Abstract
Vascular growth and remodeling are complex processes that depend on the proper spatial and temporal regulation of many different signaling molecules to form functional vascular networks. The ability to understand and regulate these signals is an important clinical need with the potential to treat a wide variety of disease pathologies. Current approaches have focused largely on the delivery of proteins to promote neovascularization of ischemic tissues, most notably VEGF and FGF. Although great progress has been made in this area, results from clinical trials are disappointing and safer and more effective approaches are required. To this end, biological agents used for therapeutic neovascularization must be explored beyond the current well-investigated classes. This review focuses on potential pathways for novel drug discovery, utilizing small molecule approaches to induce and enhance neovascularization. Specifically, four classes of new and existing molecules are discussed, including transcriptional activators, receptor selective agonists and antagonists, natural product-derived small molecules, and novel synthetic small molecules.
Introduction
The vasculature is the first organ to develop and vigorous growth of new blood vessels is essential for the formation of all other organs. Advances in embryology and experiments conducted in a variety of developmental models, including avian, murine and fish embryos, have helped to identify many of the molecular regulators of vascular development and to aid in the understanding of vascular remodeling in adult tissues. While the study of blood vessel growth was once more narrowly focused on the effects of a few known diffusible signals such as vascular endothelial growth factor-A (VEGF-A), researchers are now increasingly aware of the complexity of vascular growth and remodeling, as well as the vast array of both divergent and cooperative molecular signals governing these processes, including numerous soluble effectors and their receptors, mediators of cell-cell and cell-matrix interactions, and matrix degrading enzymes.Citation1 Proper spatial and temporal regulation of these signals is critical to the formation of functional vascular networks, spanning the various arterial, venous, capillary and collateral vessel systems. In addition, impaired or abnormal function of the vasculature is associated with a wide range of disease pathologies, such as coronary artery disease, peripheral vascular disease and osteonecrosis. Animal models such as the dorsal skin and cranial windows, hind limb ischemia and corneal micro pocket have provided insight into the effective coordination of vascular remodeling during injury and adaptive responses. But, despite enormous expenditures supporting basic science research and increasing numbers of patients suffering from ischemia-related vascular diseases, available treatments to address such vascular deficiencies have shown limited effectiveness.
The growth and remodeling of vascular networks in the adult has been described as a continuum of processes spanning angiogenesis, arteriogenesis and vessel maintenance.Citation2 Whereas early studies focused on inducing angiogenesis (formation of new vessels from pre-existing vessels) in areas of ischemia to increase capillary density, it is increasingly recognized that arteriogenesis (remodeling of collateral vessels to accommodate increased flow) is the critical adaptive response to the occlusion of a major arteriole.Citation3 The targeted remodeling of larger collateral vessels via arteriogenesis may also facilitate superior network resistance reduction and a more homogeneous blood flow distribution to sites downstream of an arterial occlusion. Similarly, there is an emerging appreciation of the importance of maintenance via recruitment of pericytes and smooth muscle cells. Numerous studies suggest that without recruitment of such mural support cells, vessels are prone to regression. Thus, the coordination of the processes of angiogenesis, arteriogenesis and vessel maintenance to form an organized network containing arterioles and venules (in addition to capillaries), is absolutely critical to sustaining long-term patency and functionality of microvascular networks. In this review, the term “neovascularization” will be used as an umbrella terminology to describe the stimulation of vascular network growth particularly in circumstances in which it is uncertain whether an intervention is eliciting angiogenesis, arteriogenesis, maintenance or some combination of the three.
Therapeutic Neovascularization
Therapeutic neovascularization is the delivery of growth factors, small molecules, genes or cells to stimulate the growth of new vascular networks in injured, hypoxic or ischemic tissues for the purpose of restoring physiological tissue function. Such strategies seek to enhance the natural process of tissue repair and remodeling, with primary emphasis on ischemic tissue diseases. To date, there have been several randomized, double-blind, controlled phase II/III clinical trials evaluating both protein and gene delivery strategies (outlined in ). These studies evaluated the efficacy of FGF-2 or VEGF165 protein, adenoviral vectors encoding VEGF121 or FGF-4, and VEGF164 plasmid in cases of coronary artery disease (CAD) or peripheral vascular disease (PVD). Although the results point to possible efficacy, there are several areas for improvement, including the mode and method of delivery. For example, intracoronary or intravenous administration of large peptide growth factors may have resulted in ineffective levels of protein in the tissue space. Furthermore, these proteins are known to have a very short half-life.Citation4 Conversely, gene-based approaches yielded longer-term expression of angiogenic factors, but the results were still not definitive. So although there have been several clinical trials to evaluate growth-factor induced therapeutic neovascularization, the trials thus far have failed to show clear evidence of efficacy and no factors to date have been approved for clinical use.
These disappointing outcomes exist despite high expectations of earlier animal studies that show clear revascularization potential in ischemic tissues. VEGF remains the most well-studied angiogenic growth factor for therapeutic neovascularization, but concerns persist regarding its efficacy due to observations of poorly disorganized, leaky and/or hemorrhagic blood vessel formation.Citation2,Citation6 More recent studies now highlight the necessity to stabilize newly formed capillaries via proliferation and recruitment of mural cells to form functional microvascular networks.Citation7 Increasing research emphasis is being directed toward the therapeutic induction of arteriogenesis through the delivery of one or more well-established polypeptide growth factors, such as PDGF-BB (platelet-derived growth factor), Ang1 (angiopoiten 1) and TGFβ (transforming growth factor).Citation8 Temporal delivery of growth factors, such as VEGF164 and Ang1 or VEGF165 and PDGF-BB, can be utilized to successfully arteriolize nascent vascular networks in adult tissues.Citation7,Citation9 But, while growth factors are powerful and promising tools for developing effective strategies in regenerative medicine, they also possess several potential drawbacks, such as high costs associated with recovery and purification of recombinant proteins and susceptibility to aggregation and degradation.Citation10,Citation11 The central theme of this review is that biological agents used for therapeutic neovascularization must be explored beyond the current well-investigated classes.
Recent advances in medicinal chemistry utilizing computer-assisted combinatorial chemistry and traditional high-throughput screening of synthetic and natural product libraries have resulted in a variety of non-peptide small molecules that are capable of regulating cell and tissue function. The use of small molecules to promote neovascularization is an advantageous strategy to overcome the cost and classical limitations of protein-based therapies. In this review, specific emphasis is placed on potential pathways for novel drug discovery and utilization of small molecule approaches to enhance the native biological revascularization response in ischemic tissues. Four classes of new and existing molecules are reviewed: (1) transcriptional activators with multiple gene targets, including HIF-1α stabilizers; (2) receptor selective compounds that permit greater control over the manipulation of cell fate responses, specifically agonists to G protein-coupled receptors; (3) natural product-derived small molecules, including common dietary supplements; and (4) novel synthetic small molecules resulting from drug discovery programs.
Transcriptional Activators
HIF-1 (hypoxia inducible factor-1) is a heterodimeric transcription factor that is composed of two basic-helix-loop-helix (bHLH) subunits, HIF-1α and HIF-1β. Although the β subunit is constitutively expressed, the α subunit is regulated by oxygen levels; it is degraded rapidly under normoxic conditions and stabilized by hypoxic conditions. In normoxia, the HIF-1α subunit is a target for prolyl hydroxylation of two proline residues by HIF prolyl-4-hydroxylases (PHDs), a process that requires oxygen, iron (II), vitamin C and α-ketogluterate.Citation12 The prolyl-hydroxylated HIF-1α promotes binding of the E3 ubiquitin ligase, von Hippel-Lindau protein (vHLP), targeting HIF-1α for quick degradation by the 26S proteasome. The oxygen-dependent hydroxylation of the asparagine residue in the transactivation domain of HIF-1α also blocks the binding of the coactivator proteins CREB and p300, which are required for transcription.Citation13 Taken together, activation of HIF-1α depends on both proline hydroxylation and asparagine hydroxylation.
