abstract
The protein kinase mTOR is the central player within a pathway, which is known to be involved in the regulation of e.g., cell size, cell cycle, apoptosis, autophagy, aging and differentiation. mTOR activity responds to many signals, including cellular stress, oxygen, nutrient availability, energy status and growth factors. Deregulation of this enzyme is causatively involved in the molecular development of monogenic human diseases, cancer, obesity, type 2 diabetes or neurodegeneration. Recently, mTOR has also been demonstrated to control stem cell homeostasis. A more detailed investigation of this new mTOR function will be of highest relevance to provide more explicit insights into stem cell regulation in the near future. Different cellular tools, including adult stem cells, embryonic stem cells or induced pluripotent stem cells could be used to investigate the role of mTOR in mammalian stem cell biology. Here we discuss the potential of amniotic fluid stem cells to become a promising cellular model to study the role of signaling cascades in stem cell homeostasis.
Introduction
Isolation, characterization, in vitro cultivation and modulation of stem cells have been the focus of interest for many years, because stem cells are considered as the bases of new biological therapy concepts for a wide variety of different human diseases.Citation1,Citation2 However, besides their putative usage for therapies, stem cells are also a promising tool for functional studies of genes involved in the molecular development of human genetic diseases. Studies using in vitro cultivation of human stem cell models are of importance for both, the investigation of stem cell homeostasis and differentiation and the clarification of the underlying molecular disease causing processes. This is of special interest since it became evident that due to fundamental biological differences many phenotypes of human genetic diseases fail to be successfully replicated in mice.Citation1-Citation4 Here we briefly review the concept to use human amniotic fluid stem (AFS) cells as a cellular tool to study the biochemical signaling switches being involved in these processes.
Amniotic Fluid Stem Cells
The history from the discovery of human AFS cells to today’s knowledge about this cell type can be summarized in different steps: (1) in the year 2003 the first report was published describing the existence of stem cells in human amniotic fluid.Citation5-Citation7 (2) Thereafter, the original finding of a highly proliferative Oct-4 positive stem cell type in human amniotic fluid was confirmed by different groups and the differentiation potential of AFS cells upon hematopoietic, neurogenic, osteogenic, chondrogenic, adipogenic, renal and hepatic lineages was demonstrated.Citation8-Citation17 (3) In the years 2006 and 2007 it was shown for the first time that descending from one single AFS cell it was possible to induce differentiation into cells of all three germ layers.Citation13,Citation17 (4) In the year 2010, monoclonal human AFS cells were proven to harbor the potential to form three-dimensional multicellular aggregates, called embryoid bodies. This feature is known to be a hallmark of pluripotent stem cells because it is accompanied by a decrease of stem cell marker expression and by the induction of differentiation into different lineages.Citation18 (5) In between, a very promising and fast growing stem cell research field has been established supporting the notion that AFS cells could be used for both, regenerative therapy and basic molecular science.Citation19-Citation30 In summary, monoclonal AFS cells can efficiently be grown in vitro, are pluripotent and non-tumorigenic, do not raise ethical concerns and are genomically stable.Citation24-Citation30
Tools to Study Genetic Modifications in Amniotic Fluid Stem Cells
The characteristics of AFS cells described above clearly argue for their usage as in vitro models to study cell signaling cascades being of relevance for stem cell homeostasis and molecular disease development. However, we want to point out another important advantage of AFS cells: they have been proven to be a very useful tool to study endogenous gene modulations. For this purpose two different approaches can be chosen. First, AFS cells harboring natural occurring disease causing genetic aberrations are available. Second, recently a protocol for efficient prolonged knockdown of endogenous gene expression in AFS cells has been established.
