Abstract
Introduction
(1) Human embryonic stem (ES) cells are pluripotent but are difficult to be used for therapy because of immunological, oncological and ethical barriers. (2) Pluripotent cells exist in vivo, i.e., germ cells and epiblast cells but cannot be isolated without sacrificing the developing embryo. (3) Reprogramming to pluripotency is possible from adult cells using ectopic expression of OKSM and other integrative and non-integrative techniques. (4) Hurdles to overcome include i.e stability of the phenotype in relation to epigenetic memory.
Sources of data
We reviewed the literature related to reprogramming, pluripotency and fetal stem cells.
Areas of agreement
(1) Fetal stem cells present some advantageous characteristics compared with their neonatal and postnatal counterparts, with regards to cell size, growth kinetics, and differentiation potential, as well as in vivo tissue repair capacity. (2) Amniotic fluid stem cells are more easily reprogrammed to pluripotency than adult fibroblast. (3) The parental population is heterogeneous and present an intermediate phenotype between ES and adult somatic stem cells, expressing markers of both.
Areas of controversy
(1) It is unclear whether induced pluripotent stem (iPS) derived from amniotic fluid stem cells are fully or partially reprogrammed. (2) Optimal protocols to ensure highest efficiency and phenotype stability remains to be determined. (3) The “level” of reprogramming, fully vs partial, of iPS derived from amniotic fluid stem cells remain to be determined.
Growing points
Banking of fully reprogrammed cells may be important both for (1) autologous and allogenic applications in medicine, and (2) disease modeling.
Introduction
Human embryonic stem (hES) cells are pluripotent and can be used for disease modeling, for drug screening, and to develop cell-based therapies to treat diseases and tissue injuries. However, the ethical problems linked to the derivation of hES cells from the inner cell mass of the embryos and the recent progress in stem-cell biology have lead to the development of induced-pluripotent stem cells. Reprogramming human cells by defined factors allowed for the first time the generation of patient-specific pluripotent cell lines without somatic cell nuclear transfer (SCNT), which was first used in 1958 to create pluripotent cells from adult somatic cells.Citation1 In the latter, the nuclear material of a somatic cell was transferred into an oocyte and pluripotency was induced by chemical and electrical stimuli.Citation2 The system was initially abandoned because of being considered inefficient, but it has been recently improved by leaving the oocyte nucleus in place, allowing derivation of triploid pluripotent stem cells.Citation3 In 2006, Takahashi and Yamanaka showed that pluripotent stem cells could be generated from mouse fibroblasts by ectopic expression of the OKSM factors: i.e., OCT3/4, SOX2, CMYC, and KLF4Citation4 These cells, designated as induced pluripotent stem (iPS) cells, have similar, but not identical, morphology, growth properties and genetic profile of mES cells. Pluripotency of iPS cells is assessed by their capacity to form teratomas in vivo following subcutaneous transplantation into immunocompromised mice and to contribute to embryonic development following injection into blastocysts.Citation4 This was later followed by the derivation of iPS cells using retroviral transduction of the OKSM factors in human dermal fibroblasts demonstrating.Citation5 At the same time, James Thomson’s group also reported the generation of human iPSCs using a different combination of factors.Citation6 They identified another set of 4 genes (OSNL factors), i.e., OCT4, SOX2, NANOG and LIN28, capable of reprogramming somatic cells to full pluripotency at a clonal level.Citation7 Thus, many ways to generate integration-free iPSCs have subsequently been tested: plasmids,Citation8 Sendai virus,Citation9 adenovirus,Citation10 synthesized RNAs,Citation11 and proteins.Citation12 However, the non-integrative methods still have pitfalls, being mainly associated with poor efficiency of iPS generation.
The similarity of the phenotype of iPS derived from human somatic cells compared with hES cells is a challenge. It is well accepted that the iPS are not identical to ES cells. With increasing evidence showing that iPS cells are distinct from hES cells, albeit both being pluripotent, as defined by their capacity to differentiate into lineages of the three germ layers ().Citation6 For example, differences have been described at the level of gene expression, DNA methylation, and stability of the pluripotent phenotype over time, as well as the epigenetic memory. These may be attributed to somatic mutations,Citation13 copy number variationsCitation14 and immunogenicity,Citation15 which could be altered in iPS cells. Moreover, the factor combinations, gene delivery methods, and culture conditions might also contribute to the differences obtained between the different iPS cell populations generated. Finally, some variations may be attributed to stochastic events during reprogramming, which cannot be controlled.Citation6 Thus, increasing efforts now focus on finding the “best candidate parental population” to generate iPS cells for in vitro studies and future clinical applications. The sequencing of the majority of the protein-coding exons of 22 human iPS lines and the nine parental fibroblast revealed that some of the reprogramming-associated mutations were likely to pre-exist in the starting fibroblast cultures, while the others occurred during reprogramming and subsequent culturing.Citation13 The comparison of copy number variations (CNVs) of different passages of human iPS cells with their fibroblast cell population and with ES cells showed that the reprogramming process is associated with high mutation rates characterized by an increased levels of CNVs and genetic mosaics in particular during early-passage of human iPS lines.Citation14 Immunologically, it has been observed that mouse embryonic fibroblasts (MEFs), reprogrammed into iPSCs by either retroviral approach (ViPS) cells or a novel episomal approach (EiPS cells) generated an immunoresponse when transplanted in B6 mice.Citation15 In contrast to B6-derived ESCs, teratomas formed by B6 (ViPS) cells transplantation were mostly immune-rejected by B6 recipients, and the majority of teratomas formed by B6-derived iPS cells were immunogenic, with T cell infiltration and apparent tissue damage observed in a small fraction of teratomas.Citation15
Table 1. Number of ESC and somatic-derived iPSC clones compared in published studies. The table summarizes the conclusion reported by different studies about the relationship between ESC and iPSC with the author’s name, the year of the article publication and the number of the clones analyzed, modified from ref. Citation6
In this context, fetal stem cells (FSCs) have emerged as an ‘intermediate phenotype’ between embryonic and adult stem cells.Citation16 FSCs are neither fully pluripotent nor multipotent; when compared with their adult counterparts, FSCs appear to be more primitive, with higher growth kinetics, smaller cell size, active telomerase and greater plasticity; while lacking tumorogenicity.Citation16,Citation17 These features may represent an advantage for regenerative medicine because they might be easier to reprogram.Citation18
Moreover, one of the major limitations related to iPS cell generation has been the use of retroviruses or lentiviruses, which could cause mutagenesis leading to a risk for teratogenesis and other adverse effects like those seen in some attempts at gene therapy.Citation19 Therefore, it has been reported that to ameliorate the efficiency of iPS generated from somatic cells, some groups have tried to modulate key component of the cell cycle like repression of the Ink4a/Arf locus or downregulation of the p53–p21 pathway; nevertheless, p53 suppression can lead to increased levels of DNA damage and genomic instability.Citation14 FSCs represent an alternative source for cell reprogramming and regenerative medicine since they are easily achievable, they show high proliferation rate, negligible immunogenity and demonstrate no evidence for teratoma formation and no ethical concerns.Citation20 Here, we review the generation of iPS cells from fetal tissues and their future applications.
