Abstract
Division of large, immature alveolar structures into smaller, more numerous alveoli increases the surface area available for gas exchange. Alveolar division requires precise epithelial-mesenchymal interactions. However, few experimental models exist for studying how these cell-cell interactions produce changes in 3-dimensional structure. Here we report an epithelial-mesenchymal cell co-culture model where 3-dimensional peaks form with similar cellular orientation as alveolar structures in vivo. Co-culturing fetal mouse lung mesenchyme with A549 epithelial cells produced tall peaks of cells covered by epithelia with cores of mesenchymal cells. These structures did not form when using adult lung fibroblasts. Peak formation did not require localized areas of cell proliferation or apoptosis. Mesenchymal cells co-cultured with epithelia adopted an elongated cell morphology closely resembling myofibroblasts within alveolar septa in vivo. Because inflammation inhibits alveolar formation, we tested the effects of E. coli lipopolysaccharide on 3-dimensional peak formation. Confocal and time-lapse imaging demonstrated that lipopolysaccharide reduced mesenchymal cell migration, resulting in fewer, shorter peaks with mesenchymal cells present predominantly at the base. This epithelial-mesenchymal co-culture model may therefore prove useful in future studies of mechanisms regulating alveolar morphogenesis.
Abbreviations
| 3-D | = | 3-dimensional |
| α-SMA | = | alpha-smooth muscle actin |
| ATCC | = | American Type Culture Collection |
| BALB/cJ | = | Bagg Albino |
| BMP4 | = | bone morphogenetic protein 4 |
| CO2 | = | carbon dioxide |
| DAPI | = | 4′, 6-Diamidino-2-Phenylindole, Dihydrochloride |
| DEVD | = | acetyl-Asp-Glu-Val-Asp p-nitroanilide |
| DiI | = | 1, 1′-dioctadecyl-3, 3, 3′3′-tetramethylindocarbocyanine perchlorate |
| DMEM | = | Dulbecco's modified eagle medium |
| E-cad | = | e-cadherin |
| E. coli | = | Escherichia coli |
| E15 | = | embryonic day 15 |
| FBS | = | fetal bovine serum |
| FGF | = | fibroblast growth factor |
| LPS | = | lipopolysaccharide |
| PDGF | = | platelet derived growth factor |
| SHH | = | sonic hedgehog |
| TGF-β | = | transforming growth factor beta |
| TO-PRO-3 | = | 4-[3-(3-methyl-2(3H)-benzothiazolylidene)-1-propenyl]-1-[3-(trimethylammonio)propyl]-, diiodide |
| VEGF | = | vascular endothelial growth factor |
| Z-VAD-FMK | = | Z-Val-Ala-Asp-CH2F |
Introduction
Interactions between epithelia and surrounding mesenchyme guide the 3-dimensional (3-D) formation of airway and alveolar structures in the developing lung. During the initial embryonal stage of lung development, epithelial tubes extend from the endoderm into a mass of surrounding mesenchymal cells.Citation1,2 As the developing airways undergo multiple iterations of branching morphogenesis during the pseudoglandular and canalicular stages, the lung mesenchyme is steadily displaced by more numerous airspaces. By the end of the saccular stage, airway branching completes and each terminal air sac is surrounded by a thin layer of mesenchymal cells.Citation3,4 As opposed to airway branching, a different developmental paradigm divides these immature air sacs into smaller, more numerous alveolar structures. During alveolar formation, mesenchymal myofibroblasts divide air sacs by migrating into divisions or septa while maintaining epithelial-mesenchymal orientations. The newly formed alveolar septa contain cores of mesenchymal cells surrounded by alveolar epithelia. This process continues until the final, mature lung structure is formed.Citation5-8
Signaling between epithelia and mesenchyme guides lung morphogenesis. Compartmentalization and spatial restriction of morphogens and growth factors control the pattern of airway branching. Among the growth factors expressed in the lung mesenchyme, FGF-7 and FGF-10 promote cell proliferation and airway morphogenesis by binding FGF receptors localized to airway epithelia.Citation9-11 The epithelial cells in the developing lung in turn express factors including SHH, BMP4, and VEGF that regulate differentiation and dynamics of adjacent mesenchymal cells.Citation12-16 While the molecular mechanisms regulating airway branching morphogenesis have been well studied, the processes regulating epithelial-mesenchymal interactions during alveolar septa formation are less understood. During septa formation, epithelial cells maintain their apical orientations with the airspace with the underlying mesenchymal cells pushing up from the basal side.Citation6,17,18 Current experimental models have not been able to study the dynamics of septa formation. While genetic mouse models have proven excellent for studying developmental mechanisms during lung morphogenesis,Citation19,20 direct visualization of cellular processes and detailed mechanistic studies are often difficult to study in vivo. Culturing explanted fetal mouse lung tissue has also been very useful for investigating airway morphogenesis in the embryonal through saccular stages of fetal lung development.Citation21-24 However, maintaining and culturing the air filled alveolar stage lungs from mice and rats ex vivo has been difficult.
A high throughput experimental model system for studying alveolar morphogenesis would facilitate a more detailed dissection of the processes required for this latest stage of lung development. Here we report an in vitro epithelial-mesenchymal co-culture system that models the development of 3-dimensional structures during alveolar septa formation. As an epithelial cell source, we chose the human bronchioalveolar carcinoma cell line A549, which shares properties with immature alveolar Type II cells.Citation25-27 The use of this particular immortalized cell line also provides experimental reproducibility compared with isolates of primary alveolar epithelia. We co-cultured A549 cells on a confluent layer of primary fetal mouse lung mesenchyme,Citation28,29 keeping the apical surface of the epithelial cells exposed to the media and the basal surface of the epithelia in contact with the underlying mesenchyme. As these cells were co-cultured, they spontaneously formed 3-dimensional peaks but maintained this epithelial-mesenchymal orientation. These peaks had a similar appearance and orientation as alveolar septa in vivo. By imaging co-cultures using live-cell microscopy, we were able to investigate the dynamics of this process. This epithelial-mesenchymal co-culture system may therefore prove useful for studying the basic molecular mechanisms regulating alveolar septa formation.