Amniotic fluid cell sampling via amniocentesis is a very widespread, standardly used procedure. Indications for amniocentesis are always situations with increased risk for fetal mutations. These include numerical and structural chromosome aberrations in cases of increased maternal age, specific ultrasound signs or e.g., paternal carriers of balanced translocations. In addition, today several thousands of gene mutations causatively involved in monogenic diseases can prenatally be diagnosed. In such cases the indication for amniocentesis is an already estimated increased familiar risk for this monogenic disease.Citation31-Citation33
We have already suggested elsewhere to establish AFS cell banks for research purposes.Citation4 All the different monoclonal AFS cell lines, which are currently available for research worldwide, have been generated via single cell isolation from amniocentesis in prenatal medicine. Since in the course of clinical routine diagnosis hundreds of thousands of amniocentesis are performed every year, the generation and banking of normal human AFS cell lines, of AFS cell lines with chromosomal aberrations, as well as of AFS cell lines with specific monogenic disease mutations could be a straightforward approach. So obtained monoclonal AFS cell lines would be a powerful tool for stem cell research and disease modeling.Citation4,Citation30
At this point another advantage of AFS cells must be highlighted. AFS cells are primary cells of a very early stage of human development and very likely did not have time to accumulate many somatic mutations yet. This is a problem that occurs with induced pluripotent stem (iPS) cells generated from patients with genetic diseases. Furthermore, in contrast to iPS cells, AFS cells do not accumulate karyotypic aberrations and gene mutations during cultivation and do not need ectopic treatment to induce pluripotency. In addition, during reprogramming iPS cells do not perfectly erase the epigenetic pattern of the original differentiated cells they are derived from.Citation2-Citation4,Citation24-Citation30,Citation34-Citation42
Primary cells are hard to transfect, which was always considered to be a limiting factor with respect to their usage for endogenous gene modulation experiments, such as siRNA approaches. siRNA-based experiments are reversible and enable the investigation of modifications of essential genes, without the limiting problems of clonal variations, selection procedures or inducing agents. Another relevant advantage of AFS cells, which must be pointed out in this review, is the existence of a very efficient protocol for the lipid-based forward transfection of AFS cells. This protocol allows efficient, functional and reproducible silencing of endogenous genes for prolonged time periods. Using this approach, transfection efficiencies of 94% transfected cells on average, as well as a 96–98% downregulation of the endogenous target protein can be achieved. This cost-effective and time-efficient experimental procedure was proven to allow the analysis of the cellular effects of simultaneously knocking down two genes in human AFS cells over a time period of about 14 d.Citation43
The mTOR Pathway
mTOR (originally “mammalian target of rapamycin,” but now officially “mechanistic target of rapamycin”) is the key enzyme component of the insulin signaling cascade. The fact that currently worldwide an enormous amount of scientific groups with interest in very many different areas are working on the investigation of this kinase has recently been explained by the joke “mTOR regulates everything.”Citation44 It is indeed fascinating how many important processes the mTOR pathway does control. By affecting the activity of a wide variety of substrates, including e.g., HIF1α, S6K, Grb10, 4E-BP, SREBP1/2, ULK/Atg13/FIP200, SGK1 or PKCα, the mTOR pathway is involved in the regulation of cell growth and protein synthesis, cell cycle, energy metabolism, transcription, survival, autophagy, aging, stem cell homeostasis, differentiation, oncogenesis, lipid synthesis and cytoskeletal organization ().Citation44-Citation50
Figure 1. The mTOR pathway. In mammalian cells the mTOR protein is part of two enzyme complexes, mTORC1 (containing raptor) and mTORC2 (containing rictor), with different substrates. For details see the text.