Fetal Stem Cells (FSCs): A Potential Source for Cellular Reprogramming
In recent few years, fetal stem cells (FSCs) have emerged as an alternative cell type in regenerative medicine. Stem cells can be isolated from fetal tissues such as blood, liver, bone marrow (BM), pancreas, spleen and kidneyCitation21 and from the supportive extra-embryonic structures such as placenta, cord blood and Wharton jelly from the umbilical cord, and amniotic fluid ().Citation22 FSCs can be obtained from termination of pregnancy (BM or liver) or during an on going pregnancy (fetal blood during the first trimester), although the latter is an invasive and technically challenging procedure.Citation16 In contrast, mid-trimester amniotic fluid and first trimester placenta samples can be obtained during amniocentesis and chorionic villus sampling during prenatal screening.
Figure 1. Schematic drawing of fetal stem cells localization derived from the extra embryonic tissues such as human placenta consisting in amnion, chorion (fetal parts) and deciduas (maternal part), amniotic fluid and umbilical cord blood.
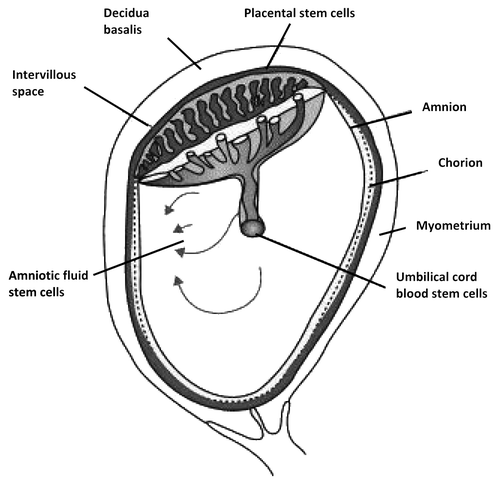
Fetal stem cells populations are heterogeneous with respect to phenotypic feature, properties and cell markers expression, which depend on their tissue of origin and gestational age. They include stromal/mesenchymal stem cells (MSCs), hematopoietic stem cells (HSCs), and pro-pluripotent cells, (). Herein we list the different properties of FSCs, their role on iPS cells generation and their possible application in regenerative medicine ().
Table 2. Characteristics of stem cells from embryonic fetal and extra embryonic fetal tissues. E, embryonic; MSC, mesenchymal; H, hematopoietic; EP, epithelial; VSEL, very small embryonic like; P, pluripotent; M, multipotent; U, unipotent; n.d, not determined, modified from ref. Citation16
Table 3. Some therapeutic applications of fetal stem cells isolated from fetal and extra embryonic tissues, modified from ref. Citation16
Human Fetal Mesenchymal Stem Cells (MSCs)
Mesenchymal stromal / stem cells (MSCs) were isolated initially from adult BM. They are plastic adherent and show a fibroblast-like morphology, express SH2, SH3, CD29 (also expressed by ES), CD44, CD71, CD90, CD106, CD120a, CD124 but not markers of the hematopoietic lineage CD14, CD34, and CD45Citation23 and not OCT4, or markers of pluripotency. MSC can differentiate into osteogenic (bone), chondrogenic (cartilage), and adipogenic (BM stroma) lineages. Some studies demonstrated that MSC can also differentiate to other cell types of mesodermal origin (skeletal muscle, smooth muscle, cardiac muscle, endothelial cells) but there is little evidence of their capacity to undergo terminal functional differentiation in vivo.Citation24 MSCs have also been found earlier in gestation, circulating in human first-trimester fetal blood, and present in first-trimester liver and bone marrow.Citation25 All three fetal sources of first trimester MSC have similar growth kinetics,Citation26 whereas a different growth potential of human fetal MSC compared with adult MSC has been showed by microarray data.Citation27 Contrary to adult BM MSCs, first-trimester fetal cells have more primitive characteristics, although they are unable to form embryoid bodies in vitro,Citation25 they retain a stable phenotype in culture, are more expandable to therapeutic scales for either pre- or postnatal ex vivo gene or cell therapyCitation25 and for their plasticity which may go beyond the mesodermal lineages.Citation28,Citation29 The potential therapeutic advantages of fetal MSC over adult MSC are not restricted to differentiation potential and growth kinetics.Citation25 Indeed, some studies have been showed that fetal MSC from fetal liver when transplanted into the fetus of immunodeficient SCID mice, showed a 10-fold engraftment advantage over those from adult bone marrow.Citation30 Moreover, in preimmune fetal sheep, fetal MSC engrafted in multiple tissues showed multilineage differentiation like their adult counterparts, but unlike adult bone marrow MSC, they appeared to contribute hematopoiesis.Citation31
Human Fetal Hematopoietic Stem Cells (HSCs)
HSCs are multipotent stem cells involved in the maintenance of hematopoiesis by generation of all hematopoietic lineages throughout fetal and adult life.Citation32 They are characterized by the expression of CD34 and CD45 antigens, and the absence of markers such as CD38 and human leucocyte antigen (HLA)/DRE.Citation33 The specific localization of HSCs in the BM “niche” allows HSC self-renewal and differentiation throughout the adult life. Before reaching the BM, during development, newly formed HSCs migrate to the fetal liver, which is the main hematopoietic organ before birth.Citation34 The migration of HSCs during the fetal life is accompanied with a modification in the percentage of the cells in each regions of hematopoiesis: first-trimester fetal blood contains more CD34+ cells than term gestation blood,Citation26 in which CD34+ cells constitute 4% of cells in blood, 16.5% in BM, 6% in liver, 5% in spleen and 1.1% the thymus.Citation35 Moreover, during the third trimester the frequency of CD34+ cells in the blood gradually decreases probably because the marrow is the primary site of hematopoiesis.Citation36 Fetal HSCs show a greater proliferative capacity, lower immunological reactivity and lower risk of graft-vs.-host disease (GVHD) respect to HSCs from adult BM;Citation37 for their repopulating capacity following intra-bone injection of severe combined immunodeficiency mice,Citation38,Citation39 these cells can be used as an alternative cell source respect to adult BM HSCs.