In contrast, during hypoxic conditions, the activity of HIF prolylhydroxylase is inhibited, since it utilizes oxygen as a cosubstrate. HIF α and β subunits dimerize in the presence of coactivators CREB and p300, leading to the transcription of over 100 genes that promote survival in low-oxygen conditions. These genes encode proteins that are important in the various stages of angiogenesis, including but not limited to, VEGF, placental growth factor (PLGF), angiopoietin-1 and -2 (Ang1, Ang2) and PDGF, as well as their respective receptors.Citation14 Thus, HIF-1 is sometimes referred to as a “master regulator” because of its ability to either directly or indirectly regulate the cell type-specific expression of multiple angiogenic growth factors or cytokines. Because the limited efficacy of previous clinical trials may in part be attributed to the delivery of only a single factor, an emerging consensus among researchers in this field suggests that a combination of two or more stimulatory molecules, or the use of transcription factors, might yield a more robust induction of new blood vessel growth.Citation15 To this end, strategies designed to increase HIF-1 activity acknowledge the complexity of neovascularization and aim to enhance the expression of multiple factors in order to elicit a more natural and coordinated response.
The potential for a new pharmacological strategy to treat ischemic tissue diseases based on the regulation of HIF pathways has gained momentum over the past decade. The importance of HIF-1 in vascular development was initially realized when HIF-1α-/- mice were found to be embryonic lethal due to dramatic vascular regression resulting from extensive endothelial cell death.Citation16 Transgenic mice constitutively expressing HIF-1α demonstrated hypervascularity of intact vessels without the associated edema, inflammation or vessel leakage as characterized by VEGF-overexpressing transgenic mice.Citation17 Gene transfer approaches of constitutively active HIF-1 have been shown to be effective in reducing infarct size and enhancing neovascularization in an acute ischemic myocardiumCitation18 and in improving perfusion in a rabbit hind-limb ischemic model.Citation19 These results underscore the importance of multiple signaling cues involved in vascular network formation and maintenance of newly formed vessels and highlight a potential niche for targeting transcription factors instead of individual genes. Although gene therapy approaches can be powerful tools to understanding the role of HIF signaling in ischemic tissue diseases, pharmacological approaches may provide cheaper more imminently translatable therapies to induce HIF-1 activation.
Because HIF-1 levels in vivo are determined by the rate of hydroxylation-dependent degradation, the prolyl hydroxylase domain proteins (PHD1, PHD2 and PHD3) hold promise as good targets for therapeutic neovascularization. Prolyl hydroxylases require iron as a cofactor to hydroxylate the two proline residues on the HIF-1α subunit, and thus iron-chelators have been proposed as HIF-1 activators. Recent studies have investigated the role of iron-chelation in inducing angiogenesis in a sponge model.Citation20 Both L-mimosine and ethyl 3,4 dihydroxybenzoate (3,4 DHB) were repeatedly injected into polyurethane sponges and implanted subcutaenously in adult rats. After sponge excision and histological analysis, the cross-sections revealed significant differences in the extent of vascular infiltration into the center of the sponges between those treated with L-mimosine or 3,4 DHB and the vehicle control. Additionally, the induction of HIF-1 target genes within 16 hours of treatment demonstrated the ability of these iron chelators to mediate acute protection from hypoxia, which is clinically relevant in cases of acute ischemia or organ transplants. Dibenzoylmethane (DBM) is a nontoxic bioactive chemical compound found in low levels in licorice plants which also acts as iron chelator and may be a carcinogen antagonist.Citation21 DBM dramatically increased HIF-1α protein levels in both cancerous and non-cancerous cells by preventing proteosomal degradation at a step prior to ubiquitination, resulting in a concomitant increase in VEGF secretion under normoxic conditions.Citation22 Another iron chelator, ciclopirox olamine, has been found to also stimulate HIF-induced transcription of VEGF, leading to enhanced angiogenesis in vivo in both a murine skin wound model and the chicken chorioallantoic membrance model.Citation23
However, the most-established iron chelators to date with the ability to activate HIF-1 are desferoxamine (DFO) and cobalt chloride (CoCl2). Wang and Semenza and then Gleadle et al. first demonstrated the connection between DFO and HIF-1α stabilization under normoxia. DFO induced high-level expression of both VEGF and erythropoietin mRNA in a dose-dependent manner with kinetics similar to the induction of HIF-1 by hypoxia or CoCl2. These results were attenuated in the presence iron.Citation24,Citation25 A decade later, the efficacy of DFO in enhancing the expression of HIF-1 regulated genes is still under investigation. Local application of DFO to mobilized latissimus dorsi muscles of adult sheep enhanced neovascularization, specifically capillary density, and recovery of skeletal muscle ischemia.Citation26 Most recently, in vivo administration of DFO into a distraction gap model of bone repair resulted in increased angiogenesis and markedly improved bone regeneration.Citation27 The authors first showed that DFO (and to a lesser extent L-mimosine) increased the formation of tube-like structures in a standard Matrigel tube formation assay with HUVECs and promoted endothelial outgrowth from fetal mouse metatarsels in explant culture in vitro. Subsequently, DFO delivery to the distraction gap every other day for a period of 10 days significantly increased both vessel number and vessel connectivity as well as bone volume compared with controls, all with a lack of overt toxicity. DFO is currently the only iron chelator available for clinical use in the US and is being used for the treatment of iron overload, including acute iron poisoning and chronic iron overload from routine blood transfusions.Citation28 However, new studies such as the ones by Wan et al. and Chekanov et al. also highlight DFO as a potential angiogenic agent to improve blood supply to ischemic tissues.
The iron chelator CoCl2 was the first transition metal shown to activate HIF-1.Citation29 However, hypoxia-like effects resulting from cobalt treatment were realized much earlier (1950s) and exploited on the US market as a drug for treating anemia in adults and children. It was later pulled from the market due to relative ineffectiveness and high renal toxicity, most likely due to increased oxidative stress in cells.Citation30 Since then, much work has been done to understand the role of cobalt in activation of HIF-1 transcription and its effects on angiogenesis. Tanaka et al. investigated the effects of cobalt on tubulointerstitial hypoxia, a hurdle in progressive renal diseases. The authors found that administration of cobalt to rats that had undergone subtotal nephrectomy significantly improved the tubulointerstitial hypoxia by stimulating endothelial cell proliferation and resultant capillary network formation, while maintaining endothelial barrier integrity.Citation31 CoCl2 was also found to increase VEGF generation in human microvascular endothelial cells in a HIF-1 dependent manner.Citation32 Furthermore, chemical preconditioning with CoCl2 induced a delayed preconditioning-like cardioprotection against ischemia-reperfusion injury.Citation33 Specifically, CoCl2 mimicked acute systemic hypoxia, a current cardioprotective treatment modality,Citation34 reducing myocardial infarct size in a HIF-1α-dependent mechanism. Similarly, chronic treatment with CoCl2 increased both arteriolar and capillary supply in the rat myocardium.Citation35 Various other small molecule PHD inhibitors with pro-angiogenic potential have also been reported that act through iron chelation, including FG-0041, S956711 and baicalein.Citation20,Citation36,Citation37 Taken together, these studies promote CoCl2, a low-cost water-soluble iron chelator, as a potential therapeutic in ischemic tissues as it may be an effective inducer of an ischemia-resistant phenotype in various organs and tissues.
Although the aforementioned iron chelators show promise as HIF-1 activators, it is important to note that not all iron chelators stimulate the HIF-1 pathway, two of which include curcuminCitation22 and HBSer.Citation38 Furthermore, iron is a necessary cofactor for many cellular functions, including oxidative phosphorylation and arachidonic acid signaling, so the relative efficacy of iron chelators may be hindered by potential side effects.Citation39 Additional exploration into the mechanisms and promiscuity of iron chelators is warranted.
In addition to iron (II), the HIF prolyl and asparaginyl hydroxylases are also dependent on 2-oxoglutarate (2OG) as a cofactor for the hydroxylation of the HIF-1α subunit. 2OG analogues have been identified that structurally mimic 2OG (a methylene group is replaced by an amine group at position 3), but are unable to stimulate hydroxylation of the primary substrate.Citation40 N-oxaloglycine (NOG) does not permeate cell membranes and has been shown to inhibit enzymes other than the 2OG oxygenases, posing a problem of specificity.Citation41 The ester form of NOG, dimethyloxalylglycine or DMOG, has an increased membrane permeability and is a potent PHD inhibitor. Recent work has shown that inhibiting PHD with DMOG resulted in enhanced HIF-1α activity and subsequent VEGF expression in both human adult lung microvascular endothelial cells and human alveolar epithelial cells.Citation42 Furthermore, DMOG stabilized HIF-1α in human microvascular endothelial cells in vitro and attenuated cytokine-induced neutrophil infiltration into the myocardium, leading to a significant reduction in myocardial infarct size in vivo.Citation43 In a mouse model of hindlimb ischemia, DMOG inhibited HIF PHDs, significantly increasing HIF-1α protein levels, as well as the expression of VEGF and its receptor Flk-1, and stimulated neovascularization in the hindlimb muscles.Citation44 Similarly, in a rat model of ischemia, increased VEGF levels alone did not enhance angiogenesis, but were instead dependent on enhanced levels of Flk-1 as well.Citation45 Recently, a synthetic PHD inhibitor (TM6008) has been discovered that stimulates HIF-1 activity without a co-dependence on iron chelation.Citation39 FibroGen, Inc. (South San Francisco, CA) is interested in the development of HIF prolyl hydroxylase inhibitors related to erythropoiesis, cytoprotection, metabolism and vascular biology and has filed a patent on several compounds that stabilize HIF-1α by inhibiting 2OG dioxygenase enzyme activity.Citation46 Taken together, these studies suggest that stabilization and activation of HIF-1 is a promising strategy for the treatment of ischemic diseases. Potential HIF-1α stabilizers are outlined in .