Results
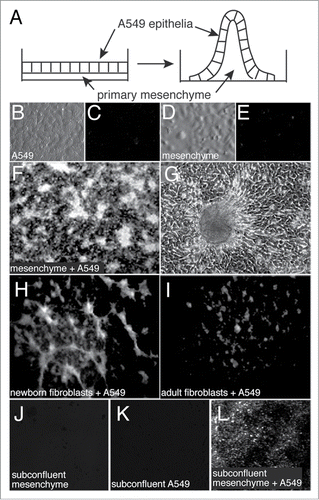
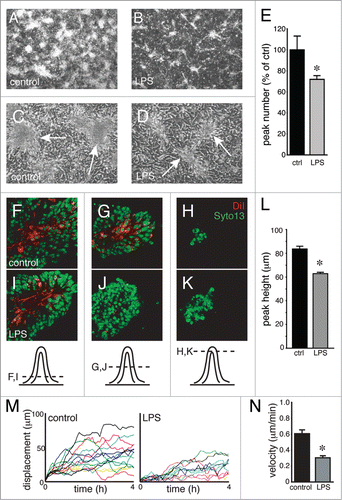
To develop a model system for studying epithelial-mesenchymal interactions during lung morphogenesis, we first isolated cultures of primary mesenchyme from E15 fetal mouse lungs. When grown to confluence, both the mesenchymal and A549 cells formed flat monolayers without any observable 3-dimensional structure (). A549 epithelial cells were then overlaid onto confluent mesenchymal cells at high density. Following 3 d of co-culture, 3-D peaks and ridges formed as seen by both dark-field and phase-contrast microscopy (). Using primary newborn mouse lung fibroblasts in place of E15 mesenchyme produced fewer ridges and peaks, and primary adult lung fibroblasts rarely gave rise to any structures detectable by dark field microscopy (). Plating of sub-confluent amounts of E15 mesenchyme and A549 cells resulted in some cell ridges and mounds, but not the tall peaks seen with confluent cell cultures ().
Figure 1. Formation of 3-dimensional structures in epithelial-mesenchymal co-cultures. (A) Diagram of epithelial-mesenchymal co-culture system. (B–E). Images of A549 epithelia and primary mesenchyme cultured alone and imaged by varel optics (B and D) or dark field microscopy (C and E). (F) Dark field image of epithelial-mesenchymal co-culture following three days of co-culture. Bright structures are peaks that rise from the culture dish. (G) Phase contrast image of a 3-dimensional peak with adjacent monolayer of cells. (H and I) Dark field images of co-cultures using newborn lung fibroblasts (H) or adult lung fibroblasts (I). (J–L) Fewer 3-dimensional structures were observed when using subconfluent cultures.
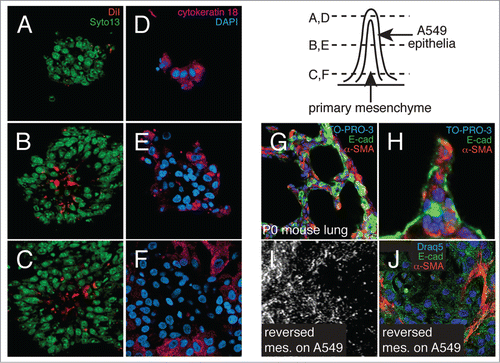
To determine cellular orientation within these structures, we differentially labeled the individual cell populations and imaged co-cultures by confocal microscopy (). Mesenchymal cells labeled with DiI prior to co-culture localized to the inner core of 3-D peaks and were covered by DiI-negative cells (). The cells along the outside and top of the cell peaks expressed the epithelial marker cytokeratin18 (), confirming that the peaks contained central cores of mesenchyme covered by A549 epithelia. This orientation resembled the epithelial-mesenchymal orientation present in the newborn mouse lung, where septa dividing immature alveoli contain cores of α-SMA-expressing myofibroblasts covered by epithelial cells (). The cellular orientation of the co-cultures may be important, as reversing the procedure and adding mesenchymal cells to confluent A549 monolayers did not form the same large 3-D structures (). Interestingly, we did observe more linear morphology in mesenchymal cells added to A549 epithelia, suggesting epithelial-mesenchymal interactions influenced mesenchymal cell shape and morphology.
Figure 2. Orientation of epithelial and mesenchymal cells within 3-dimensional peaks. (A–C) Confocal sections through co-cultures in which mesenchyme was labeled with DiI prior to addition of epithelial cells. Nuclei were labeled with SYTO 13. (D–F) Confocal images of co-cultures immunostained for cytokeratin 18 expression. Nuclei were labeled with DAPI. 3-dimensional peaks have cores of mesenchyme covered by epithelial cells. (G and H) Confocal sections through newborn mouse lungs showing developing alveolar septa are comprised of mesenchymal cell cores (α-SMA-positive cells, red) covered by epithelia (E-cad, green). Nuclei labeled with TO-PRO-3. (I and J) Adding mesenchymal cells to confluent monolayers of A549 epithelia (reversing the order) produced ridges containing elongated mesenchymal cells, but not the tall peaks and numerous ridges seen in A-F. (I) Darkfield image. (J) Confocal image of immunostained co-culture. (E-cadherin, green; α-SMA, red; nuclei labeled with Draq5).