iPS from HSCs
Generation of human iPSCs from blood HSCs offers some advantages, such as the more convenient and less invasive procedure to obtain peripheral blood (PB) than dermal fibroblasts, where several weeks are required to establish a primary cell culture from skin biopsy. A recent study reported the generation of iPSCs from human immature mononuclear cells (MNCs) expressing the hematopoietic markers CD34 or CD133 isolated from umbilical cord blood (CB), adult peripheral blood (PB) and BMCitation40 using a set of EBNA1/OriP plasmidsCitation40 using inclusion of the EBNA1 gene and the OriP DNA sequence from the Epstein-Barr virus (EBV) enables a plasmid, after one-time DNA transfection, to replicate extra-chromosomally in many types of primate cells as a circular episome. In particular, they adopted two sets of plasmids to transduce the cells. In the first EBNA1/OriP plasmid (called pEB-C5), 5 reprogramming factors (OCT4, SOX2, KLF4, c-MYC and LIN28) are expressed as a single poly-cistronic unit; in the second set of EBNA1/OriP plasmids, SV40 Large T antigen (Tg), NANOG or a small hairpin RNA targeting p53 (p53shRNA) is individually expressed.Citation40 They observed a highly efficient reprogramming of blood MNCs. Within 14 d of one-time transfection by one plasmid, up to 1000 iPSC-like colonies per 2 million transfected CB MNCs were generated. Although the efficiency of deriving iPSCs from adult PB MNCs was approximately 50-fold lower, could be enhanced by inclusion of a second EBNA1/OriP plasmid for transient expression of additional genes such as SV40 T antigen. The time of obtaining iPSC colonies from adult PB MNCs was reduced to half (~14 d) as compared with adult fibroblastic cells (28–30 d). More than 9 human iPSC lines derived from PB or CB cells are extensively characterized, including those from PB MNCs of an adult patient with sickle cell disease. They lack V(D)J DNA rearrangements and vector DNA after expansion for 10–12 passages. This method of generating human iPSCs from blood MNCs will accelerate their use in both research and future clinical applications.Citation40
Fetal HSCs can also be derived at birth from the umbilical cord blood (UCB). Around 1% of the cells isolated from UCB express the CD34 surface marker, the pivotal marker of hHSCs and negative expression for CD38. The frequency of CD34+ cells in cord blood is higher than that of adult BM or peripheral blood following cytokine mobilizationCitation20 and compared with BM cells, CD34+/CD38− UCB cells proliferate more rapidly and generate larger numbers of progeny cells;Citation41 longer telomere lengths of UCB cells have been proposed as a possible explanation for the greater proliferative capacity of UCB.Citation41 Besides, it was demonstrated that cord blood HSCs express neuronal proteins and can differentiate into neuronal-like cells or glial cells.Citation42 Altogether, these properties designate UCB has an alternative source of HSCs for transplantation. Despite this, obtaining an adequate cell dose from a single UCB unit is difficult, also because the homing and engraftment capacity of HSCs seems to be dependent from cytokines release, molecular and cellular factors.Citation43
Besides the previously mentioned study, other studies reported the generation of iPS from human CD34+ UCB cells using different transduction systems. A brief report described the iPS production from fresh CB and CB cryopreserved for 5–8 y. Oct4, KLF-4, SOX2, and c-Myc reprogramming lentiviral vector was employed to transduce CD34+ cells. iPS cell colonies stained positive for OCT4, NANOG, TRA-1–60, SSEA-4, alkaline phosphatase and they were also characterized by quantitative RT-PCR for the expression of endogenous OCT4, SOX2 and NANOG. The expression of ectodermal, mesodermal, and endodermal proteins was confirmed by Embryoid bodies (EBs) and teratoma formation. Therefore, generation of iPS from frozen CB produced cells expressing TRA-1–60, SSEA-4, NANOG, and OCT4, and able to form teratomas with expression of endoderm, mesoderm, and ectoderm markers; at last, the efficiency of iPS cell from thawed CB ranged from 0.027–0.05% per CD34+ cell, similar to cultured CD34+ cells from freshly isolated or shorter-term frozen CB.Citation44 In another interesting study it was shown that the frequency of formation of iPS-like colonies from CD34+ cells could be increased when p53 expression is repressed. Since it has been reported that the absence of the p53 gene results in spontaneous reversion of germ cell stem cells in culture to a pluripotent state,Citation16 the authors investigated if the repression of p53 expression has the potential to mediate induction of pluripotency in cord blood cells. A shTP53 RNA construct expressing a short-hairpin RNA (shRNA) sequence that can reduce the amount of endogenous p53 transcripts was introduced in addition to SOX2, OCT3/4, KLF4, and C-MYC factors. With this system they obtained a number of bona fide iPS cell clones from 2X10Citation4 virus-infected cells.Citation43
Placental Stem Cells
The placenta is the organ involved in maintaining fetal tolerance and allows nutrient uptake and gas exchange with the mother, but it is now clear that progenitors and stem cells are also present.Citation45 Placenta consists in amnion, chorion (fetal sides) and deciduas (the maternal side), each of these parts is characterized by the presence of different stem cells populations. From amniotic membrane it is possible to isolate both amniotic epithelial cells (AECs) and amniotic mesenchymal cells (AMSC). AECs are plastic adherent and grow under MSC conditions; evidence suggests that they express pluripotency markers and have the ability, in vitro, to form xenogeneic chimera with mouse ES cells.Citation46 The cells have subsequently been differentiated into cell types from all three germ layers.Citation47,Citation48 Amniotic mesenchymal (AMSC) and chorionic (CSC) cells have been widely characterizedCitation49 and can be isolated throughout gestation from first trimester to delivery. AMSC and CSC display a fibroblastoid phenotype upon adherence to plastic like BM MSCs, can form typical colonies, show a differentiation potential toward mesodermal lineages and express the range of markers used to characterize MSCs. Furthermore these cells express markers such as SSEA-4, TRA-1–61, and TRA-1–80. Nevertheless, there are some differences between AMSCs and CSCs regarding their differentiation potential; indeed, AMSCs seem to be more directed to the adipogenic lineage whereas CMSCs more to chrondo-, osteo-, myo- and neurogenic.Citation50 On the other hand, chorionic villi (CVS) cells express the pluripotency markers OCT4, ALP, NANOG and SOX2Citation51 and not only have differentiation potential toward adipogenic, chondrogenic and osteogenic cellsCitation52,Citation53 but, in vitro, they can also give rise to cells with hepatocytes-like phenotype with the ability to store glycogen.Citation54,Citation55 Finally, in our recent studyCitation49 we has compared the phenotype of first trimester and term fetal placental chorionic stem cells (e-CSC and l-CSC respectively) and has shown that compared with l-CSC, e-CSC are smaller cells with faster growth kinetics, and higher levels of pluripotency marker expression. We also found that e-CSC uniquely expressed OCT4A variant 1 and had potential to differentiate into lineages of the three germ layers in vitro. In addition e-CSC and l-CSC express markers associated with primordial germ cells (PGC) and thus may share a developmental origin with these cells. Finally, they showed that e-CSC demonstrate higher tissue repair in vivo.
iPS from placental stem cells
Human amnion-derived cells (hADCs) are a heterogeneous group of multipotent progenitor cells that can be readily derived from placental tissue after delivery. It was recently demonstrated the capability of hADCs to give rise to iPS using lentivirus expressing OCT4, SOX2 and NANOG as transduction system. Staining of hADC–iPS colonies revealed the positive expression of AP, OCT4, SOX2, NANOG, SSEA-3, SSEA-4, TRA-1–60, and TRA-1–81 expression; moreover, hADc-iPS were able to form EBs expressing markers of the three embryonic germ layers. Teratoma-like masses containing mesoderm, ectoderm and endoderm proteins were observed 6–8 weeks after the injection of hADc-iPS into immunodeficient mice.Citation56 In conclusion, hADCs could be an ideal source to efficiently reprogram into individual-specific iPS cells.
Amniotic fluid stem cells (AFSC)
Human amniotic fluid (hAF) contains lines of broadly multipotent cells (hAFS cells) that can give rise to adipogenic, osteogenic, myogenic, endothelial, neurogenic and hepatic lineages, inclusive of all embryonic germ layers. hAFS cells grow easily in culture maintaining a stable phenotype and genotype. Approximately 1% of AF cells express the surface antigen c-Kit (CD117); these cells express a number of surface markers characteristic of mesenchymal and/or neural stem cells, but not embryonic stem (ES) cells, including CD44 (hyaluronan receptor), CD73, CD105 (endoglin);Citation17 90% of hAFSC express the pluripotency marker OCT4, NANOG and SSEA-4,Citation41 but they did not express other surface markers characteristic of embryonic stem cells as SSEA-3 and Tra-1-81.Citation17 As mentioned above, hAFSC had multipotent properties and exhibited the intrinsic capacity to differentiate into cell types indicative of the three germ layers. Since these cells did not form teratomas upon transplantation into mice, they could be considered for therapeutic applications.