Receptor Selective Agonists and Antagonists
G protein-coupled receptors (GPCRs) have been identified as molecular targets because they represent one of the largest gene families in the human genome.Citation47 As of 2006, GPCRs were the target of more than 30% of currently marketed drugs (>200).Citation48 These drugs target only a small fraction of the total members in the GPCR superfamily, so there is tremendous potential within the pharmaceutical industry to develop small molecule therapeutics that target the remaining family members, including more than 100 orphan receptors for which their ligands have yet to be identified.Citation49 GPCRs are activated by various stimuli, such as hormones, nucleosides, neurotransmitters, and lipid mediators to name a few, and initiate second messenger cascades that result in diverse cellular functions. Receptor selective compounds permit greater control over the manipulation of cell fate responses, several of which are discussed here and outlined in .
Sphingosine 1-Phosphate
Sphingosine 1-phosphate (S1P) is a bioactive sphingolipid that is now in the spotlight for its role in many critical cellular processes, including cell proliferation, migration and survival,Citation50,Citation51 cytoskeletal arrangements and cell motility,Citation52–Citation54 angiogenesis and vascular maturation,Citation55,Citation56 and trafficking of immune cells,Citation57 among others. S1P has been shown to play such diverse roles because it not only functions inside of the cell, but can be secreted extracellularly and act as a ligand of the S1P receptors, a family of GPCRs designated as S1P1–5.Citation58 The importance of S1P in vascular development was elucidated using global S1P1-/- mice. The fate of these mice was embryonic lethality at E12.5–14.5 due to massive hemorrhaging as a result of aberrant smooth muscle cell (SMC) recruitment to nascent endothelial tubes.Citation56 These results suggest that S1P1 is critical for vascular SMC migration in vivo and that stimulating S1P signaling may prove to be an effective strategy for promoting therapeutic neovascularization in regions of ischemia or tissue insult.
Exogenous delivery of S1P in vitro has been shown to enhance both proliferation and migration of vascular endothelial cells (ECs) and vascular SMCs,Citation59–Citation61 as well as reduce oxygen- and nutrient-deprived cell death.Citation62 Furthermore, S1P has been shown to promote vascular stabilization by recruitment of pericytes and SMCs to newly-formed vesselsCitation63 and to stimulate SMC differentiation into a more contractile phenotype.Citation61 Although there are numerous studies performed in vitro that highlight the potential therapy of S1P, few studies have been published that evaluate the efficacy of S1P delivery on neovascularization or wound healing in vivo. Wacker et al. were the first group to develop biomaterials for the controlled delivery of S1P.Citation64 The authors showed that sustained delivery of S1P from poly(ethylene glycol) (PEG) hydrogels enhanced EC migration in vitro and stimulated angiogenesis in a chick chorioallantoic membrance (CAM) assay in vivo, demonstrating that S1P maintained its bioactivity after encapsulation in the hydrogels. A year later, the effects of S1P in wound healing were reported. Daily injection of S1P into full-thickness dorsal wounds of diabetic mice enhanced the rate and extent of wound closure and stimulated neovascularization in the wound area.Citation65 Then in early 2008, the angiogenic actions of S1P in a model of hindlimb ischemia were published. Administration of S1P intramuscularly into the mouse hindlimb dose-dependently stimulated blood flow recovery and increased intramuscular capillary density without increasing vascular permeability.Citation66 Additionally, SPHK1-transgenic mice that overexpress the S1P-synthesizing enzyme sphingosine kinase-1 (SPHK1) showed accelerated blood flow recovery and increased capillary density, compared to controls, due to a 1.8-fold increase in intramuscular S1P levels. To this end, local increases in available S1P, regardless of delivery mode, stimulated therapeutic neovascularization in an ischemic zone.
Although previous studies support the therapeutic use of S1P, the results may have been enhanced using a sustained delivery system that obviates the need for multiple exogenous injections. Most recently, our group evaluated the effects of sustained release of S1P from biodegradable poly(lactic-co-glycolic acid) (PLAGA) films on microvascular remodeling and bone regeneration in vivo.Citation67 Release of S1P from PLAGA significantly enhanced lumenal diameter expansion of arterioles after 3 days, as well as the number of proliferating SMCs on smooth muscle α-actin-stained microvessels, compared to unloaded PLAGA controls. Furthermore, sustained release of S1P in a rat cranial defect model resulted in significantly greater amounts of new bone formation after 2 and 6 weeks of healing that was accompanied by significant increases in the numbers of blood vessels located within the defect area, compared to controls. These experiments, as well as those by Wacker et al. demonstrate that S1P maintains its bioactivity following encapsulation and release from polymeric biodegradable biomaterials and can be utilized as a therapeutic to improve healing outcomes.
There is strong evidence that vascular SMCs are highly specialized cells that retain remarkable plasticity and can undergo profound phenotypic changes during development and neovascularization, adult microvacular remodeling and wound repair.Citation68 However, in contrast to the desirable pro-proliferative effects of S1P on vSMCs in the aforementioned examples, inhibition of vSMC proliferation may also be advantageous in certain disease states. A classic example of SMC phenotypic modulation in adult blood vessels occurs during macrovessel injury. Balloon angioplasty causes an acute vascular injury, resulting in phenotypic switching of medial SMCs.Citation69 These SMCs proliferate and migrate towards the lumen, ultimately leading to a narrowing of the vessel wall and neointimal hyperplasia. S1P has many diverse effects on vascular cells, including SMCs, and these actions are tailored by activation of specific S1P receptors.Citation70 Analysis of S1P signaling in vascular SMCs suggests that the S1P1 receptor plays a role in SMC proliferation and migration,Citation60,Citation61 whereas the S1P2 receptor inhibits SMC migration by inhibiting small GTPase RacCitation71 and stimulates SMC differentiation to a more contractile phenotype.Citation61 Wamhoff et al. recently evaluated the role of S1P receptors in acute vascular injury using specific receptor antagonists.Citation72 Daily injection of S1P1/S1P3 antagonist VPC44116 significantly decreased neointimal hyperplasia by approximately 50% following an acute balloon injury of the rat carotid artery compared to vehicle control. Conversely, inhibiting S1P2 with antagonist JTE013 further increased S1P-induced proliferation of rat aortic SMCs in vitro, supporting the belief of S1P1/S1P3-mediated proliferation. Meanwhile, Reidy and colleagues demonstrated that the expression levels of specific S1P receptors vary within inbred mouse strains, leading to significantly different responses to acute vascular injury. S1P2-null mice exhibited larger neointimal lesions after ligation of the carotid artery compared to wild-type mice, suggesting that S1P2 normally acts to suppress SMC proliferation.Citation73,Citation74 Taken together, these studies highlight the potential therapeutic benefits of S1P receptor agonists and antagonists to modulate SMC phenotype to a pro- or anti-proliferative state depending on the disease state and desired outcome. For example, activating the S1P1 and S1P3 receptors on vSMCs may be an effective strategy for promoting arteriogenesis in ischemic tissues by inducing vSMC proliferation and increasing the diameter of arterioles and blood flow to the area. Conversely, inhibiting the S1P1 and S1P3 receptors with a pharmacological antagonist may be advantageous following coronary balloon angioplasty where attenuating vSMC proliferation and neointimal hyperplasia are desirable. highlights potential S1P receptor agonists and antagonists and their effects on the vasculature.