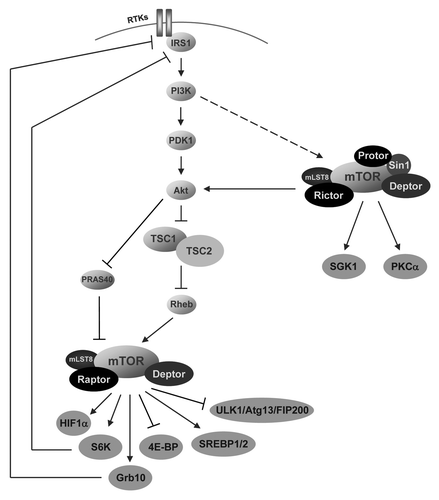
In mammalian cells the serine/threonine protein kinase mTOR is part of two complexes, mTORC1 and mTORC2, with different upstream regulators and downstream targets. mTORC1 consists of mTOR protein, the scaffold protein raptor, the two inhibitors PRAS40 and deptor and mLST8, which still is of unknown function for mTORC1. Besides mTOR protein, mTORC2 contains the two scaffold proteins rictor and sin1, the regulating element protor, the inhibitor deptor, and mLST8, which is essential for mTORC2 activity (but not for mTORC1) (). In addition, both complexes contain the scaffold proteins tti1 and tel2, which regulate the assembly and stability of both, mTORC1 and mTORC2.Citation44,Citation50
Whereas the upstream regulators of mTORC2 still need to be identified, in the last years a clear picture of the cascade upstream of mTORC1 has been drawn. The Akt kinase is activated via the enzyme cascade of PI3K (phosphatidylinositol-3-kinase) and PDK1 (phosphoinositide-dependent kinase-1). Full activation of Akt is mediated via both, phosphorylation at T308 by PDK1 and phosphorylation at S473 by mTORC2. So activated Akt triggers phosphorylation of the tumor suppressor protein tuberin (TSC2) causing downregulation of tuberin’s GTPase-activating potential toward Rheb, which is a potent regulator of mTOR ().Citation44-Citation46,Citation50
One could argue mTOR “not only regulates everything, but is also regulated by everything.” A large network of regulators and targets puts mTOR in the center of the molecular development of many types of cancer and human genetic diseases. Upstream regulators of mTOR, including Wnt, Ras, TNF-α, PI3K or Akt have been implicated in the molecular processes of the development of many tumors. Mutations in the mTOR pathway component genes TSC1, TSC2, LKB1, PTEN, VHL, NF1 and PKD1 trigger the development of the monogenic diseases Tuberous sclerosis, Peutz-Jeghers syndrome, Cowden syndrome, Bannayan-Riley-Ruvalcaba syndrome, Lhermitte-Duclos disease, Proteus syndrome, von Hippel-Lindau disease, neurofibromatosis type 1 and polycystic kidney disease. In addition, recently it became clear that mTOR is also involved in the development of complex diseases, such as cardiac hypertrophy, obesity or type 2 diabetes.Citation44,Citation48,Citation49
mTOR in Stem Cell Biology
Knockout mice revealed that mTOR is required for embryonic gastrulation. mTOR deleted embryos die shortly after implantation at embryonic day 5.5−6.5. Inner cell mass cells from explanted blastocysts from these knockout mice failed to proliferate, what suggested that mTOR is crucial for the viability of murine embryonic stem (ES) cells.Citation50-Citation52 Since then, different studies mainly using the inhibitor rapamycin to modulate endogenous mTOR activity in hematopoietic stem cells provided some first evidence for a role of mTOR in stem cell physiology.Citation50
But only recently, the potential of mTOR to control stem cell homeostasis and differentiation has been proven using different pluripotent cellular models. The mTOR cascade was already earlier shown to be fully active in human AFS cells.Citation53 The process of embryoid body formation was used to investigate the role of mTOR for the stemness of human AFS cells. Embryoid body formation is a commonly used in vitro approach to recapitulate and investigate the three-dimensional and tissue level contexts of the cell differentiation phenomena mimicking early