Two different strategies to use AFSC in transplantation studies exist. One approach is based on the application of undifferentiated AFSC in the animal model; upon transplantation, hAFSC receive specific-tissue signals and are able to migrate to a specific microenvironment, proliferate and produce different progeny adapted to the tissue context. AFSC could contribute to the replacement of the specific cell types loss after organ or tissue damage.Citation57 The second approach regards the in vitro differentiation of the AFSC before transplantation. As reported in several studies, AFSC can be cultured in vitro for several passages and can be differentiated toward adipogenic, osteogenic, myogenic, and endothelial lineages.Citation17
Several studies indicated that AFSC could be largely expanded and have the capacity to attach and proliferate on biodegradable scaffolds. The expansion of AFSC can be achieved simultaneously with gestation and support the desired cells in time for surgical implantation in utero or after birth; this methods was used to the generation of cartilage grafts Citation58,Citation59 and tendon grafts for diaphragmatic hernia repair,Citation60,Citation61 with ovine mesenchymal AF-derived cells. AFSC osteogenically differentiated are able to give rise to tissue-engineered bone grafts after subcutaneous transplantation into immune-deficient mice.Citation17 Moreover, AFSC can be use also in congenital malformations of the heart to regenerate the functionality of the heart valves.Citation62 hAFSC were selected for the expression of CD133 surface marker in order to obtain the two cell types found in heart valves, namely myofibroblast like cells (CD133-) and endothelial cells (CD133+). The valve showed opening and closing capability after seeding of these cells on the heart valve scaffolds. Some applications of fetal stem cells isolated from human amniotic fluid in tissue engineering and cell replacement therapies are listed in the .
iPS from AFSC
The panel of genes expression characteristic of hAFSC designs these cells as “precursor” stem cells and, because the “precursor” state could be reprogrammed rapidly (6 days after infection) and efficiently, Citation17 hAFSCs seem to be a good candidate for cell reprogramming. Moreover, the simplicity of the collection of human amniotic fluid (hAF) specimens makes these cells an in vitro attractive model.
The reason for which amniocytes could be easier to reprogram to the iPS cell state than to somatic cell types is related to the similarity of their transcriptional and epigenetic states to early embryonic cell types.Citation63,Citation64 Thanks to their early embryonic origin, amniocytes may have accumulated less genetic damage or somatic mutation than older cell types. Moreover, amniocytes are autologous to the foetus and semi-allogeneic to each parent, thereby expanding the potential utility of AFiPS cells to other family members.Citation64 The main aim of the researchers in the field of iPSCs generation is to identify not only the “best” cell type but also the right protocol that guarantees the maximum efficiency, viability and safe of iPS colonies. Recently, the capacity of hAFSCs to generate iPSCs has been reported in several studies using defined protocols. For instance, to solve the problem of reprogramming efficiencies (~0.001%), several small molecular drugs, such as histone methyltransferase inhibitors,Citation65 an L-channel calcium agonist,Citation66,Citation67 Wnt inhibitors,Citation67 zinc finger nucleases,Citation68 rapamycin,Citation69 lithium,Citation70 and vitamin C,Citation71 have been used to increase the efficiency of reprogramming during the generation of iPS cells. CD34+ subpopulation cells isolated from hAFCs could generate iPS cell lines after infection with lentiviral constructs encoding only OCT4. The results showed high levels of AP in these cells and, immunofluorescence staining revealed increasing in the expression levels of NANOG, OCT4, SOX2, and REX1. The expression of these stem cells markers was ~5 to 120-fold higher in human iPS cells than in hAFCs after qRT-PCR analysis. Moreover, the in vivo study demonstrated that the injection of iPS cells into the hind leg of mice gave rise to teratomas contained cellular type representatives of all 3 germ layers. In another recent article iPS cells were derived by transduction of hAFSCs with a retroviral cocktail consisting of OCT4, SOX2, KLF4 and c-MYC.Citation72 AFiPSCs were characterized by analysis of alkaline phosphatase (AP) activity, expression of several markers of the undifferentiated state, including NANOG, OCT4, SOX2, SSEA-4, TRA-1-60, TRA-1-81; therefore, AFiPSCs exhibited a normal karyotype several passages after their generation and their genetic relatedness to primary AFCs cells was confirmed by DNA fingerprinting analysis. AFiPSCs were able to form derivatives of the three embryonic germ layers but also of the extra embryonic trophoblast lineage activating of BMP signaling cascades and blocking of TGFbeta/Activin/Nodal signalling. Although the generation of a nonviral iPSCs particularly from hAFSCs still remains a challenge, it Citation18 has been shown for the first time that functional AFiPS which express OCT4, SOX2, KLF4, C-MYC, and hESC-specific surface antigens, can be generated without ectopic reprogramming factors by culture on Matrigel in hESC medium supplemented with the histone deacetylase inhibitor (HDACi) valproic acid (VPA). Besides the expression of some MSC markers, such as CD73, CD44, CD105, fibronectin and laminin, the authors demonstrated in this paper that c-Kit+ human first-trimester AFSCs showed 82% transcriptome identity with hESCs and contained a subset of cells expressing the hESC-specific markers OCT4, NANOG, SSEA4, SOX2, KLF4, and C-MYC with 60% of the cells coexpressing SSEA-3, TRA-1-60, TRA-1-81 and ALP at clonal level. Moreover, AFSCs are able of forming embryoid bodies (EBs) in vitro and teratomas in vivo and capacity to differentiate into lineages of the three germ layers, such as definitive endoderm, hepatocytes, bone, fat, cartilage, neurons, and oligodendrocytes. Interestingly, the genetic stability, expression of key pluripotency factors, high cell-division kinetics, telomerase activity is maintained also after passages in culture. Citation18 Regarding the potential implication in regenerative medicine of AFSC_VPA, their results showed that upon differentiation, the levels of C-MYC expression are downregulated, indicating that differentiated AFSC_VPA may not be oncogenic and they could be used potentially in cell-based therapies. Citation18 In another fascinating work the same authors isolated hAFSC from 15-18 weeks of gestation (mid-trimester) showing that mid-trimester hAFSC express the MSC markers CD105, CD90, CD73, CD44 and CD29 along with a subset of cells expressing OCT4A, C-MYC and SSEA-4. Citation73 Compare to their previously findings on first-trimester AFSC,Citation18 mid-trimester hAFSC in MSC media showed low/null levels of NANOG, SOX2, KLF4, SSEA-3, TRA-1-60 or TRA-1-81. Citation73 Nevertheless, the culture in ES conditions and VPA supplementation for 5 days induced major upregulation of OCT4, SOX2, C-MYC and KLF4, with cells expressing NANOG, SSEA-3, SSEA-4, TRA-1-60 and TRA-1-81, gaining EBs and teratoma formation competency, showing that a chemical approach can also be used on this cell type.Citation73
Together, these data show that AFSC can be used to generate patient-specific iPS cells for use in regenerative medicine, pharmaceutical screening, and in disease modelling. In particular, the VPA treatment put attention on the existence of a reprogramming system in which the use of retroviral vectors can be avoided in order to guarantee the safety for a future clinical application of AFSC-derived cells.