Lysophosphatidic Acid
Similar to S1P, lysophosphatidic acid (LPA) is also a bioactive lipid with important biological roles, including the stimulation of cell proliferation, migration, differentiation and survival.Citation75 LPA activates four GPCRs, designated as LPA1–LPA4; unlike S1P, however, the physiological roles of individual LPA receptors are not well understood and there have been far fewer studies reporting on the angiogenic potential of LPA. LPA dose-dependently increased EC proliferation and, utilizing an in vitro wound healing model (scratch assay), LPA significantly enhanced EC migration and wound closure compared to control.Citation76 Wu and colleagues demonstrated that EC migration may be enhanced with LPA treatment by regulating the expression of matrix metalloproteinase-2 (MMP-2), an enzyme involved in degradation of the extracellular matrix during angiogenesis.Citation77 Furthermore, LPA was found to stabilize barrier function of ECs in culture, an important event in late stage angiogenesis.Citation78 LPA has also been implicated to play important roles in vascular development, as described using transgenic mouse models. LPA1-null mice are not embryonic lethal, in contrast to S1P1 knockouts, but are characterized as semi-lethal resulting from impaired suckling behavior, craniofacial dysmorphism, and decreased postnatal growth rate.Citation79 LPA2-/- mice are viable with no obvious phenotypical differences, but exhibit reduced second messenger signaling cascades in response to LPA. Furthermore, LPA1-/-–LPA2-/- double knockout mice phenocopy LPA1-null mice, as they are semi-lethal with increased rates of frontal hematomas.
Nearly half a decade ago, researchers showed that autotaxin, a LPA-producing enzyme, is angiogenic itself. ATX incorporation into Matrigel plugs enhanced endothelial tube formation, compared to controls, in a similar fashion to VEGF delivery.Citation80 ATX is a secreted lysophospholipase D that generates LPA and promotes stabilization of nascent vessels; ATX-null mice are embryonic lethal at day E9.5 because properly formed vessels failed to mature.Citation81,Citation82 ATX heterozygous mice were found to have only half the lysophospholipase D activity and LPA levels of wild type mice, demonstrating the importance of ATX in producing LPA.Citation82 Although all of these studies suggest that LPA signaling is critical for normal vascular development, none have directly demonstrated that the lysophospholipid is angiogenic in vivo.
Lynch and colleagues recently evaluated the direct angiogenic actions of LPA, the first study of its kind.Citation83 Utilizing the CAM assay, the authors showed that LPA induced a robust angiogenic response similar to VEGF or S1P treatment. Furthermore, LPA-treated vessels appeared larger than VEGF-treated vessels, suggesting an arteriogenic effect of LPA. LPA-induced angiogenesis was found to be mediated by the LPA1 and LPA3 receptors, since delivery of LPA1/LPA3 antagonist VPC32183 blocked the response. The angiogenic actions of LPA were direct and were not a result of a non-specific inflammatory response; the anti-inflammatory corticosteroid hydrocortisone was unable to block LPA-induced angiogenesis. Although it is now promising that LPA is angiogenic in vivo, more experiments should be performed in rodents using selective pharmacological compounds to target specific LPA receptors to further elucide the role of LPA in microvascular remodeling. outlines LPA receptor agonists and antagonists that are currently available.
Adenosine
Adenosine is a potent vasodilator produced in response to tissue hypoxia or ischemia that acts to maintain oxygen delivery within a physiological range. Adenosine signals through a family of GPCRs, of which four receptor subtypes have been identified: A1, A2A, A2B and A3.Citation84 Although well-known for its vasodilatory effects, adenosine also maintains oxygen levels in ischemic tissues by promotoing the formation of new blood vessels. Over two decades ago, adenosine was shown to stimulate angiogenesis in a CAM assay.Citation85 Since then, many investigators have shown that adenosine or adenosine analogues stimulate EC migration and proliferation in vitro in a concentration-dependent manner.Citation86 Similarly, adenosine promotes vascular growth in vivo in several tissues, including skeletal muscle, myocardium and wound beds.Citation87,Citation88 For example, Ziada et al. showed that intravenous delivery of adenosine in rabbits resulted in approximately a 27% increase in capillary density in the myocardium and in approximately a 26% increase capillary-to-muscle fiber ratio in skeletal muscles.Citation89
The main pro-angiogenic effects of adenosine have so far been attributed to adenosine-induced secretion of pro-angiogenic growth factors, such as VEGF and bFGF in an A2A receptor-mediated fashion.Citation90 Importantly, Lebovich and colleagues identified a unique mechanism whereby activation of A2A converts macrophages from a pro-inflammatory state that generates tumor necrosis factor-α (TNFα) and interleukin-6 (IL-6) to a pro-angiogenic phenotype that primarily secretes VEGF.Citation91 The A1 receptor was also shown to play a role in angiogenesis as A1 receptor agonist CPA enhanced neovascularization by 40% compared to controls in a mechanism that appeared to involve A1-mediated VEGF secretion from monocytes.Citation92 Taken together, adenosine receptor agonists could play a role as therapeutic targets to enhance angiogenesis either directly or indirectly through production of pro-angiogenic factors. To this end, small molecule agonists and antagonists to adenosine receptors have been cloned and developed and their efficacy in promoting therapeutic neovascularization is under investigation.Citation93
Natural Product-Derived Small Molecules
Natural product-derived small molecules are an attractive option to treat various diseases. In general, they have wide acceptability, and are well tolerated and economical. Historically, natural compounds have provided structural platforms upon which significant volume of current drug products are derived. Between 1981 and 2002, 10% of new products introduced to the drug market were natural products and 61% were natural product-derived.Citation94,Citation95 Some of the most commonly prescribed antibiotics are derived from natural products including â-lactams (cf. penicillins and cephalosporin C), macrolides (i.e., erythromycin), and aminoglycosides (i.e., streptomycin).Citation96–Citation100 More recently, epidemiologists have uncovered new plant-based therapies by identifying dietary habits of various ethnic groups and correlating them to diseases such as coronary artery disease or cancer.Citation94 Others have taken the strategy to perform large scale screening of traditional Chinese medicinal herbs to determine possible angiogenic effects. For example, 47 crude plant extracts were recently tested in a CAM assay and in vitro bovine aortic endothelial cell (BAEC) proliferation assays. Of these, 24 demonstrated angiogenic properties with Epimedium sagittatum, Trichosanthes kirilowii and Dalbergia odorifera performing the strongest in both CAM and BAEC models.Citation101
Resveratrol
The “French paradox” named by epidemiologists describes the unique observation that, despite a diet known to be high in both cholesterol and saturated fats, people of southern France are less prone to ischemic heart disease. Subsequently, a link between moderate consumption of red wine and low incidence of coronary artery disease was hypothesized.Citation102,Citation103 The medicinal component of red grapes, resveratrol, was first identified in 1940 and recent emerging evidence has supported the diverse biochemical and physiological effects of this compound including estrogenic, anti-platelet, anti-inflammatory, anti-apoptosis, ROS scavenging and angiogenic properties.Citation104 Specifically, in terms of angiogenic activity, endothelial progenitor cells exposed to resveratrol showed enhanced proliferation, migration and adhesion. Enhanced tube formation was accompanied by increased VEGF expression. Cell cycle analysis suggested mitogenic effects of resveratrol are achieved via a shift to the G1/S transition.Citation105 The therapeutic effects of resveratrol in improving cardiac function following infarction are most potent when used as a preventative treatment to exert such pre-conditioning effects increased capillary density, improved left ventricular function, and increased expression of anti-apoptotic and pro-angiogenic factors NFκB and Sp-1.Citation106 Oral administration of resveratrol showed increased VEGF and tyrosine kinase receptor Flk-1 expression three weeks following myocardial infarction in a rat model. Exogenous incubation with resveratrol leads to an increase in both eNOS protein expression and eNOS-derived nitric oxide production in human umbilical vein endothelial cells (HUVEC).Citation107 Three-day incubation with exogenous resveratrol lead to increased iNOS expression in cultured bovine pulmonary artery endothelial cells.Citation108 Furthermore, increased expression of iNOS, VEGF, Flk-1 and eNOS occurred in a time-specific manner to resveratrol. Taken together, these results suggest resveratrol may be a promising candidate for therapeutic neovascularization strategies.