mammalian embryogenesis. siRNA-mediated knockdown of raptor or rictor demonstrated that embryoid body formation of AFS cells depends on both, mTORC1 and mTORC2.Citation18 To the best of our knowledge, these data together with similar results in human ES cellsCitation54 were the first in vitro demonstration of the role of mTOR for EB formation, regulation of stemness and for the differentiation potential of pluripotent stem cells. Recently, it was further demonstrated that siRNA-mediated knockdown of endogenous tuberin or PRAS40, the two major negative regulators of mTOR, leads to massive apoptotic cell death during EB formation of AFS cells without affecting the endodermal, mesodermal and ectodermal cell differentiation spectrum. Co-knockdown of mTOR showed these effects to be mTOR-dependent, proving the mTOR pathway to be a major regulator of stem cell maintenance and differentiation.Citation55
In between, both renal and osteoblastic differentiation of pluripotent stem cells have been demonstrated to be controlled by mTOR. Different independent studies have shown that AFS cells are able to contribute to the formation process of renal tissues.Citation56-Citation60 Recently, murine embryonic kidneys were dissociated into a single-cell suspension and then reaggregated to form organotypic renal structures. Using this approach it was possible to form chimeric renal structures via mixing murine embryonic kidney cells with monoclonal human AFS cells. Monoclonal, pluripotent Oct4 and CD117 positive AFS cells were able to participate in the formation process of different renal tissues accompanied by the induction of the expression of well-known renal markers. Furthermore, the recently established protocol for siRNA-mediated gene silencing in human monoclonal AFS cellsCitation43 allowed to demonstrate the pivotal role of mTOR component genes for renal differentiation of pluripotent stem cells.Citation58 Using the inhibitor rapamycin, it was also recently shown that downregulation of endogenous mTOR activity promotes the osteoblastic differentiation of human embryonic stem cells.Citation61
Concluding Remarks
Although currently the mTOR cascade is emerging as an important pathway playing a role in stem cell homeostasis and differentiation, many open questions must be addressed in the near future. Conflicting results have been reported with regard to which extent mTOR supports or blocks stem cell differentiation.Citation50 Most importantly, experiments are warranted to investigate which lineage-specific differentations are mTOR-dependent and which mTOR substrates are involved. In this review we argue that human AFS cells are a very potent cellular model for such investigations of mTOR’s role for stemness. AFS cells are pluripotent, do not raise ethical concerns, can be cultivated with high efficiency and are genomically stable. AFS cells can easily be obtained harbouring natural occurring disease causing genetic modifications, which are of relevance for certain human pathological phenotypes. Finally, with regard to the topic of this review it is of highest importance that the relevance of endogenous gene functions can very efficiently be studied in human AFS cells via siRNA approaches.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- Zhu H, Lensch MW, Cahan P, Daley GQ. Investigating monogenic and complex diseases with pluripotent stem cells. Nat Rev Genet 2011; 12:266 - 75; http://dx.doi.org/10.1038/nrg2951; PMID: 21386866