Conclusions
The previously reported studies indicates that hAFSCs are easily reprogrammed by primary infection with a latency of 5–6 d, compared with about 10 d to induce iPS cell colonies from keratinocytes and 2 weeks or more from mouse embryonic fibroblasts (MEFs). hAFSCs offer several potential advantages for the generation of iPS cells compared with other somatic cell types,Citation74 such as adult human fibroblasts, MEFs, blood cells, adipose stem cells and keratinocytes. Moreover, the source of cells used to generate iPSCs may have an important impact on safety; for example, skin keratinocytes, although utilized by several groups for obtaining disease- and patient-specific iPSC lines, may have potential disadvantages. First, they have a considerably higher probability of harbouring silent genetic aberrations. Second, the establishment of keratinocyte or fibroblast cultures from patient skin biopsy specimens is a relatively lengthy procedure that could allow the accumulation and enrichment of cellular subpopulations harbouring mutations that may either hinder subsequent reprogramming or encourage clonal dominance.Citation75 hAFSC provide a safe source of cells that permit the generation of iPS cells with a significantly higher and efficiency, by more than 10-fold, relative to human dermal fibroblasts (HDFs); the efficiencies reported for adult human fibroblasts, MEFs, blood cells, adipose stem cells and keratinocytes are: 0.01, 0.001, 0.001, 0.2 and 0.002%, respectively.Citation64 Further, the pluripotent potential of hAFSCs due to the similarity of their transcriptional and epigenetic state to early embryonic cell types,Citation64 the non-invasive procedure of isolation from amniotic fluid and the simplicity of cell culturing make them an advantaged and safety source of iPSCs compare with the iPS from fetal MSCs, HSCs and placental stem cells, previously discussed. Thanks to their early embryonic origin, amniocytes may have accumulated less genetic damage or somatic mutation than the older FSCs. Not only iPSCs generation from hAFSCs do not require feeder cells but it is also possible using nonviral reprogramming methods, as recently described.Citation18 In conclusion, fetal tissues are a highly efficient target for iPS cells derivation; contains the major studies on reprogramming fetal cells and cells from extraembryonic tissues. Among different sources, the AF has the advantage to be taken during gestation with minimal risks both for the fetuses and the mother. Beside therapeutic use, isolation of hAFSCs from fetal with chromosomal anomalies such as Down Syndrome, Trisomy 18 or Trisomy 13, the consequently generation of AFiPSCs and their, in vitro differentiation, could be an interesting model to predict the outcome of these pathologies; not only, AFiPSCs could be used to identify novel pharmacological targets and to develop new therapeutic strategies due to improve the quality of life of affected newborns.
Table 4. Reprogramming of fetal cells and cells from extraembryonic tissues. The table summarizes the major studies on reprogramming fetal cells and cells from extraembryonic tissues using different methods
| Abbreviations: | ||
| hESCs | = | Human embryonic stem cells |
| SCNT | = | somatic cell nuclear transfer |
| OKSM | = | OCT3/4, SOX2, C-MYC, KLF4 |
| OSNL | = | OCT4, SOX2, NANOG, LIN28 |
| iPSCs | = | induced pluripotent stem cells |
| CNVs | = | copy number variations |
| MEFs | = | mouse embryonic fibroblasts |
| ViPS | = | retroviral iPS cells |
| EiPS | = | episomal iPS cells |
| FSCs | = | fetal stem cells |
| BM | = | bone marrow |
| HSCs | = | haematopoietic stem cells |
| GVHD | = | graft-versus-host disease |
| PB | = | peripheral blood |
| MNCs | = | human immature mononuclear cells |
| CB | = | umbilical cord blood |
| PB | = | adult peripheral blood |
| EBV | = | Epstein-Barr virus |
| UCB | = | umbilical cord blood |
| EBs | = | Embryoid bodies |
| shRNA | = | short-hairpin RNA |
| AMSC | = | amniotic mesenchymal cells |
| CSC | = | chorionic stem cells |
| CVS | = | chorionic villi cells |
| e-CSC | = | first trimester chorionic stem cells |
| l-CSC | = | term fetal chorionic stem cells |
| PGC | = | primordial germ cells |
| hADCs | = | Human amnion-derived cells |
| AFSC | = | Amniotic fluid stem cells |
| hAF | = | Human amniotic fluid |
| HDACi | = | histone deacetylase inhibitor |
| VPA | = | valproic acid |
| TERT | = | telomerase reverse transcriptase |
| Q-PCR | = | quantitative polymerase chain reaction |
| HDFs | = | human dermal fibroblasts |
Submitted
01/18/13
Revised
05/24/13
Accepted
05/28/13
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- Gurdon JB, Elsdale TR, Fischberg M. Sexually mature individuals of Xenopus laevis from the transplantation of single somatic nuclei. Nature 1958; 182:64 - 5; http://dx.doi.org/10.1038/182064a0; PMID: 13566187
- Chung Y, Bishop CE, Treff NR, Walker SJ, Sandler VM, Becker S, et al. Reprogramming of human somatic cells using human and animal oocytes. Cloning Stem Cells 2009; 11:213 - 23; http://dx.doi.org/10.1089/clo.2009.0004; PMID: 19186982
- Noggle S, Fung HL, Gore A, Martinez H, Satriani KC, Prosser R, et al. Human oocytes reprogram somatic cells to a pluripotent state. Nature 2011; 478:70 - 5; http://dx.doi.org/10.1038/nature10397; PMID: 21979046
- Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 2006; 126:663 - 76; http://dx.doi.org/10.1016/j.cell.2006.07.024; PMID: 16904174
- Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 2007; 131:861 - 72; http://dx.doi.org/10.1016/j.cell.2007.11.019; PMID: 18035408
- Yamanaka S. Induced pluripotent stem cells: past, present, and future. Cell Stem Cell 2012; 10:678 - 84; http://dx.doi.org/10.1016/j.stem.2012.05.005; PMID: 22704507
- Yu J, Vodyanik MA, Smuga-Otto K, Antosiewicz-Bourget J, Frane JL, Tian S, et al. Induced pluripotent stem cell lines derived from human somatic cells. Science 2007; 318:1917 - 20; http://dx.doi.org/10.1126/science.1151526; PMID: 18029452
- Aoi T, Yae K, Nakagawa M, Ichisaka T, Okita K, Takahashi K, et al. Generation of pluripotent stem cells from adult mouse liver and stomach cells. Science 2008; 321(5889): 699-702.12.