Interestingly, emerging evidence from literature suggests that resveratrol also plays an effective role in anti-cancer therapies. This compound has been shown to effectively kill cancer cells by inhibiting cell survival and anti-apoptotic signaling pathways. These contrasting physiological effects of resveratrol can be correlated to differences in dosing concentration. At low dosage (2 ng/mL), resveratrol induces angiogenic actions, whereas at higher dosages (1.2 mg/L), it can inhibit cell survival and angiogenic pathways. Furthermore, pharmacokinetics, bioavailability and metabolism likely play a critical role in appropriate delivery strategies to produce appropriate therapeutic outcomes. Specifically, oral administration leads to rapid absorption to plasma, liver, kidney and heart.Citation109 Therefore, tissue-engineered controlled release systems may be particular advantageous for sustained low dosage delivery of resveratrol to areas of ischemia insult.
Ginseng
Ginseng, named by botanist Carl Meyer, was derived from Greek to mean all-healing (pan akos). Ginseng has historically been a key ingredient to Chinese medicine and is also the most extensively used herbal medicine in the western world.Citation110 Annual sales exceed $200 million in the US alone.Citation94,Citation111 To date, more than 30 ginsenosides, a class of steroid-like compounds that are the principle active components in ginseng, have been identified. Interestingly, differences in vascular response to various extracts of ginseng have recently been correlated with different ratios of ginsenosides. HPLC studies coupled with neovascularization assays revealed different ratios in ginsenoside subgroups based on geographic location of the plant resulting in altered physiologic effect. Specifically, Panax and American ginseng have a predominance of Rb1, effective in preventing cancer in multiple animal models.Citation112,Citation113 In contrast, the predominant ginsenoside in Sanqi ginseng is Rg1, demonstrating proangiogenic activity. A standardized avascular polyether polyurethane scaffold implant was used to assess morphological and functional neovascularization in which strong immunostaining for von Willebrand Factor (vWF), a blood glycoprotein required for normal hemostasis, was observed in Rg1 treated groups compared with controls. Variations in Rg1:Rb1 dosing ratios demonstrated that larger percentages of Rg1 resulted in significant induction of angiogenesis within the implant compared with controls. In contrast, co-delivery of large amounts of Rb1 inhibited Rg1-induced neovascularization.Citation110 This study confirms that ginseng subtype Rb1 is a promising candidate for wound healing and tissue regeneration applications.
VEGF-mediated angiogenesis depends on increased NO expression. In a scratch wound assay, Rg1 exerted a concentration dependent effect on HUVEC proliferation compared with controls.Citation110 Rg1-induced HUVEC proliferation was abolished in the presence of L-NAME, a non-selective inhibitor of NOS, thus suggesting an NO-dependent proliferative response. Others have demonstrated Rg1 treatment activates eNOS in HUVEC thus enhancing production of NO following treatment.Citation114 Increased VEGF and NO expression in HUVECs following Rg1 treatment is mediated via the glucocorticoid receptor (GR).Citation114 Others have also confirmed enhanced HUVEC proliferation in response to exogenous Rg1 in addition to demonstrating enhanced tube formation, microvascular outgrowth from aortic rings, and neovascularization in a subdermal implant in response to Rg1 delivery. Furthermore, gene expression profiling in Rg1-treated HUVEC suggest upregulation of genes involved in cell adhesion, migration and cytoskeleton restructuring (RhoA, RhoB, IQGAP1, CALM2, Vav2 and LAMA4).Citation115 The ginsenoside, Re, has also shown many similar pro-angiogenic properties to that of Rg1.Citation116,Citation117
Traditionally, steroids such as dexamethasone are principally thought to disrupt capillary function and abate endothelial cell proliferation.Citation118 However, as discussed above, Rg1 ginsenoside treatment promotes neovascularization. This discrepancy between steroid classes may be related to dosage. It is thought that high steroid dosage may lead to inhibition of neovascularization whereas low steroid dosage may promote angiogenic activities.Citation119 Interestingly, comparisons of binding affinity for GRs show that Rg1 is 10-fold less likely to bind to its GR than dexamethasone and 100-fold less likely to activate gene transcription when in direct competition with dexamethasone.Citation114,Citation120 Furthermore, differences in the processing of raw ginseng extracts can also alter the structural fingerprint, and thus phenotypic outcome.Citation110 To this end, observations of diverse physiological effects resulting from differences in species, geography location, processing technique, and dosage will require further investigation and greater government regulation if the successful implementation of ginseng derivatives as angiogenic therapeutics is to occur.
Curcumin
Active compounds in turmeric, a yellow-colored spice prevalent in both Asian and Indian cooking, are curcumoids. These are divided into three subgroups; curcumin (curcumin I), demethoxycurcumin (curcumin II) and bisdemethoxycurcumin (curcumin III).Citation121 Both oral and topically treatment of curcumin have demonstrated a variety of pharmalogical effects including anti-inflammatory,Citation122,Citation123 anti-cancer,Citation124 anti-oxidantCitation125,Citation126 and anti-microbialCitation127 properties. Several studies have highlighted the pro-angiogenic properties of curcumin by both oral and topical application. Specifically, serum-free curcumin application showed significant upregulation of CD31,Citation128 E-selectin,Citation129 and both VEGF and VEGFR-2.Citation121 Additionally, Kiran and colleagues showed significant capillary network formation in HUVEC and RAEC cultures, vessel sprouting in aortic ring assays, and increased vascular density in CAM assays in response to curcumin treatment.Citation121 In contrast, HUVEC cultured in serum-containing media with curcumin application demonstrated anti-angiogenic properties including a decreased expression of CD31, E-selectin, Ang-1, VEGF and VEGFR-2 as well as a lack of capillary network formation.Citation121 Therefore, knowledge of the local microenvironment and possible interactions with co-delivered compounds will be critical to elicit an appropriate biological response. Additionally, appropriate dosing for therapeutic application is particular challenging given than turmeric may exist in the diet and is known to produce effects when administered both orally and systemically. Furthermore, both oral consumption and i.p. injection of curcumin resulted in approximately 75% excretion suggesting low absorption within the GI tract, highlighting a potential opportunity for a tissue-engineering controlled release system.Citation130,Citation131
Curcumin is also an effective treatment for accelerating skin wound closure in both diabetic rats and genetically diabetic mice. Specifically, both oral and topical treatment with curcumin showed enhanced neovascularization, increased migration of macrophages and dermal myofibroblasts to the wound zone, and higher levels of collagen and TGFβ expression within the wound zone.Citation132 Others have also demonstrated enhanced production of TGFβ and extracellular matrix protein and increased formation of granulation tissue in rat and guinea pig models.Citation133 In mice receiving whole-body γ-radiation, pretreatment with oral doses of curcumin accelerated cutaneous wound closure and significantly increased vascular density, collagen production, nitric oxide expression and DNA synthesis following within the wound zone following irradiation.Citation134 Others have developed slow release systems using collagen gels were developed to provide controlled delivery of curcumin to the wound site. Results showed application of curcumin-loaded gels accelerated wound closure, enhanced cell proliferation, and was effective in scavenging free radicals compared with untreated and collagen-treated controls.Citation135
Sokotrasterol Sulfate
Currently, there are more than a dozen natural products isolated from marine sponges undergoing clinical or advanced pre-clinical assessment. Sponges are chemically diverse and physiological activity is well-documented. Therefore, Karsan et al. screened a library of crude marine extracts to target possible candidates with the ability to stimulate vessel remodeling for therapeutic angiogenesis. Screening led to identification of a promising target, sokotrasterol, a sulfated steroid. Preliminary experiments demonstrated that sokotrasterol induced sprouting of HUVECs with a bell curve dosedependency.Citation136 Maximal sprouting occurred at 5 µg/ml loading. Murphy and colleagues demonstrated sokotrasterol-loaded gelatin sponges stimulated new vessel growth in a CAM assay similar to levels of FGF-2. Furthermore, local delivery of sokotrasterol to a hindlimb ischemia model accelerated reperfusion and increased vascular density compared with vehicle controls. Vessel sprouting via sokotrasterol stimulation was determined to be dependent on cycloxygenase-2 (COX-2) activation, VEGF expression, and the integrin αV-β3. Furthermore, fibroblasts did not respond to sokotrasterol stimulation suggesting that the angiogenic effects of this compound are selective. The synthetic analog of sokotrasterol, cholestanetrisulfate, was equally affective in restoring reperfusion to occluded hindlimbs. Additionally, this analog can be easily synthesized in a six-step process with 90% yield.Citation137 These results suggest marine sponge extracts may be promising natural-based candidates for therapeutic vascularization applications.