- Mattis VB, Svendsen CN. Induced pluripotent stem cells: a new revolution for clinical neurology?. Lancet Neurol 2011; 10:383 - 94; http://dx.doi.org/10.1016/S1474-4422(11)70022-9; PMID: 21435601
- Wu SM, Hochedlinger K. Harnessing the potential of induced pluripotent stem cells for regenerative medicine. Nat Cell Biol 2011; 13:497 - 505; http://dx.doi.org/10.1038/ncb0511-497; PMID: 21540845
- Rosner M, Dolznig H, Schipany K, Mikula M, Brandau O, Hengstschläger M. Human amniotic fluid stem cells as a model for functional studies of genes involved in human genetic diseases or oncogenesis. Oncotarget 2011; 2:705 - 12; PMID: 21926447
- Prusa AR, Hengstschläger M. Amniotic fluid cells and human stem cell research: a new connection. Med Sci Monit 2002; 8:RA253 - 7; PMID: 12444390
- Prusa AR, Marton E, Rosner M, Bernaschek G, Hengstschläger M. Oct-4-expressing cells in human amniotic fluid: a new source for stem cell research?. Hum Reprod 2003; 18:1489 - 93; http://dx.doi.org/10.1093/humrep/deg279; PMID: 12832377
- Prusa AR, Marton E, Rosner M, Freilinger A, Bernaschek G, Hengstschläger M. Stem cell marker expression in human trisomy 21 amniotic fluid cells and trophoblasts. J Neural Transm Suppl 2003; 67:235 - 42; http://dx.doi.org/10.1007/978-3-7091-6721-2_21; PMID: 15068255
- In ’t Anker PS, Scherjon SA, Kleijburg-van der Keur C, Noort WA, Claas FHJ, Willemze R, et al. Amniotic fluid as a novel source of mesenchymal stem cells for therapeutic transplantation. Blood 2003; 102:1548 - 9; http://dx.doi.org/10.1182/blood-2003-04-1291; PMID: 12900350
- Tsai M-S, Lee J-L, Chang Y-J, Hwang S-M. Isolation of human multipotent mesenchymal stem cells from second-trimester amniotic fluid using a novel two-stage culture protocol. Hum Reprod 2004; 19:1450 - 6; http://dx.doi.org/10.1093/humrep/deh279; PMID: 15105397
- Prusa AR, Marton E, Rosner M, Bettelheim D, Lubec G, Pollack A, et al. Neurogenic cells in human amniotic fluid. Am J Obstet Gynecol 2004; 191:309 - 14; http://dx.doi.org/10.1016/j.ajog.2003.12.014; PMID: 15295384
- Karlmark KR, Freilinger A, Marton E, Rosner M, Lubec G, Hengstschläger M. Activation of ectopic Oct-4 and Rex-1 promoters in human amniotic fluid cells. Int J Mol Med 2005; 16:987 - 92; PMID: 16273276
- Bossolasco P, Montemurro T, Cova L, Zangrossi S, Calzarossa C, Buiatiotis S, et al. Molecular and phenotypic characterization of human amniotic fluid cells and their differentiation potential. Cell Res 2006; 16:329 - 36; http://dx.doi.org/10.1038/sj.cr.7310043; PMID: 16617328
- Tsai M-S, Hwang S-M, Tsai Y-L, Cheng F-C, Lee J-L, Chang YJ. Clonal amniotic fluid-derived stem cells express characteristics of both mesenchymal and neural stem cells. Biol Reprod 2006; 74:545 - 51; http://dx.doi.org/10.1095/biolreprod.105.046029; PMID: 16306422
- Kim J, Lee Y, Kim H, Hwang KJ, Kwon HC, Kim SK, et al. Human amniotic fluid-derived stem cells have characteristics of multipotent stem cells. Cell Prolif 2007; 40:75 - 90; http://dx.doi.org/10.1111/j.1365-2184.2007.00414.x; PMID: 17227297
- Rehni AK, Singh N, Jaggi AS, Singh M. Amniotic fluid derived stem cells ameliorate focal cerebral ischaemia-reperfusion injury induced behavioural deficits in mice. Behav Brain Res 2007; 183:95 - 100; http://dx.doi.org/10.1016/j.bbr.2007.05.028; PMID: 17619060
- Kolambkar YM, Peister A, Soker S, Atala A, Guldberg RE. Chondrogenic differentiation of amniotic fluid-derived stem cells. J Mol Histol 2007; 38:405 - 13; http://dx.doi.org/10.1007/s10735-007-9118-1; PMID: 17668282