- Fusaki N, Ban H, Nishiyama A, Saeki K, Hasegawa M. Efficient induction of transgene-free human pluripotent stem cells using a vector based on Sendai virus, an RNA virus that does not integrate into the host genome. Proc Jpn Acad Ser B Phys Biol Sci 2009; 85:348 - 62; http://dx.doi.org/10.2183/pjab.85.348; PMID: 19838014
- Stadtfeld M, Nagaya M, Utikal J, Weir G, Hochedlinger K. Induced pluripotent stem cells generated without viral integration. Science 2008; 322:945 - 9; http://dx.doi.org/10.1126/science.1162494; PMID: 18818365
- Warren L, Manos PD, Ahfeldt T, Loh YH, Li H, Lau F, et al. Highly efficient reprogramming to pluripotency and directed differentiation of human cells with synthetic modified mRNA. Cell Stem Cell 2010; 7:618 - 30; http://dx.doi.org/10.1016/j.stem.2010.08.012; PMID: 20888316
- Kim D, Kim CH, Moon JI, Chung YG, Chang MY, Han BS, et al. Generation of human induced pluripotent stem cells by direct delivery of reprogramming proteins. Cell Stem Cell 2009; 4:472 - 6; http://dx.doi.org/10.1016/j.stem.2009.05.005; PMID: 19481515
- Gore A, Li Z, Fung HL, Young JE, Agarwal S, Antosiewicz-Bourget J, et al. Somatic coding mutations in human induced pluripotent stem cells. Nature 2011; 471:63 - 7; http://dx.doi.org/10.1038/nature09805; PMID: 21368825
- Hussein SM, Batada NN, Vuoristo S, Ching RW, Autio R, Närvä E, et al. Copy number variation and selection during reprogramming to pluripotency. Nature 2011; 471:58 - 62; http://dx.doi.org/10.1038/nature09871; PMID: 21368824
- Zhao T, Zhang ZN, Rong Z, Xu Y. Immunogenicity of induced pluripotent stem cells. Nature 2011; 474:212 - 5; http://dx.doi.org/10.1038/nature10135; PMID: 21572395
- Abdulrazzak H, Moschidou D, Jones G, Guillot PV. Biological characteristics of stem cells from foetal, cord blood and extraembryonic tissues. J R Soc Interface 2010; 7:Suppl 6 S689 - 706; http://dx.doi.org/10.1098/rsif.2010.0347.focus; PMID: 20739312
- De Coppi P, Bartsch G Jr., Siddiqui MM, Xu T, Santos CC, Perin L, et al. Isolation of amniotic stem cell lines with potential for therapy. Nat Biotechnol 2007; 25:100 - 6; http://dx.doi.org/10.1038/nbt1274; PMID: 17206138
- Moschidou D, Mukherjee S, Blundell MP, Drews K, Jones GN, Abdulrazzak H, et al. Valproic acid confers functional pluripotency to human amniotic fluid stem cells in a transgene-free approach. Mol Ther 2012; 20:1953 - 67; http://dx.doi.org/10.1038/mt.2012.117; PMID: 22760542
- Hacein-Bey-Abina S, Von Kalle C, Schmidt M, McCormack MP, Wulffraat N, Leboulch P, et al. LMO2-associated clonal T cell proliferation in two patients after gene therapy for SCID-X1. Science 2003; 302:415 - 9; http://dx.doi.org/10.1126/science.1088547; PMID: 14564000
- Pappa KI, Anagnou NP. Novel sources of fetal stem cells: where do they fit on the developmental continuum?. Regen Med 2009; 4:423 - 33; http://dx.doi.org/10.2217/rme.09.12; PMID: 19438317
- Marcus AJ, Woodbury D. Fetal stem cells from extra-embryonic tissues: do not discard. J Cell Mol Med 2008; 12:730 - 42; http://dx.doi.org/10.1111/j.1582-4934.2008.00221.x; PMID: 18194447
- Cananzi M, Atala A, De Coppi P. Stem cells derived from amniotic fluid: new potentials in regenerative medicine. Reprod Biomed Online 2009; 18:Suppl 1 17 - 27; http://dx.doi.org/10.1016/S1472-6483(10)60111-3; PMID: 19281660
- Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, et al. Multilineage potential of adult human mesenchymal stem cells. Science 1999; 284:143 - 7; http://dx.doi.org/10.1126/science.284.5411.143; PMID: 10102814
- Pozzobon M, Ghionzoli M, De Coppi PES. ES, iPS, MSC, and AFS cells. Stem cells exploitation for Pediatric Surgery: current research and perspective. Pediatr Surg Int 2010; 26:3 - 10; http://dx.doi.org/10.1007/s00383-009-2478-8; PMID: 19727766
- Guillot PV, Gotherstrom C, Chan J, Kurata H, Fisk NM. Human first-trimester fetal MSC express pluripotency markers and grow faster and have longer telomeres than adult MSC. Stem Cells 2007; 25:646 - 54; http://dx.doi.org/10.1634/stemcells.2006-0208; PMID: 17124009
- Campagnoli C, Roberts IA, Kumar S, Bennett PR, Bellantuono I, Fisk NM. Identification of mesenchymal stem/progenitor cells in human first-trimester fetal blood, liver, and bone marrow. Blood 2001; 98:2396 - 402; http://dx.doi.org/10.1182/blood.V98.8.2396; PMID: 11588036
- Götherström C, West A, Liden J, Uzunel M, Lahesmaa R, Le Blanc K. Difference in gene expression between human fetal liver and adult bone marrow mesenchymal stem cells. Haematologica 2005; 90:1017 - 26; PMID: 16079100
- Mackenzie TC, Flake AW. Multilineage differentiation of human MSC after in utero transplantation. Cytotherapy 2001; 3:403 - 5; http://dx.doi.org/10.1080/146532401753277571; PMID: 11953022
- Kennea NL, Waddington SN, Chan J, O’Donoghue K, Yeung D, Taylor DL, et al. Differentiation of human fetal mesenchymal stem cells into cells with an oligodendrocyte phenotype. Cell Cycle 2009; 8:1069 - 79; http://dx.doi.org/10.4161/cc.8.7.8121; PMID: 19270512
- Taylor PA, McElmurry RT, Lees CJ, Harrison DE, Blazar BR. Allogenic fetal liver cells have a distinct competitive engraftment advantage over adult bone marrow cells when infused into fetal as compared with adult severe combined immunodeficient recipients. Blood 2002; 99:1870 - 2; http://dx.doi.org/10.1182/blood.V99.5.1870; PMID: 11861310
- MacKenzie TS, Campagnoli C, Almeida-Porada G. Circulating human fetal stromal cells engraft and differentiate in multiple tissues following transplantation into pre-immune fetal lambs. Blood 2001; 98:328a
- Weissman IL, Shizuru JA. The origins of the identification and isolation of hematopoietic stem cells, and their capability to induce donor-specific transplantation tolerance and treat autoimmune diseases. Blood 2008; 112:3543 - 53; http://dx.doi.org/10.1182/blood-2008-08-078220; PMID: 18948588
- Huss R. Perspectives on the morphology and biology of CD34-negative stem cells. J Hematother Stem Cell Res 2000; 9:783 - 93; http://dx.doi.org/10.1089/152581600750062228; PMID: 11177591
- Bigas A, D’Altri T, Espinosa L. The Notch pathway in hematopoietic stem cells. Curr Top Microbiol Immunol 2012; 360:1 - 18; http://dx.doi.org/10.1007/82_2012_229; PMID: 22692832
- Lim FT, Kanhai HH, Falkenburg JH. Characterization of the human CD34+ hematopoietic progenitor cell compartment during the second trimester of pregnancy. Haematologica 2005; 90:173 - 9; PMID: 15710568
- Wagers AJ, Allsopp RC, Weissman IL. Changes in integrin expression are associated with altered homing properties of Lin(-/lo)Thy1.1(lo)Sca-1(+)c-kit(+) hematopoietic stem cells following mobilization by cyclophosphamide/granulocyte colony-stimulating factor. Exp Hematol 2002; 30:176 - 85; http://dx.doi.org/10.1016/S0301-472X(01)00777-9; PMID: 11823053
- Broxmeyer HE. Cord blood hematopoietic stem cell transplantation. StemBook [Internet]. Cambridge (MA): Harvard Stem Cell Institute 2008.