Synthetic Small Molecules
Recent advances in computer assisted drug discovery and high-throughput phenotype screens of synthetic and natural product libraries have resulted in a variety of non-peptide small molecules that are capable of regulating cell and tissue function. The discovery of new small molecule therapies that specifically promote neovascularization may allow for the creation of functional vascular networks without the cost and classical limitations of protein-based therapies. Our colleagues have previously reported on the discovery of novel modulators of angiogenesis for treatment of cancers.Citation138,Citation139 From this program emerged phthalimide neovascularization factor (PNF1), a novel small molecule inducer of angiogenesis (). PNF1 is a structural analog of thalidomide with a cyclopentanone substitution on the imide group. PNF1 possesses a molecular weight similar to that of known small molecule therapeutics. In contrast to endogenous peptide-based growth factors, including VEGF, PNF1 is a much smaller molecule (38.2 kDa and 229 amu, respectively). In addition, this small organic molecule might better withstand processing methods commonly used for incorporation into and sterilization of polymeric biomaterials that may compromise peptide-based growth factors.Citation140–Citation142
In vitro evaluation of the compound demonstrated efficacy in enhancing proliferation, survivability, and capillary network formation by human microvascular endothelial cells (HMVEC). Preliminary in vivo studies also indicated an early stage angiogenic response induced by the drug in rat mesenteric windows.Citation143 Subsequently, network analysis tools were utilized to identify genetic networks of the global biological processes involved in PNF1 stimulation, and to describe known molecular and cellular functions that were most highly regulated by the drug. The most significantly perturbed networks identified gene products associated with transforming growth factor-β (TGFβ) after 24 hours, a protein which has many known implications on angiogenesis.Citation144 Further interrogation of PNF1's mechanism using a novel compendium method identified both TNFα and TGFβ as signaling pathways associated with drug function over 1–48 hours.Citation145 In vivo delivery of PNF1 at 5% loading was examined in the dorsal skinfold window chamber model. Results show that structural microvascular network remodeling induced by PNF1 is both angiogenic and arteriogenic. Specifically, controlled release of this drug led to spatial and temporal increases in location-dependent vessel density and microvessel diameter in treated tissues, with maintenance of functional vascular patterns. Furthermore previously observed increase in microvessel length density induced by PNF1 was abolished in CCR2-/- mouse chimeras, suggesting a critical role for monocyte recruitment and promotion of arteriogenic processes on PNF1 function. Collectively, these experiments suggest that PNF1 is effective for therapeutic induction of neovascularization for possible treatment of ischemic tissue disorders and vascularization of engineered tissues.Citation146
Conclusions
Many significant age-associated diseases, such as myocardial infarction, peripheral limb ischemia or osteonecrosis, arise from impaired or abnormal function of the microvasculature, the complex network of small vessels transporting blood and nutrients to and from various tissues in the body. As a consequence, the development of effective strategies to promote the growth of mature vascular networks is an important clinical need. The small molecule approaches reviewed here hold promise as successful inducers of therapeutic neovascularization for the treatment of ischemic tissue diseases and provide clear alternatives to protein- and gene-based approaches. Further investigation into the basic science of how natural product-derived small molecules elicit cellular responses, as well as cultivating novel drug discovery programs for synthetic small molecules, is needed to realize the full promise of new pharmacological approaches for induction of neovascularization.
Abbreviations
| VEGF | = | vascular endothelial growth factor |
| FGF | = | fibroblast growth factor |
| PDGF | = | platelet derived growth factor |
| Ang1 | = | 2, angiopoietin 1, 2 |
| TGFβ | = | transforming growth factor-β |
| HIF-1 | = | hypoxia inducible factor-1 |
| PHD | = | prolyl-4-hydroxylases |
| vHLP | = | von Hippel-Lindau protein |
| PLGF | = | placental growth factor |
| 3,4 DHB | = | 3, 4 dihydroxybenzoate |
| DBM | = | dibenzoylmethane |
| DFO | = | desferoxamine |
| 2OG | = | 2-oxoglutarate |
| NOG | = | N-oxaloglycine |
| DMOG | = | dimethyloxalylglycine |
| GPCR | = | G protein-coupled receptor |
| S1P | = | sphingosine 1-phosphate |
| LPA | = | lysophosphatidic acid |
| SMC | = | smooth muscle cell |
| EC | = | endothelial cell |
| PEG | = | poly(ethylene glycol) |
| SPHK1 | = | sphingosine kinase 1 |
| PLAGA | = | poly(lactic-co-glycolic acid) |
| MMP-2 | = | matrix metalloproteinase-2 |
| ATX | = | autotaxin |
| TNFα | = | tumor necrosis factor-α |
| IL-6 | = | interleukin-6 |
| BAEC | = | bovine aortic endothelial cell |
| HUVEC | = | human umbilical vein endothelial cell |
| NO | = | nitric oxide |
| PNF1 | = | phthalimide neovascular factor 1 |
| HMVEC | = | human microvascular endothelial cell |
Figures and Tables
Figure 1 Synthesis of phthalimide neovascular factor 1 (PNF1), a synthetic small molecule with angiogenic properties.Citation143
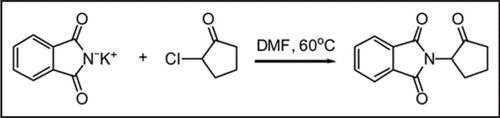
Table 1 Summary of select clinical trials of therapeutic angiogenesis
Table 2 Chemical structures of HIF-1αstabilizers
Table 3 Chemical structures of receptor selective compounds
Table 4 Vascular-related effects of S1P receptor agonists and antagonists
Table 5 Vascular-related effects of LPA receptor agonists and antagonists
Table 6 Chemical structures of natural small molecules
Acknowledgements
This work was supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases grant AR-052352-01A1 to Dr. Botchwey. Lauren Sefcik has been supported by predoctoral fellowships from the National Science Foundation and American Heart Association and Dr. Petrie Aronin by the University of Virginia Biotechnology Training Program grant 5T32GM008715-08.