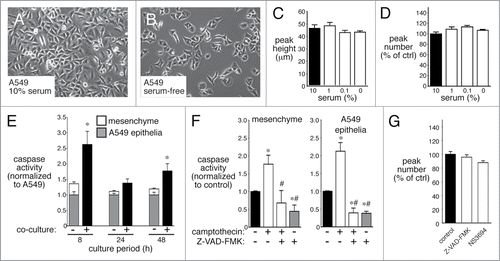
The 3-D peaks of cells could result from localized areas of increased proliferation. We therefore tested if serum deprivation and subsequent inhibition of cell proliferation could reduce epithelial-mesenchymal 3-D peak formation. Reducing serum concentration from 10% to 0% reduced overall cell proliferation, but had no effect on either peak number or peak height (). In the presence of serum, only epithelia comprised the areas between peaks. To test the possible role of apoptosis, we first measured caspase 3/7 activity in fetal lung mesenchymal cells, A549 epithelia alone, and co-culture. As seen in , co-culturing epithelial and mesenchymal cells did increase caspase 3/7 activity above levels measured when either cell type was cultured separately. While Z-VAD-FMK did inhibit caspase 3/7 activity in both cells cultured alone and in co-cultures, it had no effects on formation of 3-D peaks (). These data suggest that 3-D epithelial-mesenchymal peak formation did not require cell proliferation or apoptosis.
Figure 3. 3-dimensional peak formation does not require cell proliferation or apoptosis. (A–D) Serum deprivation for 48 h slows epithelial proliferation (B), but does not alter 3-dimensional peak height (C) or peak number (D) (n = 6). (E). Co-culturing A549 epithelia with mesenchymal cells increases apoptosis as measured by caspase 3/7 activity. Apoptosis was measured both in cells cultured alone (white and gray bars) and in co-cultures (black bar) (* P < 0.05; n = 6). (F and G). Apoptosis inhibitors Z-VAD-FMK and NS3694 reduce caspase 3/7 activity (F; * P < 0.05 compared with control, # P < 0.05 compared with camptothecin alone, n = 12), but have no effect on 3-dimensional peak number (G; n = 20).
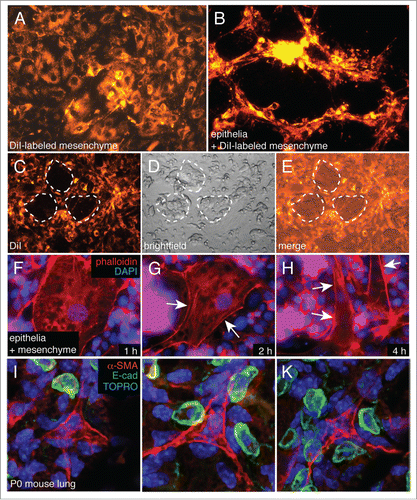
Mesenchymal cells grew as flat monolayers. Once epithelia were added, we observed mesenchymal cells in 3-D peaks and ridges (). To assess the dynamics of these epithelial-mesenchymal interactions, we added a reduced number of epithelial cells to DiI labeled mesenchyme As seen in , mesenchymal cells vacated locations where epithelial cells initially attached, suggesting cell repulsion away from areas of epithelial-mesenchymal contact. Higher magnification fluorescence imaging of epithelial-mesenchymal co-cultures demonstrated that addition of epithelial cells altered mesenchymal cell morphology, causing cells to become more elongated (). This interaction closely resembled the changes in mesenchymal cell morphology observed in the newborn mouse lung, where alveolar myofibroblasts become elongated with cellular processes localized between epithelial Type II cells (). These spatial relationships suggest that epithelial cells repel mesenchymal cell attachment, promoting migration of elongated cells into 3-D structures.
Figure 4. Epithelial cells appear to repel mesenchymal cells in co-culture. (A and B) DiI labeled mesenchymal cells begin to form 3-dimensional peaks and ridges following 18 h of co-culture. (C–E) DiI labeled mesenchyme was co-cultured with a reduced number of epithelia, allowing islands of epithelia to form within the co-cultures (dotted lines). Mesenchymal cells were excluded from these islands (C). (F–H) Epithelial cells stimulate mesenchymal cell elongation. Mesenchymal cells were cultured with reduced numbers of cells as in (C and D). Actin cytoskeleton was visualized using Alexa594-phalloidin. Nuclei were labeled with DAPI. Arrows indicate areas of apparent membrane retraction. (I–K) Orientation of alveolar Type II cells (E-cad positive, green) with mesenchymal cells (α-SMA positive, red) in newborn mouse lungs. Mesenchymal cell membrane processes extend between Type II cells, suggesting possible epithelial-mesenchymal cell repulsion.
We have previously shown that inflammatory mediators alter fetal lung mesenchymal cell phenotype and reduce expression of genes critical for normal epithelial-mesenchymal interactions during lung development.Citation23,28,29 To test if inflammatory mediators might also affect 3-D structure formation, we added E. coli LPS to epithelial-mesenchymal co-cultures. LPS reduced both the number and apparent size of 3-D peaks (). Confocal imaging showed that mesenchymal cells in LPS-treated co-cultures remained near the bases, with fewer mesenchymal cells visualized high within epithelial-covered peaks (). LPS treated peaks were also shorter than controls (). Measuring migration of DiI labeled mesenchymal cells by live cell microscopy showed that LPS appeared to inhibit overall mesenchymal cell displacement and reduced cell velocity in co-culture (). LPS therefore may inhibit 3-D structure formation by altering mesenchymal cell migration.
Figure 5. LPS inhibits 3-dimensional peak formation and mesenchymal cell migration. (A–D) Dark field (A and B) and phase contrast (C and D) images of control and LPS-treated epithelial-mesenchymal co-cultures. LPS treatment resulted in fewer 3-D peaks (E; * P < 0.05, n = 6) that also appeared smaller in size (B and D). (F–K) Confocal images show that DiI-labeled mesenchymal cells (red) did not extend as high into 3-D peaks following LPS treatment (I–K) compared with controls (F–H). (L) Reduced peak height in LPS-treated co-cultures (* P < 0.05, n = 30). (M and N) Live cell imaging of DiI-labeled mesenchymal cells within co-cultures measured reduced cell migration with LPS treatment. Positional traces of individual cells that demonstrate displacement from the starting position within a culture are shown in (M). Average velocity is reduced by LPS treatment (N; * P < 0.05, n = 14).