- De Coppi P, Bartsch G Jr., Siddiqui MM, Xu T, Santos CC, Perin L, et al. Isolation of amniotic stem cell lines with potential for therapy. Nat Biotechnol 2007; 25:100 - 6; http://dx.doi.org/10.1038/nbt1274; PMID: 17206138
- Valli A, Rosner M, Fuchs C, Siegel N, Bishop CE, Dolznig H, et al. Embryoid body formation of human amniotic fluid stem cells depends on mTOR. Oncogene 2010; 29:966 - 77; http://dx.doi.org/10.1038/onc.2009.405; PMID: 19935716
- Orciani M, Emanuelli M, Martino C, Pugnaloni A, Tranquilli AL, Di Primio R. Potential role of culture mediums for successful isolation and neuronal differentiation of amniotic fluid stem cells. Int J Immunopathol Pharmacol 2008; 21:595 - 602; PMID: 18831926
- Ditadi A, de Coppi P, Picone O, Gautreau L, Smati R, Six E, et al. Human and murine amniotic fluid c-Kit+Lin- cells display hematopoietic activity. Blood 2009; 113:3953 - 60; http://dx.doi.org/10.1182/blood-2008-10-182105; PMID: 19221036
- Jezierski A, Gruslin A, Tremblay R, Ly D, Smith C, Turksen K, et al. Probing stemness and neural commitment in human amniotic fluid cells. Stem Cell Rev 2010; 6:199 - 214; http://dx.doi.org/10.1007/s12015-010-9116-7; PMID: 20221716
- Mareschi K, Rustichelli D, Comunanza V, De Fazio R, Cravero C, Morterra G, et al. Multipotent mesenchymal stem cells from amniotic fluid originate neural precursors with functional voltage-gated sodium channels. Cytotherapy 2009; 11:534 - 47; http://dx.doi.org/10.1080/14653240902974024; PMID: 19548144
- Pfeiffer S, McLaughlin D. In vitro differentiation of human amniotic fluid-derived cells: augmentation towards a neuronal dopaminergic phenotype. Cell Biol Int 2010; 34:959 - 67; http://dx.doi.org/10.1042/CBI20090445; PMID: 20388119
- Pappa KI, Anagnou NP. Novel sources of fetal stem cells: where do they fit on the developmental continuum?. Regen Med 2009; 4:423 - 33; http://dx.doi.org/10.2217/rme.09.12; PMID: 19438317
- Dobreva MP, Pereira PNG, Deprest J, Zwijsen A. On the origin of amniotic stem cells: of mice and men. Int J Dev Biol 2010; 54:761 - 77; http://dx.doi.org/10.1387/ijdb.092935md; PMID: 20446274
- Pozzobon M, Ghionzoli M, De Coppi P. ES, iPS, MSC, and AFS cells. Stem cells exploitation for Pediatric Surgery: current research and perspective. Pediatr Surg Int 2010; 26:3 - 10; http://dx.doi.org/10.1007/s00383-009-2478-8; PMID: 19727766
- Abdulrazzak H, Moschidou D, Jones G, Guillot PV. Biological characteristics of stem cells from foetal, cord blood and extraembryonic tissues. J R Soc Interface 2010; 7:Suppl 6 S689 - 706; http://dx.doi.org/10.1098/rsif.2010.0347.focus; PMID: 20739312
- Da Sacco S, Sedrakyan S, Boldrin F, Giuliani S, Parnigotto PP, Habibian R, et al. Human amniotic fluid as a potential new source of organ specific precursor cells for future regenerative medicine applications. J Urol 2010; 183:1193 - 200; http://dx.doi.org/10.1016/j.juro.2009.11.006; PMID: 20096867
- Rosner M, Mikula M, Preitschopf A, Feichtinger M, Schipany K, Hengstschläger M. Neurogenic differentiation of amniotic fluid stem cells. Amino Acids 2012; 42:1591 - 6; http://dx.doi.org/10.1007/s00726-011-0929-8; PMID: 21573873
- Gundacker C, Dolznig H, Mikula M, Rosner M, Brandau O, Hengstschläger M. Amniotic fluid stem cell-based models to study the effects of gene mutations and toxicants on male germ cell formation. Asian J Androl 2012; 14:247 - 50; http://dx.doi.org/10.1038/aja.2011.170; PMID: 22231297