- Mazurier F, Doedens M, Gan OI, Dick JE. Rapid myeloerythroid repopulation after intrafemoral transplantation of NOD-SCID mice reveals a new class of human stem cells. Nat Med 2003; 9:959 - 63; http://dx.doi.org/10.1038/nm886; PMID: 12796774
- Wang J, Kimura T, Asada R, Harada S, Yokota S, Kawamoto Y, et al. SCID-repopulating cell activity of human cord blood-derived CD34- cells assured by intra-bone marrow injection. Blood 2003; 101:2924 - 31; http://dx.doi.org/10.1182/blood-2002-09-2782; PMID: 12480697
- Chou BK, Mali P, Huang X, Ye Z, Dowey SN, Resar LM, et al. Efficient human iPS cell derivation by a non-integrating plasmid from blood cells with unique epigenetic and gene expression signatures. Cell Res 2011; 21:518 - 29; http://dx.doi.org/10.1038/cr.2011.12; PMID: 21243013
- Delaney C, Ratajczak MZ, Laughlin MJ. Strategies to enhance umbilical cord blood stem cell engraftment in adult patients. Expert Rev Hematol 2010; 3:273 - 83; http://dx.doi.org/10.1586/ehm.10.24; PMID: 20835351
- McGuckin CP, Forraz N, Allouard Q, Pettengell R. Umbilical cord blood stem cells can expand hematopoietic and neuroglial progenitors in vitro. Exp Cell Res 2004; 295:350 - 9; http://dx.doi.org/10.1016/j.yexcr.2003.12.028; PMID: 15093735
- Takenaka C, Nishishita N, Takada N, Jakt LM, Kawamata S. Effective generation of iPS cells from CD34+ cord blood cells by inhibition of p53. Exp Hematol 2010; 38:154 - 62; http://dx.doi.org/10.1016/j.exphem.2009.11.003; PMID: 19922768
- Broxmeyer HE, Lee MR, Hangoc G, Cooper S, Prasain N, Kim YJ, et al. Hematopoietic stem/progenitor cells, generation of induced pluripotent stem cells, and isolation of endothelial progenitors from 21- to 23.5-year cryopreserved cord blood. Blood 2011; 117:4773 - 7; http://dx.doi.org/10.1182/blood-2011-01-330514; PMID: 21393480
- Parolini O, Alviano F, Bergwerf I, Boraschi D, De Bari C, De Waele P, et al. Toward cell therapy using placenta-derived cells: disease mechanisms, cell biology, preclinical studies, and regulatory aspects at the round table. Stem Cells Dev 2010; 19:143 - 54; http://dx.doi.org/10.1089/scd.2009.0404; PMID: 19947828
- Tamagawa T, Oi S, Ishiwata I, Ishikawa H, Nakamura Y. Differentiation of mesenchymal cells derived from human amniotic membranes into hepatocyte-like cells in vitro. Hum Cell 2007; 20:77 - 84; http://dx.doi.org/10.1111/j.1749-0774.2007.00032.x; PMID: 17645727
- Miki T, Lehmann T, Cai H, Stolz DB, Strom SC. Stem cell characteristics of amniotic epithelial cells. Stem Cells 2005; 23:1549 - 59; http://dx.doi.org/10.1634/stemcells.2004-0357; PMID: 16081662
- Miki T, Strom SC. Amnion-derived pluripotent/multipotent stem cells. Stem Cell Rev 2006; 2:133 - 42; http://dx.doi.org/10.1007/s12015-006-0020-0; PMID: 17237552
- Jones GN, Moschidou D, Puga-Iglesias TI, Kuleszewicz K, Vanleene M, Shefelbine SJ, et al. Ontological differences in first compared to third trimester human fetal placental chorionic stem cells. PLoS One 2012; 7:e43395; http://dx.doi.org/10.1371/journal.pone.0043395; PMID: 22962584
- Bieback K, Brinkmann I. Mesenchymal stromal cells from human perinatal tissues: From biology to cell therapy. World J Stem Cells 2010; 2:81 - 92; http://dx.doi.org/10.4252/wjsc.v2.i4.81; PMID: 21607124
- Spitalieri P, Cortese G, Pietropolli A, Filareto A, Dolci S, Klinger FG, et al. Identification of multipotent cytotrophoblast cells from human first trimester chorionic villi. Cloning Stem Cells 2009; 11:535 - 56; http://dx.doi.org/10.1089/clo.2009.0046; PMID: 20025524
- Portmann-Lanz CB, Schoeberlein A, Huber A, Sager R, Malek A, Holzgreve W, et al. Placental mesenchymal stem cells as potential autologous graft for pre- and perinatal neuroregeneration. Am J Obstet Gynecol 2006; 194:664 - 73; http://dx.doi.org/10.1016/j.ajog.2006.01.101; PMID: 16522395
- Ilancheran S, Michalska A, Peh G, Wallace EM, Pera M, Manuelpillai U. Stem cells derived from human fetal membranes display multilineage differentiation potential. Biol Reprod 2007; 77:577 - 88; http://dx.doi.org/10.1095/biolreprod.106.055244; PMID: 17494917
- Chien CC, Yen BL, Lee FK, Lai TH, Chen YC, Chan SH, et al. In vitro differentiation of human placenta-derived multipotent cells into hepatocyte-like cells. Stem Cells 2006; 24:1759 - 68; http://dx.doi.org/10.1634/stemcells.2005-0521; PMID: 16822884
- Huang HI. Isolation of human placenta-derived multipotent cells and in vitro differentiation into hepatocyte-like cells. Curr Protoc Stem Cell Biol 2007; Chapter 1:1; PMID: 18785166
- Zhao HX, Li Y, Jin HF, Xie L, Liu C, Jiang F, et al. Rapid and efficient reprogramming of human amnion-derived cells into pluripotency by three factors OCT4/SOX2/NANOG. Differentiation 2010; 80:123 - 9; http://dx.doi.org/10.1016/j.diff.2010.03.002; PMID: 20510497
- Klemmt PA, Vafaizadeh V, Groner B. The potential of amniotic fluid stem cells for cellular therapy and tissue engineering. Expert Opin Biol Ther 2011; 11:1297 - 314; http://dx.doi.org/10.1517/14712598.2011.587800; PMID: 21623704
- Kunisaki SM, Jennings RW, Fauza DO. Fetal cartilage engineering from amniotic mesenchymal progenitor cells. Stem Cells Dev 2006; 15:245 - 53; http://dx.doi.org/10.1089/scd.2006.15.245; PMID: 16646670
- Kunisaki SM, Fuchs JR, Steigman SA, Fauza DO. A comparative analysis of cartilage engineered from different perinatal mesenchymal progenitor cells. Tissue Eng 2007; 13:2633 - 44; http://dx.doi.org/10.1089/ten.2006.0407; PMID: 17655491
- Kunisaki SM, Fuchs JR, Kaviani A, Oh JT, LaVan DA, Vacanti JP, et al. Diaphragmatic repair through fetal tissue engineering: a comparison between mesenchymal amniocyte- and myoblast-based constructs. J Pediatr Surg 2006; 41:34 - 9, discussion 34-9; http://dx.doi.org/10.1016/j.jpedsurg.2005.10.011; PMID: 16410104
- Fuchs JR, Kaviani A, Oh JT, LaVan D, Udagawa T, Jennings RW, et al. Diaphragmatic reconstruction with autologous tendon engineered from mesenchymal amniocytes. J Pediatr Surg 2004; 39:834 - 8, discussion 834-8; http://dx.doi.org/10.1016/j.jpedsurg.2004.02.014; PMID: 15185207