Discussion
We demonstrate here that co-culturing primary fetal mouse lung mesenchyme with epithelial cells uniquely resulted in formation of 3-D peaks and ridges. These 3-D structures were covered by epithelia with underlying cores of mesenchymal cells. The epithelial-mesenchymal orientation in co-culture resembled the in vivo situation during alveolar septa formation. Interestingly, we did not observe similar 3-D morphogenesis when using adult lung fibroblasts. Localized changes in cell proliferation and apoptosis did not appear to cause these 3-D changes, but cell density and orientation did contribute at least partially to 3-D development. Few mesenchymal cells were found in the areas between peaks and ridges. Live cell imaging experiments demonstrated that mesenchymal cells actively migrated into 3-D peaks and suggested that active repulsion by epithelial cells contributed to peak formation. We propose that this epithelial-mesenchymal co-culture system could be useful in studying the molecular and cellular mechanisms regulating alveolar morphogenesis in the lung.
Epithelial-mesenchymal interactions guide airway branching and alveolar formation in the developing lung. However, understanding the temporal and spatial mechanisms regulating alveolar formation has been limited by the lack of in vitro experimental models. During alveolar septation, multicellular peaks containing alveolar epithelia, mesenchyme, and capillary endothelia divide the immature alveolar space into smaller, more numerous air spaces. Formation of alveolar structures occurs not only during normal lung development, but also in lung repair and regrowth following injury. New alveolar structures form in mature adult lungs following necrotizing pneumonia and lung regrowth following pneumonectomy.Citation30,31 Here we describe an epithelial-mesenchymal co-culture model that may be useful for understanding the 3-D formation of alveolar septa. Interaction with epithelial cells induced changes in mesenchymal cell morphology and stimulated migration of mesenchyme into consolidations beneath the epithelial monolayer. These epithelial-covered 3-D peaks with mesenchymal cores contain similar cellular orientations and arrangements as alveolar septa in vivo, potentially making this model useful for studying specific aspects of alveolar development.
This co-culture system was designed for large-scale experiments, high-throughput screens, and live-cell imaging studies. Using consistent, expandable cell populations are important to ensure consistency and reproducibility. We therefore employed the human A549 epithelial cell line for these initial studies. Many features of A549 cells resemble alveolar Type II cells, including cuboidal morphology, cell-cell junctions along their lateral membranes, and expression of surfactant protein genes, thyroid transcription factor-1, VEGF, and epidermal growth factor receptors.Citation26,Citation32-34 In comparison, the inherent variability in primary alveolar epithelial isolates and contamination with additional cell populations limit the usefulness of primary cells in this type of experimental model system. Bronchioalveolar stem cell progeny may be useful for future work; however, these cell lines require specialized media that may alter primary mesenchyme phenotype and growth. In contrast, primary fetal mouse lung mesenchymal cells can be readily isolated and expanded. Primary mesenchymal cells are relatively slow growing, but have consistent morphology and cell behavior. The unique properties in fetal lung mesenchyme that allow 3-D morphogenesis compared with newborn or adult lung fibroblasts are not yet understood. Additionally, whether epithelial differentiation into Type I or II cells would alter 3-D peak formation is not known.
Three-dimensional co-culture model systems have proven very useful in studying invasion of epithelial cells and epithelial tubes into mesenchymal cell-containing matrices.Citation35-37 In vitro multicellular systems have been useful for studying cell assembly during epithelial cyst formation or branching morphogenesis.Citation38,39 To model alveolar morphogenesis, we tested multiple 3-D epithelial-mesenchymal co-culture approaches using various matrix compositions, including collagen gels, matrigel, and synthetic hydrogels. Because alveolar development appears to involve the migration of mesenchymal-derived myofibroblasts into septa that divide the immature saccular airway,Citation6,18,40 we were particularly interested in model systems that would allow us to model both epithelial mesenchymal interactions and mesenchymal cell migration. We therefore selected an approach that would allow direct epithelial-mesenchymal cell contact and interactions. This system allows high-resolution imaging of cell dynamics and could be useful for studying how factors implicated in alveolar development, including PDGF,Citation18,40,41 TGF-β,Citation42-44 VEGF,Citation45,46 and FGFCitation47,48 influence epithelial-mesenchymal interactions and the migration of mesenchymal cells into 3-D peaks.
Defects in epithelial-mesenchymal interactions and alveolar septation contribute to bronchopulmonary dysplasia pathogenesis.Citation49,50 In extremely preterm infants, inflammation and infection increase the risk of developing bronchopulmonary dysplasia.Citation51,52 Inflammation also arrests late stage lung development in animal models.Citation22,53,54 Our data using the epithelial-mesenchymal co-culture system show addition of E. coli LPS inhibited mesenchymal cell migration and caused formation of fewer, shorter 3-D peaks. Whether abnormal mesenchymal cell migration plays a role in bronchopulmonary dysplasia pathogenesis will require further experimentation. Cell-based experimental models may provide new insight into how defects in cell-cell interactions and dynamics contribute to developmental disease origins.