References
- Carmeliet P. Mechanisms of angiogenesis and arteriogenesis. Nat Med 2000; 6:389 - 395
- Peirce SM, Skalak TC. Microvascular remodeling: a complex continuum spanning angiogenesis to arteriogenesis. Microcirculation 2003; 10:99 - 111
- Heil M, Schaper W. Insights into pathways of arteriogenesis. Current Pharm Biotech 2007; 8:35 - 42
- Lazarous DF, Shou M, Stiber JA, Dadhania DM, Thirumurti V, Hodge E, et al. Pharmacodynamics of basic fibroblast growth factor: route of administration determines myocardial and systemic distribution. Cardiovasc Res 1997; 36:78 - 85
- Lekas M, Lekas P, Latter DA, Kutryk MB, Stewart DJ. Growth factor-induced therapeutic neovascularization for ischaemic vascular disease: time for a re-evaluation?. Curr Opin Cardiol 2006; 21:376 - 384
- Takeshita S, Zheng LP, Brogi E, Kearney M, Pu LQ, Bunting S, et al. Therapeutic angiogenesis—a single intraarterial bolus of vascular endothelial growth-factor augments revascularization in a rabbit ischemic hind-limb. J Clin Invest 1994; 93:662 - 670
- Peirce SM, Price RJ, Skalak TC. Spatial and temporal control of angiogenesis and arterialization using focal applications of VEGF164 and Ang-1. Am J Physiol Heart Circ Physiol 2004a; 286:918 - 925
- Jain RK. Molecular regulation of vessel maturation. Nature Medicine 2003; 9:685 - 693
- Chen RR, Silva EA, Yuen WW, Mooney DJ. Spatio-temporal VEGF and PDGF delivery patterns blood vessel formation and maturation. Pharm Res 2007; 24:258 - 264
- Henry TD, Annex BH, McKendall GR, Azrin MA, Lopez JJ, Giordano FJ, et al. The VIVA trial: vascular endothelial growth factor in ischemia for vascular angiogenesis. Circulation 2003; 107:1359 - 1365
- Barnard GC, McCool JD, Wood DW, Gerngross TU. Integrated recombinant protein expression and purification platform based on ralstonia eutropha. Appl Environ Microbiol 2005; 71:5735 - 5742
- Ivan M, Kondo K, Yang HF, Kim W, Valiando J, Ohh M, et al. HIF alpha targeted for VHL-mediated destruction by proline hydroxylation: Implications for O-2 sensing. Science 2001; 292:464 - 468
- Mahon PC, Hirota K, Semenza GL. FIH-1: a novel protein that interacts with HIF1-alpha and VHL to mediate repression of HIF-1 transcriptional activity. Genes Dev 2001; 15:2675 - 2686
- Kelly BD, Hackett SF, Hirota K, Oshima Y, Cai Z, Berg-Dixon S, et al. Cell type-specific regulation of angiogenic growth factor gene expression and induction of angiogenesis in nonischemic tissue by a constitutively active form of hypoxia-inducible factor 1. Circ Res 2003; 93:1074 - 1081
- Simons M, Bonow RO, Chronos NA, Cohen DJ, Giordano FJ, Hammond HK, et al. Clinical trials in coronary angiogenesis: issues, problems, consensus: an expert panel summary. Circulation 2000; 102:73 - 86
- Kotch LE, Iyer NV, Laughner E, Semenza GL. Defective vascularization of HiF-1α-null embryos is not associated with VEGF deficiency but with mesenchymal cell death. Develop Biol 1999; 209:254 - 267
- Elson DA, Thurston G, Huang LE, Ginzinger DG, McDonald DM, Johnson RS, et al. Induction of hypervascularity without leakage or inflammation in transgenic mice overexpressing hypoxia-inducible factor-1α. Genes Dev 2001; 15:2520 - 2532
- Shyu KG, Wang MT, Wang BW, et al. Intramyocardial injection of naked DNA encoding HIF-1alpha/VP16 hybrid to enhance angiogenesis in an acute myocardial infarction model in the rat. Cardiovasc Res 2000; 54:576 - 583
- Vincent KA, Shyu KG, Luo Y, Magner M, Tio RA, Jiang C, et al. Angiogenesis is induced in a rabbit model of hindlimb ischemia by naked DNA encoding an HIF-1α/VP16 hybrid transcription factor. Circulation 2000; 102:2255 - 2261
- Warnecke C, Griethe W, Weidemann A, Jurgensen JS, William C, Bachmann S, et al. Activation of the hypoxia-inducible factor-pathway and stimulation of angiogenesis by application of prolyl hydroxylase inhibitors. FASEB J 2003; 17:1186 - 1188
- Singletary K, MacDonald C, Iovinelli M, Fisher C, Wallig M. Effect of the β-diketones diferuloulmethane (curcumin) and dibenzoylmethane on rat mammary DNA adducts and tumors induced by 7,12-dimethylbenz[a]anthracene. Carcinogenesis 1998; 19:1039 - 1043
- Mabjeesh, Willard MT, Harris WB, Sun HY, Wang R, Zhong H, Umbreit JN, et al. Dibenzoylmethane, a natural dietary compound, induces HIF-1alpha and increases expression of VEGF. Biochem Biophys Res Commun 2003; 303:279 - 286
- Linden T, Katschinski DM, Eckhardt K, Scheid A, Pagel H, Wenger RH. The antimycotic ciclopirox olamine induces HIF-1alpha stability, VEGF expression and angiogenesis. FASEB J 2003; 17:761 - 763
- Gleadle JM, Ebert BL, Firth JD, Ratcliffe PJ. Regulation of angiogenic growth factor expression by hypoxia, transition metals and chelating agents. Am J Physiol 1995; 268:1362 - 1368
- Wang GL, Semenza GL. Desferrioxamine induces erythropoietin gene expression and hypoxia-inducible factor 1 DNA-binding activity: implications for models of hypoxia signal transduction. Blood 1993; 82:3610 - 3615
- Chekanov VS, Nikolaychik V, Maternowski MA, Mehran R, Leon MB, Adamian M, et al. Deferoxamine enhances neovascularization and recovery of ischemic skeletal muscle in an experimental sheep model. Ann Thorac Surg 1003; 75:184 - 189
- Wan C, Gilbert SR, Wang Y, Cao X, Shen X, Ramaswamy G, et al. Activation of the hypoxia-inducible factor-1α pathway accelerates bone regeneration. Proc Natl Acad Sci USA 2008; 105:686 - 691
- Dragsten PR, Hallaway PE, Hanson GJ, Berger AE, Bernard B, Hedlund BE. First human studies with a high-molecular-weight iron chelator. J Lab Clinical Med 2000; 135:57 - 65
- Wang GL, Semenza GL. General involvement of hypoxia-inducible factor 1 in transcriptional response to hypoxia. Proc Natl Acad Sci USA 1993; 90:4304 - 4308
- Maxwell P, Salnikow K. HIF-1: An oxygen and metal responsive transcription factor. Cancer Biol Ther 2004; 3:29 - 35
- Tanaka T, Kojima I, Ohse T, Ingelfinger JR, Adler S, Fujita T, et al. Cobalt promotes angiogenesis via hypoxia-inducible factor and protects tubulointerstitium in the remnant kidney model. Lab Invest 2005; 85:1292 - 1307
- Loboda A, Jazwa A, Wegiel B, Jozkowicz A, Dulak J. Heme oxygenase-1-dependent and -independent regulation of angiogenic genes expression: effect of cobalt protoporphyrin and cobalt chloride on VEGF and IL-8 synthesis in human microvascular endothelial cells. Cell Mol Biol 2005; 51:347 - 355
- Xi L, Taher M, Yin C, Salloum F, Kukreja RC. Cobalt chloride induces delayed cardiac preconditioning in mcie through selective activation of HIF-1α and AP-1 and iNOS signaling. Am J Physiol Heart Circ Physiol 2004; 287:2369 - 2375
- Baker JE, Curry BD, Olinger GN, Gross GJ. Increased tolerance of chronically hypoxic immature heart to ischemia. Contribution of the KATP channel. Circulation 1997; 95:1278 - 1285
- Rakusan K, Cicutti N, Kolar F. Cardiac function, microvascular structure and capillary hematocrit in hearts of polycythemic rats. Am J Physiol Heart Circ Physiol 2001; 281:2425 - 2431
- Ivan M, Haberberger T, Gervasi DC, Michelson KS, Gunzler V, Kondo K, et al. Biochemical purification and pharmacological inhibition of a mammalian prolyl hydroxlyase acting on hypoxia-inducible factor. Proc Natl Acad Sci USA 2002; 99:13459 - 13464
- Cho H, Lee H, Ahn D, Kim SY, Kim S, Lee KB, et al. Baicalein induces functional hypoxia-inducible factor-1α and angiogenesis. Mol Pharmacol 2008; 74:70 - 81
- Creighton-Gutteridge M, Tyrrell RM. A novel iron chelator that does not induce HIF-1 activity. Free Radic Biol Med 2002; 33:356 - 363
- Nangaku M, Izuhara Y, Takizawa S, Yamashita T, Fujii-Kuriyama Y, Ohneda O, et al. A novel class of prolyl hydroxylase inhibitors induces angiogenesis and exerts organ protection against ischemia. Arterioscler Thromb Vasc Biol 2007; 27:2548 - 2554
- Hanauske-Abel HM, Gunzler V. A stereochemical concept for the catalytic mechanism of prolylhydroxylase: applicability to classification and design of inhibitors. J Theor Biol 1982; 94:421 - 455