- Schmidt D, Achermann J, Odermatt B, Breymann C, Mol A, Genoni M, et al. Prenatally fabricated autologous human living heart valves based on amniotic fluid derived progenitor cells as single cell source. Circulation 2007; 116:Suppl I64 - 70; http://dx.doi.org/10.1161/CIRCULATIONAHA.106.681494; PMID: 17846327
- Li C, Zhou J, Shi G, Ma Y, Yang Y, Gu J, et al. Pluripotency can be rapidly and efficiently induced in human amniotic fluid-derived cells. Hum Mol Genet 2009; 18:4340 - 9; http://dx.doi.org/10.1093/hmg/ddp386; PMID: 19679563
- Anchan RM, Quaas P, Gerami-Naini B, Bartake H, Griffin A, Zhou Y, et al. Amniocytes can serve a dual function as a source of iPS cells and feeder layers. Hum Mol Genet 2011; 20:962 - 74; http://dx.doi.org/10.1093/hmg/ddq542; PMID: 21156717
- Shi Y, Desponts C, Do JT, Hahm HS, Schöler HR, Ding S. Induction of pluripotent stem cells from mouse embryonic fibroblasts by Oct4 and Klf4 with small-molecule compounds. Cell Stem Cell 2008; 3:568 - 74; http://dx.doi.org/10.1016/j.stem.2008.10.004; PMID: 18983970
- Park J, Kim C, Tang Y, Amano T, Lin CJ, Tian XC. Reprogramming of mouse fibroblasts to an intermediate state of differentiation by chemical induction. Cell Reprogram 2011; 13:121 - 31; http://dx.doi.org/10.1089/cell.2010.0067; PMID: 21473689
- Ren Y, Lee MY, Schliffke S, Paavola J, Amos PJ, Ge X, et al. Small molecule Wnt inhibitors enhance the efficiency of BMP-4-directed cardiac differentiation of human pluripotent stem cells. J Mol Cell Cardiol 2011; 51:280 - 7; http://dx.doi.org/10.1016/j.yjmcc.2011.04.012; PMID: 21569778
- Sebastiano V, Maeder ML, Angstman JF, Haddad B, Khayter C, Yeo DT, et al. In situ genetic correction of the sickle cell anemia mutation in human induced pluripotent stem cells using engineered zinc finger nucleases. Stem Cells 2011; 29:1717 - 26; http://dx.doi.org/10.1002/stem.718; PMID: 21898685
- Chen T, Shen L, Yu J, Wan H, Guo A, Chen J, et al. Rapamycin and other longevity-promoting compounds enhance the generation of mouse induced pluripotent stem cells. Aging Cell 2011; 10:908 - 11; http://dx.doi.org/10.1111/j.1474-9726.2011.00722.x; PMID: 21615676
- Wang Q, Xu X, Li J, Liu J, Gu H, Zhang R, et al. Lithium, an anti-psychotic drug, greatly enhances the generation of induced pluripotent stem cells. Cell Res 2011; 21:1424 - 35; http://dx.doi.org/10.1038/cr.2011.108; PMID: 21727907
- Esteban MA, Wang T, Qin B, Yang J, Qin D, Cai J, et al. Vitamin C enhances the generation of mouse and human induced pluripotent stem cells. Cell Stem Cell 2010; 6:71 - 9; http://dx.doi.org/10.1016/j.stem.2009.12.001; PMID: 20036631
- Liu T, Zou G, Gao Y, Zhao X, Wang H, Huang Q, et al. High efficiency of reprogramming CD34⁺ cells derived from human amniotic fluid into induced pluripotent stem cells with Oct4. Stem Cells Dev 2012; 21:2322 - 32; http://dx.doi.org/10.1089/scd.2011.0715; PMID: 22264161
- Moschidou D, Mukherjee S, Blundell MP, Jones GN, Atala AJ, Thrasher AJ, et al. Human mid-trimester amniotic fluid stem cells cultured under embryonic stem cell conditions with valproic acid acquire pluripotent characteristics. Stem Cells Dev 2013; 22:444 - 58; http://dx.doi.org/10.1089/scd.2012.0267; PMID: 23050522
- Galende E, Karakikes I, Edelmann L, Desnick RJ, Kerenyi T, Khoueiry G, et al. Amniotic fluid cells are more efficiently reprogrammed to pluripotency than adult cells. Cell Reprogram 2010; 12:117 - 25; http://dx.doi.org/10.1089/cell.2009.0077; PMID: 20677926
- Mukherjee S, Thrasher AJ. iPSCs: Unstable origins?. Mol Ther 2011; 19:1188 - 90; http://dx.doi.org/10.1038/mt.2011.116; PMID: 21720379
- Fan Y, Luo Y, Chen X, Li Q, Sun X. Generation of human β-thalassemia induced pluripotent stem cells from amniotic fluid cells using a single excisable lentiviral stem cell cassette. J Reprod Dev 2012; 58:404 - 9; http://dx.doi.org/10.1262/jrd.2011-046; PMID: 22498813
- Cai J, Li W, Su H, Qin D, Yang J, Zhu F, et al. Generation of human induced pluripotent stem cells from umbilical cord matrix and amniotic membrane mesenchymal cells. J Biol Chem 2010; 285:11227 - 34; http://dx.doi.org/10.1074/jbc.M109.086389; PMID: 20139068
- Guillot PV, Abass O, Bassett JH, Shefelbine SJ, Bou-Gharios G, Chan J, et al. Intrauterine transplantation of human fetal mesenchymal stem cells from first-trimester blood repairs bone and reduces fractures in osteogenesis imperfecta mice. Blood 2008; 111:1717 - 25; http://dx.doi.org/10.1182/blood-2007-08-105809; PMID: 17967940
- Le Blanc K, Götherström C, Ringdén O, Hassan M, McMahon R, Horwitz E, et al. Fetal mesenchymal stem-cell engraftment in bone after in utero transplantation in a patient with severe osteogenesis imperfecta. Transplantation 2005; 79:1607 - 14; http://dx.doi.org/10.1097/01.TP.0000159029.48678.93; PMID: 15940052
- Ersek A, Pixley JS, Goodrich AD, Porada CD, Almeida-Porada G, Thain DS, et al. Persistent circulating human insulin in sheep transplanted in utero with human mesenchymal stem cells. Exp Hematol 2010; 38:311 - 20; http://dx.doi.org/10.1016/j.exphem.2010.02.005; PMID: 20170708
- Carraro G, Perin L, Sedrakyan S, Giuliani S, Tiozzo C, Lee J, et al. Human amniotic fluid stem cells can integrate and differentiate into epithelial lung lineages. Stem Cells 2008; 26:2902 - 11
- Yeh YC, Lee WY, Yu CL, Hwang SM, Chung MF, Hsu LW, et al. Cardiac repair with injectable cell sheet fragments of human amniotic fluid stem cells in an immune-suppressed rat model. Biomaterials 2010; 31:6444 - 53; http://dx.doi.org/10.1016/j.biomaterials.2010.04.069; PMID: 20621766
- De Coppi P, Callegari A, Chiavegato A, Gasparotto L, Piccoli M, Taiani J, et al. Amniotic fluid and bone marrow derived mesenchymal stem cells can be converted to smooth muscle cells in the cryo-injured rat bladder and prevent compensatory hypertrophy of surviving smooth muscle cells. J Urol 2007; 177:369 - 76
- Kong XY, Cai Z, Pan L, Zhang L, Shu J, Dong YL, et al. Transplantation of human amniotic cells exerts neuroprotection in MPTP-induced Parkinson disease mice. Brain Res 2008; 1205:108 - 15; http://dx.doi.org/10.1016/j.brainres.2008.02.040; PMID: 18353283