In addition to the epithelia and underlying mesenchyme, vascular endothelial cells likely contribute to the formation of mature alveoli.Citation31,55 While the data presented here do not address the potential role of endothelia, the co-culture model system should be able to characterize the interactions between endothelia, mesenchyme, and epithelia. In addition to using this model to study structural dynamics, it may also be useful for testing how cell-cell interactions regulate cellular differentiation. We have recently reported that fetal lung mesenchymal cells have stem cell or progenitor cell properties and are capable of differentiating into at least bone, cartilage, and vascular endothelia.Citation56 By using the epithelial-mesenchymal co-culture system, we will be able to test how epithelial factors and direct epithelial-mesenchymal interactions can influence differentiation of mesenchymal progenitor cells into myofibroblasts, lipid-containing fibroblast, newly formed endothelial cells, and perhaps airway smooth muscle and chondrocytes. These approaches may also be useful for studying epithelial-mesenchymal interactions during repair following lung injury. While in vitro co-culture systems cannot replace in vivo models for studying cell interactions and behavior during development, we anticipate that complimentary experimental models such as the one described here may prove useful in testing specific hypotheses and cellular mechanisms regulating alveolar formation.
Materials and Methods
Cell culture and reagents
All animal procedures were performed with approval of the Institutional Animal Care and Use Committees at the University of Alabama at Birmingham and University of California, San Diego. To isolate lung mesenchymal cells,Citation23,28,29,57 E15 lungs from BALB/cJ mice (Jackson Laboratories, 000651) were minced into 1 mm3 cubes and placed on plastic dishes covered by a minimal amount of DMEM supplemented with 10% FBS. After 5–7 d, the lung pieces were removed under a dissecting microscope, leaving behind mesenchymal cells that had grown out onto the plastic dish. A549 human epithelial cells were obtained from ATCC and cultured in DMEM with 10% FBS. All cells were maintained at 37 °C in a humidified environment of 95% air and 5% CO2. For co-culture experiments, primary fetal lung mesenchymal cells were plated at high density (3.125 × 105 cells/cm2) and grown to confluence. A549 cells were then overlaid at the same density to encourage complete coverage of the underlying mesenchyme. Following overnight attachment, the cells were washed extensively and then maintained in DMEM with 10% FBS for 3 d prior to analysis. In separate experiments to limit cell proliferation, cells were cultured in DMEM with decreasing percentages of FBS.
Gel purified E. coli LPS (strain O55:B5, L6529) and Cy3-labeled mouse anti-α smooth muscle actin (C6198) were purchased from Sigma. Camptothecin (208925), Z-Val-Ala-Asp-CH2F (Z-VAD-FMK, 219007), and NS3694 (178494) were purchased from Calbiochem/EMD. DAPI (D1306), Quinolinium (M440), 4-[3-(3-methyl-2(3H)-benzothiazolylidene)-1-propenyl]-1-[3-(trimethylammonio)propyl]-, diiodide (TO-PRO-3, T3605), SYTO 13 (S7575), Alexa-conjugated secondary antibodies (MLM20, R37119), Rat anti-E-Cadherin (ECCD-2), and 1,1'-dioctadecyl-3,3,3′3'-tetramethylindocarbocyanine perchlorate (DiI) were purchased from Invitrogen. Rabbit anti-cytokeratin 18 (SC28264) was purchased from Santa Cruz.
Microscopy and imaging
Samples were fixed, immunolabeled, and mounted for imaging using standard techniques. Nuclei were labeled with DAPI, SYTO 13, or TO-PRO-3 depending on desired detection wavelength. Phase contrast, dark field, and wide field fluorescence images were obtained using an Axiovert inverted microscope (Zeiss) and a DV885 EM-CCD camera (Andor). Confocal fluorescence images were obtained using Leica DMIRB and TCS SP2 confocal systems. All images were stored in tagged image file format (tiff) and processed in identically parallel manner using Photoshop (Adobe). For measurement of peak number, images were first imported into Histometrix (Andor). The images were threshold filtered based on background images obtained using mesenchymal cells only. The resulting visible 3-D peaks were counted and data were exported to Excel (Microsoft) for further analysis. For measurement of peak height, co-cultures were fluorescently labeled with antibodies against cytokeratin and imaged by laser scanning confocal microscopy. The distance between the top of each peak and the cells along the substrate adjacent to the peak was measured in the z-plane and recorded. For each independent experiment, at least 3 fields were imaged and at least 6 peaks per field were measured. Cell migration of DiI-labeled cells was measured using an Axiovert inverted microscopy system fitted with a live-cell imaging incubator for control of humidity, temperature, and CO2. Images were acquired using a DV885 EM-CCD camera and cell migration measured using the Tracking module within iQ software (Andor).
Cell apoptosis
Apoptosis was measured using the Caspase-Glo reagent (Promega, G8093). Cells were first lysed in Reporter Lysis Buffer (Promega, E2661). Aliquots of cell lysates were then incubated with a lumigenic caspase 3/7 substrate containing the DEVD caspase peptide recognition sequence. Upon cleavage by caspase 3/7, the substrate was then measured using a luminometer. For each experiment, light units were normalized to control samples. In co-culture experiments, samples were normalized to parallel samples from A549 cells only. When caspase activity was measured in single cell types, untreated plates of cells were used as controls. To test the effects of apoptosis inhibitors Z-VAD-FMK and NS3694 on 3-D peak number and height, plates of only mesenchymal or A549 epithelial cells were first treated with inhibitors in the absence or presence of the pro-apoptotic agent camptothecin. All samples were measured in triplicate.
Statistical analysis
Experimental data were stored in Excel and analyzed using either Excel or Prism (Graph Pad). Data were compared using the Student t test or Wilcoxon rank-sum test where appropriate.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
The authors would like to thank Albert Tousson (UAB), Sam Wells (Vanderbilt University), and Mark Brown (Andor) for their advice and assistance with imaging and image analysis. We thank our colleagues for their helpful comments regarding the experimental data and in writing and editing the manuscript. This work was supported by the NHLBI (HL086324 and HL097195, LSP), a March of Dimes Basil O’Connor Award (LSP), the American Lung Association (LSP), the Child Health Research Center at UAB, an Advancing Newborn Medicine Fellowship Grant from Ikaria (JDM), and a Neonatal Fellowship Grant from Discovery Laboratories (JDM).