- Raymond FL, Whittaker J, Jenkins L, Lench N, Chitty LS. Molecular prenatal diagnosis: the impact of modern technologies. Prenat Diagn 2010; 30:674 - 81; http://dx.doi.org/10.1002/pd.2575; PMID: 20572117
- Nicolaides KH. Turning the pyramid of prenatal care. Fetal Diagn Ther 2011; 29:183 - 96; http://dx.doi.org/10.1159/000324320; PMID: 21389681
- Nizard J. Amniocentesis: technique and education. Curr Opin Obstet Gynecol 2010; 22:152 - 4; http://dx.doi.org/10.1097/GCO.0b013e32833723a0; PMID: 20098324
- Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 2006; 126:663 - 76; http://dx.doi.org/10.1016/j.cell.2006.07.024; PMID: 16904174
- Dimos JT, Rodolfa KT, Niakan KK, Weisenthal LM, Mitsumoto H, Chung W, et al. Induced pluripotent stem cells generated from patients with ALS can be differentiated into motor neurons. Science 2008; 321:1218 - 21; http://dx.doi.org/10.1126/science.1158799; PMID: 18669821
- Park IH, Arora N, Huo H, Maherali N, Ahfeldt T, Shimamura A, et al. Disease-specific induced pluripotent stem cells. Cell 2008; 134:877 - 86; http://dx.doi.org/10.1016/j.cell.2008.07.041; PMID: 18691744
- Kim K, Doi A, Wen B, Ng K, Zhao R, Cahan P, et al. Epigenetic memory in induced pluripotent stem cells. Nature 2010; 467:285 - 90; http://dx.doi.org/10.1038/nature09342; PMID: 20644535
- Stadtfeld M, Apostolou E, Akutsu H, Fukuda A, Follett P, Natesan S, et al. Aberrant silencing of imprinted genes on chromosome 12qF1 in mouse induced pluripotent stem cells. Nature 2010; 465:175 - 81; http://dx.doi.org/10.1038/nature09017; PMID: 20418860
- Laurent LC, Ulitsky I, Slavin I, Tran H, Schork A, Morey R, et al. Dynamic changes in the copy number of pluripotency and cell proliferation genes in human ESCs and iPSCs during reprogramming and time in culture. Cell Stem Cell 2011; 8:106 - 18; http://dx.doi.org/10.1016/j.stem.2010.12.003; PMID: 21211785
- Mayshar Y, Ben-David U, Lavon N, Biancotti JC, Yakir B, Clark AT, et al. Identification and classification of chromosomal aberrations in human induced pluripotent stem cells. Cell Stem Cell 2010; 7:521 - 31; http://dx.doi.org/10.1016/j.stem.2010.07.017; PMID: 20887957
- Gore A, Li Z, Fung HL, Young JE, Agarwal S, Antosiewicz-Bourget J, et al. Somatic coding mutations in human induced pluripotent stem cells. Nature 2011; 471:63 - 7; http://dx.doi.org/10.1038/nature09805; PMID: 21368825
- Pasi CE, Dereli-Öz A, Negrini S, Friedli M, Fragola G, Lombardo A, et al. Genomic instability in induced stem cells. Cell Death Differ 2011; 18:745 - 53; http://dx.doi.org/10.1038/cdd.2011.9; PMID: 21311564
- Rosner M, Siegel N, Fuchs C, Slabina N, Dolznig H, Hengstschläger M. Efficient siRNA-mediated prolonged gene silencing in human amniotic fluid stem cells. Nat Protoc 2010; 5:1081 - 95; http://dx.doi.org/10.1038/nprot.2010.74; PMID: 20539284
- Laplante M, Sabatini DM. mTOR signaling in growth control and disease. Cell 2012; 149:274 - 93; http://dx.doi.org/10.1016/j.cell.2012.03.017; PMID: 22500797
- Polak P, Hall MN. mTOR and the control of whole body metabolism. Curr Opin Cell Biol 2009; 21:209 - 18; http://dx.doi.org/10.1016/j.ceb.2009.01.024; PMID: 19261457
- Ma XM, Blenis J. Molecular mechanisms of mTOR-mediated translational control. Nat Rev Mol Cell Biol 2009; 10:307 - 18; http://dx.doi.org/10.1038/nrm2672; PMID: 19339977
- Blagosklonny MV. Revisiting the antagonistic pleiotropy theory of aging: TOR-driven program and quasi-program. Cell Cycle 2010; 9:3151 - 6; http://dx.doi.org/10.4161/cc.9.16.13120; PMID: 20724817