References
- Morrisey EE, Hogan BL. Preparing for the first breath: genetic and cellular mechanisms in lung development. Dev Cell 2010; 18:8-23; PMID:20152174; http://dx.doi.org/10.1016/j.devcel.2009.12.010
- Warburton D, El-Hashash A, Carraro G, Tiozzo C, Sala F, Rogers O, De Langhe S, Kemp PJ, Riccardi D, Torday J, et al. Lung organogenesis. Curr Top Dev Biol 2010; 90:73-158; PMID:20691848; http://dx.doi.org/10.1016/S0070-2153(10)90003-3
- Kresch MJ, Christian C, Wu F, Hussain N. Ontogeny of apoptosis during lung development. Pediatr Res 1998; 43:426-31; PMID:9505285; http://dx.doi.org/10.1203/00006450-199803000-00020
- Collet AJ, Des Biens G. Evolution of mesenchymal cells in fetal rat lung. Anat Embryol (Berl) 1975; 147:273-92; PMID:1211654; http://dx.doi.org/10.1007/BF00315076
- Roth-Kleiner M, Post M. Similarities and dissimilarities of branching and septation during lung development. Pediatr Pulmonol 2005; 40:113-34; PMID:15965895; http://dx.doi.org/10.1002/ppul.20252
- Burri PH. Structural aspects of postnatal lung development - alveolar formation and growth. Biol Neonate 2006; 89:313-22; PMID:16770071; http://dx.doi.org/10.1159/000092868
- Hislop A, Reid L. Development of the acinus in the human lung. Thorax 1974; 29:90-4; PMID:4825556; http://dx.doi.org/10.1136/thx.29.1.90
- Hislop AA, Wigglesworth JS, Desai R. Alveolar development in the human fetus and infant. Early Hum Dev 1986; 13:1-11; PMID:3956418; http://dx.doi.org/10.1016/0378-3782(86)90092-7
- Bellusci S, Grindley J, Emoto H, Itoh N, Hogan BL. Fibroblast growth factor 10 (FGF10) and branching morphogenesis in the embryonic mouse lung. Development 1997; 124:4867-78; PMID:9428423
- Park WY, Miranda B, Lebeche D, Hashimoto G, Cardoso WV. FGF-10 is a chemotactic factor for distal epithelial buds during lung development. Dev Biol 1998; 201:125-34; PMID:9740653; http://dx.doi.org/10.1006/dbio.1998.8994
- Post M, Souza P, Liu J, Tseu I, Wang J, Kuliszewski M, Tanswell AK. Keratinocyte growth factor and its receptor are involved in regulating early lung branching. Development 1996; 122:3107-15; PMID:8898224
- Bellusci S, Furuta Y, Rush MG, Henderson R, Winnier G, Hogan BL. Involvement of Sonic hedgehog (Shh) in mouse embryonic lung growth and morphogenesis. Development 1997; 124:53-63; PMID:9006067
- Shannon JM, Hyatt BA. Epithelial-mesenchymal interactions in the developing lung. Annu Rev Physiol 2004; 66:625-45; PMID:14977416; http://dx.doi.org/10.1146/annurev.physiol.66.032102.135749
- Del Moral PM, Sala FG, Tefft D, Shi W, Keshet E, Bellusci S, Warburton D. VEGF-A signaling through Flk-1 is a critical facilitator of early embryonic lung epithelial to endothelial crosstalk and branching morphogenesis. Dev Biol 2006; 290:177-88; PMID:16375885; http://dx.doi.org/10.1016/j.ydbio.2005.11.022
- Weaver M, Dunn NR, Hogan BL. Bmp4 and Fgf10 play opposing roles during lung bud morphogenesis. Development 2000; 127:2695-704; PMID:10821767
- Shi W, Zhao J, Anderson KD, Warburton D. Gremlin negatively modulates BMP-4 induction of embryonic mouse lung branching morphogenesis. Am J Physiol Lung Cell Mol Physiol 2001; 280:L1030-9; PMID:11290528
- Blanco LN. A model of postnatal formation of alveoli in rat lung. J Theor Biol 1992; 157:427-46; PMID:1460874; http://dx.doi.org/10.1016/S0022-5193(05)80662-9
- Boström H, Willetts K, Pekny M, Levéen P, Lindahl P, Hedstrand H, Pekna M, Hellström M, Gebre-Medhin S, Schalling M, et al. PDGF-A signaling is a critical event in lung alveolar myofibroblast development and alveogenesis. Cell 1996; 85:863-73; PMID:8681381; http://dx.doi.org/10.1016/S0092-8674(00)81270-2
- Delisser HM, Helmke BP, Cao G, Egan PM, Taichman D, Fehrenbach M, Zaman A, Cui Z, Mohan GS, Baldwin HS, et al. Loss of PECAM-1 function Impairs alveolarization. J Biol Chem 2006; 281:8724-31; PMID:16377626
- Wert SE, Dey CR, Blair PA, Kimura S, Whitsett JA. Increased expression of thyroid transcription factor-1 (TTF-1) in respiratory epithelial cells inhibits alveolarization and causes pulmonary inflammation. Dev Biol 2002; 242:75-87; PMID:11820807; http://dx.doi.org/10.1006/dbio.2001.0540
- Taylor BK, Stoops TD, Everett AD. Protein phosphatase inhibitors arrest cell cycle and reduce branching morphogenesis in fetal rat lung cultures. Am J Physiol Lung Cell Mol Physiol 2000; 278:L1062-70; PMID:10781439
- Prince LS, Dieperink HI, Okoh VO, Fierro-Perez GA, Lallone RL. Toll-like receptor signaling inhibits structural development of the distal fetal mouse lung. Dev Dyn 2005; 233:553-61; PMID:15830384; http://dx.doi.org/10.1002/dvdy.20362
- Benjamin JT, Gaston DC, Halloran BA, Schnapp LM, Zent R, Prince LS. The role of integrin alpha8beta1 in fetal lung morphogenesis and injury. Dev Biol 2009; 335:407-17; PMID:19769957; http://dx.doi.org/10.1016/j.ydbio.2009.09.021
- Souza P, Sedlackova L, Kuliszewski M, Wang J, Liu J, Tseu I, Liu M, Tanswell AK, Post M. Antisense oligodeoxynucleotides targeting PDGF-B mRNA inhibit cell proliferation during embryonic rat lung development. Development 1994; 120:2163-73; PMID:7925018
- Smith BT. Cell line A549: a model system for the study of alveolar type II cell function. Am Rev Respir Dis 1977; 115:285-93; PMID:842942
- Balis JU, Bumgarner SD, Paciga JE, Paterson JF, Shelley SA. Synthesis of lung surfactant-associated glycoproteins by A549 cells: description of an in vitro model for human type II cell dysfunction. Exp Lung Res 1984; 6:197-213; PMID:6092046; http://dx.doi.org/10.3109/01902148409109248
- Greco E, Santucci MB, Sali M, De Angelis FR, Papi M, De Spirito M, Delogu G, Colizzi V, Fraziano M. Natural lysophospholipids reduce Mycobacterium tuberculosis-induced cytotoxicity and induce anti-mycobacterial activity by a phagolysosome maturation-dependent mechanism in A549 type II alveolar epithelial cells. Immunology 2010; 129:125-32; PMID:19878354; http://dx.doi.org/10.1111/j.1365-2567.2009.03145.x
- Benjamin JT, Smith RJ, Halloran BA, Day TJ, Kelly DR, Prince LS. FGF-10 is decreased in bronchopulmonary dysplasia and suppressed by Toll-like receptor activation. Am J Physiol Lung Cell Mol Physiol 2007; 292:L550-8; PMID:17071719; http://dx.doi.org/10.1152/ajplung.00329.2006
- Benjamin JT, Carver BJ, Plosa EJ, Yamamoto Y, Miller JD, Liu JH, van der Meer R, Blackwell TS, Prince LS. NF-kappaB activation limits airway branching through inhibition of Sp1-mediated fibroblast growth factor-10 expression. J Immunol 2010; 185:4896-903; PMID:20861353; http://dx.doi.org/10.4049/jimmunol.1001857
- Kumar PA, Hu Y, Yamamoto Y, Hoe NB, Wei TS, Mu D, Sun Y, Joo LS, Dagher R, Zielonka EM, et al. Distal airway stem cells yield alveoli in vitro and during lung regeneration following H1N1 influenza infection. Cell 2011; 147:525-38; PMID:22036562; http://dx.doi.org/10.1016/j.cell.2011.10.001
- Ding BS, Nolan DJ, Guo P, Babazadeh AO, Cao Z, Rosenwaks Z, Crystal RG, Simons M, Sato TN, Worgall S, et al. Endothelial-derived angiocrine signals induce and sustain regenerative lung alveolarization. Cell 2011; 147:539-53; PMID:22036563; http://dx.doi.org/10.1016/j.cell.2011.10.003
- Fujita J, Ohtsuki Y, Bandoh S, Ueda Y, Kubo A, Tojo Y, Yamaji Y, Ishida T. Expression of thyroid transcription factor-1 in 16 human lung cancer cell lines. Lung Cancer 2003; 39:31-6; PMID:12499091; http://dx.doi.org/10.1016/S0169-5002(02)00390-2
- Pertovaara L, Kaipainen A, Mustonen T, Orpana A, Ferrara N, Saksela O, Alitalo K. Vascular endothelial growth factor is induced in response to transforming growth factor-beta in fibroblastic and epithelial cells. J Biol Chem 1994; 269:6271-4; PMID:8119973
- Suzuki H, Aoshiba K, Yokohori N, Nagai A. Epidermal growth factor receptor tyrosine kinase inhibition augments a murine model of pulmonary fibrosis. Cancer Res 2003; 63:5054-9; PMID:12941834
- Liu J, Xu K, Chase M, Ji Y, Logan JK, Buchsbaum RJ. Tiam1-regulated osteopontin in senescent fibroblasts contributes to the migration and invasion of associated epithelial cells. J Cell Sci 2012; 125:376-86; PMID:22302986; http://dx.doi.org/10.1242/jcs.089466
- Andl CD, McCowan KM, Allison GL, Rustgi AK. Cathepsin B is the driving force of esophageal cell invasion in a fibroblast-dependent manner. Neoplasia 2010; 12:485-98; PMID:20563251
- Kodithuwakku SP, Pang RT, Ng EH, Cheung AN, Horne AW, Ho PC, Yeung WS, Lee KF. Wnt activation downregulates olfactomedin-1 in Fallopian tubal epithelial cells: a microenvironment predisposed to tubal ectopic pregnancy. Lab Invest 2012; 92:256-64; PMID:21968811; http://dx.doi.org/10.1038/labinvest.2011.148
- Mailleux AA, Overholtzer M, Brugge JS. Lumen formation during mammary epithelial morphogenesis: insights from in vitro and in vivo models. Cell Cycle 2008; 7:57-62; PMID:18196964; http://dx.doi.org/10.4161/cc.7.1.5150
- Curchoe CL, Russo J, Terskikh AV. hESC derived neuro-epithelial rosettes recapitulate early mammalian neurulation events; an in vitro model. Stem Cell Res 2012; 8:239-46; PMID:22265743; http://dx.doi.org/10.1016/j.scr.2011.11.003