- Wong KK, Engelman JA, Cantley LC. Targeting the PI3K signaling pathway in cancer. Curr Opin Genet Dev 2010; 20:87 - 90; http://dx.doi.org/10.1016/j.gde.2009.11.002; PMID: 20006486
- Rosner M, Hanneder M, Siegel N, Valli A, Fuchs C, Hengstschläger M. The mTOR pathway and its role in human genetic diseases. Mutat Res 2008; 659:284 - 92; http://dx.doi.org/10.1016/j.mrrev.2008.06.001; PMID: 18598780
- Russell RC, Fang C, Guan K-L. An emerging role for TOR signaling in mammalian tissue and stem cell physiology. Development 2011; 138:3343 - 56; http://dx.doi.org/10.1242/dev.058230; PMID: 21791526
- Gangloff YG, Mueller M, Dann SG, Svoboda P, Sticker M, Spetz JF, et al. Disruption of the mouse mTOR gene leads to early postimplantation lethality and prohibits embryonic stem cell development. Mol Cell Biol 2004; 24:9508 - 16; http://dx.doi.org/10.1128/MCB.24.21.9508-9516.2004; PMID: 15485918
- Murakami M, Ichisaka T, Maeda M, Oshiro N, Hara K, Edenhofer F, et al. mTOR is essential for growth and proliferation in early mouse embryos and embryonic stem cells. Mol Cell Biol 2004; 24:6710 - 8; http://dx.doi.org/10.1128/MCB.24.15.6710-6718.2004; PMID: 15254238
- Siegel N, Valli A, Fuchs C, Rosner M, Hengstschläger M. Expression of mTOR pathway proteins in human amniotic fluid stem cells. Int J Mol Med 2009; 23:779 - 84; PMID: 19424604
- Zhou J, Su P, Wang L, Chen J, Zimmermann M, Genbacev O, et al. mTOR supports long-term self-renewal and suppresses mesoderm and endoderm activities of human embryonic stem cells. Proc Natl Acad Sci U S A 2009; 106:7840 - 5; http://dx.doi.org/10.1073/pnas.0901854106; PMID: 19416884
- Fuchs C, Rosner M, Dolznig H, Mikula M, Kramer N, Hengstschläger M. Tuberin and PRAS40 are anti-apoptotic gatekeepers during early human amniotic fluid stem-cell differentiation. Hum Mol Genet 2012; 21:1049 - 61; http://dx.doi.org/10.1093/hmg/ddr535; PMID: 22090422
- Perin L, Giuliani S, Jin D, Sedrakyan S, Carraro G, Habibian R, et al. Renal differentiation of amniotic fluid stem cells. Cell Prolif 2007; 40:936 - 48; http://dx.doi.org/10.1111/j.1365-2184.2007.00478.x; PMID: 18021180
- Siegel N, Valli A, Fuchs C, Rosner M, Hengstschläger M. Induction of mesenchymal/epithelial marker expression in human amniotic fluid stem cells. Reprod Biomed Online 2009; 19:838 - 46; http://dx.doi.org/10.1016/j.rbmo.2009.09.015; PMID: 20031026
- Siegel N, Rosner M, Unbekandt M, Fuchs C, Slabina N, Dolznig H, et al. Contribution of human amniotic fluid stem cells to renal tissue formation depends on mTOR. Hum Mol Genet 2010; 19:3320 - 31; http://dx.doi.org/10.1093/hmg/ddq236; PMID: 20542987
- Perin L, Sedrakyan S, Giuliani S, Da Sacco S, Carraro G, Shiri L, et al. Protective effect of human amniotic fluid stem cells in an immunodeficient mouse model of acute tubular necrosis. PLoS One 2010; 5:e9357; http://dx.doi.org/10.1371/journal.pone.0009357; PMID: 20195358
- Rosner M, Schipany K, Gundacker C, Shanmugasundaram B, Li K, Fuchs C, et al. Renal differentiation of amniotic fluid stem cells: perspectives for clinical application and for studies on specific human genetic diseases. Eur J Clin Invest 2012; 42:677 - 84; http://dx.doi.org/10.1111/j.1365-2362.2011.02622.x; PMID: 22060053
- Lee KW, Yook JY, Son MY, Kim MJ, Koo DB, Han YM, et al. Rapamycin promotes the osteoblastic differentiation of human embryonic stem cells by blocking the mTOR pathway and stimulating the BMP/Smad pathway. Stem Cells Dev 2010; 19:557 - 68; http://dx.doi.org/10.1089/scd.2009.0147; PMID: 19642865