- Boström H, Gritli-Linde A, Betsholtz C. PDGF-A/PDGF alpha-receptor signaling is required for lung growth and the formation of alveoli but not for early lung branching morphogenesis. Dev Dyn 2002; 223:155-62; PMID:11803579; http://dx.doi.org/10.1002/dvdy.1225
- McGowan SE, McCoy DM. Fibroblasts expressing PDGF-receptor-alpha diminish during alveolar septal thinning in mice. Pediatr Res 2011; 70:44-9; PMID:21659960; http://dx.doi.org/10.1203/PDR.0b013e31821cfb5a
- Nicola T, Hagood JS, James ML, Macewen MW, Williams TA, Hewitt MM, Schwiebert L, Bulger A, Oparil S, Chen YF, et al. Loss of Thy-1 inhibits alveolar development in the newborn mouse lung. Am J Physiol Lung Cell Mol Physiol 2009; 296:L738-50; PMID:19270178; http://dx.doi.org/10.1152/ajplung.90603.2008
- Ambalavanan N, Nicola T, Hagood J, Bulger A, Serra R, Murphy-Ullrich J, Oparil S, Chen YF. Transforming growth factor-beta signaling mediates hypoxia-induced pulmonary arterial remodeling and inhibition of alveolar development in newborn mouse lung. Am J Physiol Lung Cell Mol Physiol 2008; 295:L86-95; PMID:18487357; http://dx.doi.org/10.1152/ajplung.00534.2007
- Dabovic B, Chen Y, Choi J, Davis EC, Sakai LY, Todorovic V, Vassallo M, Zilberberg L, Singh A, Rifkin DB. Control of lung development by latent TGF-β binding proteins. J Cell Physiol 2011; 226:1499-509; PMID:20945348; http://dx.doi.org/10.1002/jcp.22479
- Kasahara Y, Tuder RM, Taraseviciene-Stewart L, Le Cras TD, Abman S, Hirth PK, Waltenberger J, Voelkel NF. Inhibition of VEGF receptors causes lung cell apoptosis and emphysema. J Clin Invest 2000; 106:1311-9; PMID:11104784; http://dx.doi.org/10.1172/JCI10259
- Kunig AM, Balasubramaniam V, Markham NE, Morgan D, Montgomery G, Grover TR, Abman SH. Recombinant human VEGF treatment enhances alveolarization after hyperoxic lung injury in neonatal rats. Am J Physiol Lung Cell Mol Physiol 2005; 289:L529-35; PMID:15908474; http://dx.doi.org/10.1152/ajplung.00336.2004
- Franco-Montoya ML, Boucherat O, Thibault C, Chailley-Heu B, Incitti R, Delacourt C, Bourbon JR. Profiling target genes of FGF18 in the postnatal mouse lung: possible relevance for alveolar development. Physiol Genomics 2011; 43:1226-40; PMID:21878612; http://dx.doi.org/10.1152/physiolgenomics.00034.2011
- Perl AK, Gale E. FGF signaling is required for myofibroblast differentiation during alveolar regeneration. Am J Physiol Lung Cell Mol Physiol 2009; 297:L299-308; PMID:19502291; http://dx.doi.org/10.1152/ajplung.00008.2009
- Ahlfeld SK, Conway SJ. Aberrant signaling pathways of the lung mesenchyme and their contributions to the pathogenesis of bronchopulmonary dysplasia. Birth Defects Res A Clin Mol Teratol 2012; 94:3-15; PMID:22125178; http://dx.doi.org/10.1002/bdra.22869
- Bourbon JR, Boucherat O, Boczkowski J, Crestani B, Delacourt C. Bronchopulmonary dysplasia and emphysema: in search of common therapeutic targets. Trends Mol Med 2009; 15:169-79; PMID:19303361; http://dx.doi.org/10.1016/j.molmed.2009.02.003
- Wright CJ, Kirpalani H. Targeting inflammation to prevent bronchopulmonary dysplasia: can new insights be translated into therapies? Pediatrics 2011; 128:111-26; PMID:21646264; http://dx.doi.org/10.1542/peds.2010-3875
- Stichel H, Bäckström E, Hafström O, Nilsson S, Lappalainen U, Bry K. Inflammatory cytokines in gastric fluid at birth and the development of bronchopulmonary dysplasia. Acta Paediatr 2011; 100:1206-12; PMID:21438921; http://dx.doi.org/10.1111/j.1651-2227.2011.02286.x
- Blackwell TS, Hipps AN, Yamamoto Y, Han W, Barham WJ, Ostrowski MC, Yull FE, Prince LSNF. NF-κB signaling in fetal lung macrophages disrupts airway morphogenesis. J Immunol 2011; 187:2740-7; PMID:21775686; http://dx.doi.org/10.4049/jimmunol.1101495
- Moss TJ, Newnham JP, Willett KE, Kramer BW, Jobe AH, Ikegami M. Early gestational intra-amniotic endotoxin: lung function, surfactant, and morphometry. Am J Respir Crit Care Med 2002; 165:805-11; PMID:11897648; http://dx.doi.org/10.1164/ajrccm.165.6.2108053
- Thébaud B. Angiogenesis in lung development, injury and repair: implications for chronic lung disease of prematurity. Neonatology 2007; 91:291-7; PMID:17575472; http://dx.doi.org/10.1159/000101344
- Yamamoto Y, Baldwin HS, Prince LS. Endothelial differentiation by multipotent fetal mouse lung mesenchymal cells. Stem Cells Dev 2012; 21:1455-65; PMID:22008017; http://dx.doi.org/10.1089/scd.2011.0219
- Dieperink HI, Blackwell TS, Prince LS. Hyperoxia and apoptosis in developing mouse lung mesenchyme. Pediatr Res 2006; 59:185-90; PMID:16439576; http://dx.doi.org/10.1203/01.pdr.0000196371.85945.3a