- Jaakkola P, Mole Dr, Tian YM, Wilson MI, Gielbert J, Gaskell SJ, et al. Targeting of HIF-alpha to the von Hippel-Lindau ubiquitylation complex by O2-regulated prolyl hydroxylation. Science 2001; 292:468 - 472
- Asikainen TM, Ahmad A, Schneider BK, Ho WB, Arend M, Brenner M, et al. Stimulation of HIF-1α, HIF-2α and VEGF by prolyl 4-hydroxylase inhibition in human lung endothelial and epithelial cells. Free Radic Biol Med 2005; 38:1002 - 1013
- Okaili R, Natarajan R, Salloum F, Fisher BJ, Jones D, Fowler AA III, et al. HIF-1 activation attenuates postischemic myocardial injury: role for heme oxygenase-1 in modulating microvascular chemokine generation. Am J Physiol Heart Circ Physiol 2005; 289:542 - 548
- Milkiewicz M, Pugh CW, Egginton S. Inhibition of endogenous HIF inactivation induces angiogenesis in ischaemic skeletal muscles of mice. J Physiol 2004; 560.1:21 - 26
- Milkiewicz M, Hudlicka O, Verhaeg J, Egginton S, Brown MD. Differential expression of Flk-1 and Flt-1 in rat skeletal muscle in reponse to chronic ischaemia:favourable effect of muscel activity. Clin Sci (Lond) 2003; 105:473 - 482
- Guenzler-Pukall V, Neff TB, Wang Q, Arend MP, Flippin LA, Melekhov A. 2003; US2003017617A
- Kostenis E. G proteins in drug screening: From analysis of receptor-G protein specificity to manipulation of GPCR-mediated signaling pathways. Curr Pharma Design 2006; 12:1703 - 1715
- Gardell SE, Dubin AE, Chun J. Emerging medicinal roles for lysophospholipid signaling. Trends Mol Med 2006; 12:65 - 75
- Civelli O. GPCR deorphanizations: the novel, the known, and the unexpected transmitter. Trends Pharmacol Sci 2005; 26:15 - 19
- Zhang H, Desai NN, Olivera A, Seki T, Brooker G, Spiegel S. Spingosine 1-phosphate, a novel lipid, involved in cellular proliferation. J Cell Biol 1991; 114:155 - 167
- Culliver O, Pirianov G, Kleuser B, Vanek PG, Coso OA, Gutkind S, et al. Suppression of ceramide-mediated programmed cell death by sphingosine 1-phosphate. Nature 1996; 381:800 - 803
- Lee MJ, Thangada S, Paik JH, Sapkota GP, Ancellin N, Chae SS, et al. Akt-mediated phosphorylation of the G protein-coupled receptor EDG-1 is required for endothelial cell chemotaxis. Mol Cell 2001; 8:693 - 704
- Rosenfeldt HM, Hobson JP, Maceyka M, Olivera A, Nava VE, Milstein S, et al. EDG-1 links the PDGF receptor to Src and focal adhesion kinase activation leading to lamellipodia formation and cell migration. FASEB J 2001; 15:2649 - 2659
- Sugimoto N, Takuwa N, Okamoto H, Sakurada S, Takuwa Y. Inhibitory and stimulatory regulation of Rac and cell motility by the G12/13-Rho and Gi pathways integrated downstream of a single G protein-coupled sphingosine 1-phosphate receptor isoform. Mol Cell Biol 2003; 23:1534 - 1545
- Wang F, Van Brocklyn JR, Hobson JP, Movafagh S, Zukowska-Grojec Z, Milstein S, et al. Sphingosine 1-phosphate stimulates cell migration through a Gi-coupled cell surface receptor: potential involvement in angiogenesis. J Biol Chem 1999; 274:35343 - 35350
- Liu Y, Wada R, Yamashita T, Mi Y, Deng CX, Hobson JP, et al. Edg-1, the G protein-coupled receptor for sphingosine 1-phosphate, is essential for vascular maturation. J Clin Invest 2000b; 106:951 - 961
- Cyster JG. Chemokines, sphingosine 1-phosphate, and cell migration in secondary lymphoid organs. Annu Rev Immunol 2005; 23:127 - 159
- Pyne S, Pyne N. Sphingosine 1-phosphate signaling via the endothelial differentiation gene family of G protein-coupled receptors. Pharmacol Ther 2000; 88:115 - 131
- Kimura T, Watanabe T, Sato K, Kon J, Tomura H, Tamama K, et al. Sphingosine 1-phosphate stimulates proliferation and migration of human endothelial cells possibly through the lipid receptors, Edg-1 and Edg-3. Biochem J 2000; 348:71 - 76
- Kluk MJ, Hla T. Role of the sphingosine 1-phosphate receptor EDG-1 in vascular smooth muscle cell proliferation and migration. Circ Research 2001; 89:496 - 502
- Lockman K, Hinson JS, Medlin MD, Morris D, Taylor JM, Mack CP. Sphingosine 1-phosphate stimulates smooth muscle cell differentiation and proliferation by activating separate serum response factor co-factors. J Biol Chem 2004; 279:42422 - 42430
- Hisano N, Yatomi Y, Satoh K, Akimoto S, Mitsumata M, Fujino MA, et al. Induction and suppression of endothelial cell apoptosis by sphingolipids: A possible in vitro model for cell-cell interactions between platelets and endothelial cells. Blood 1999; 93:4293 - 4299
- Paik JH, Skoura A, Chae SS, Cowan AE, Han DK, Proia RL, et al. Sphingosine 1-phosphate receptor regulation of N-cadherin mediates vascular stabilization. Genes Dev 2004; 18:2392 - 2403
- Wacker BK, Scott EA, Kaneda MM, Alford SK, Elbert DL. Delivery of sphingosine 1-phosphate from poly(ethylene glycol) hydrogels. Biomacromolecules 2006; 7:1335 - 1343
- Kawanabe T, Kawakami T, Yatomi Y, Shimada S, Soma Y. Sphingosine 1-phosphate accelerates wound healing in diabetic mice. J Dermatol Sci 2007; 48:53 - 60
- Oyama O, Sugimoto N, Qi X, Takuwa N, Mizugishi K, Koizumi J, et al. The lysophospholipid mediator sphingosine 1-phosphate promotes angiogenesis in vivo in ischemic hindlimbs of mice. Cardiovas Res 2008; 78:301 - 307
- Sefcik LS, Petrie Aronin CE, Wieghaus KA, Botchwey EA. Sustained release of sphingosine 1-phosphate for therapeutic arteriogenesis and bone tissue engineering. Biomaterials 2008; 29:2869 - 2877
- Owens GK, Kumar MS, Wamhoff BR. Molecular regulation of vascular smooth muscle cell differentiation in development and disease. Physiol Rev 2004; 84:767 - 801
- Ferns GA, Raines EW, Sprugel KH, Motani AS, Reidy MA, Ross R. Inhibition of neointimal smooth-muscle accumulation after angioplasty by an antibody to PDGF. Science 1991; 253:1129 - 1132
- Waeber C, Blondeau N, Salomone S. Vascular sphingosine-1-phosphate S1P1 and S1P3 receptors. Drug News Perspect 2004; 17:365 - 382
- Saba JD, Hla T. Point-Counterpoint of Sphingosine 1-Phosphate Metabolism. Circ Res 2004; 94:724 - 734
- Wamhoff BR, Lynch KR, Macdonald TL, Owens GK. Sphingosine 1-phosphate receptor subtypes differentially regulate smooth muscle cell phenotype. Arterioscler Thromb Vasc Biol 2008; 28:1454 - 1461
- Inoue S, Nakazawa T, Cho A, Dastvan F, Shilling D, Daum G, et al. Regulation of arterial lesions in mice depends on differential smooth muscle cell migration: a role for sphingosine 1-phosphate receptors. J Vasc Surg 2007; 46:756 - 763
- Shimizu T, Nakazawa T, Cho A, Dastvan F, Shilling D, Daum G, et al. Sphingosine 1-phosphate receptor 2 negatively regulates neointimal formation in mouse arteries. Circ Res 2007; 101:995 - 1000
- Goetzl EJ, An SZ. Diversity of cellular receptors and functions for the lysophospholipid growth factors lysophosphatidic acid and sphingosine 1-phosphate. FASEB J 1998; 12:1589 - 1598
- Lee H, Goetzl EJ, An SZ. Lysophosphatidic acid and sphingosine 1-phosphate stimualte endothelial cell woudn healing. Am J Physiol Cell Physiol 2000; 278:612 - 618
- Wu WT, Chen CN, Lin CI, Chen JH, Lee H. Lysophospholipids enhance matrix metalloproteinase-2 expression in human endothelial cells. Endocrinology 2005; 146:3387 - 3400
- English D, Kovala AT, Welch Z, Harvey KA, Siddiqui RA, Brindley DN, et al. Induction of endothelial cell chemotaxis by sphingosine 1-phosphate and stabilization of endothelial monolayer barrier function by lysophosphatidic acid, potential mediators of hematopoietic angiogenesis. J Hematother Stem Cell Res 1999; 8:627 - 634
- Contos JJ, Ishii I, Fukushima N, Kingsbury MA, Ye X, Kawamura S, et al. Characterization of lpa(2) (Edg4) and lpa(1)/lpa(2) (Edg2/Edg4) lysophosphatidic acid receptor knockout mice: signaling deficits without obvious phenotypic abnormality attributable to lpa(2). Mol Cell Biol 2002; 22:6921 - 6929
- Nam SW, Clair T, Kim YS, McMarlin A, Schiffmann E, Liotta LA, et al. Autotaxin (NPP-2), a metastasis-enhancing motogen, is an angiogenic factor. Cancer Res 2001; 61:6938 - 6944
- Van Meeteren LA, Ruurs P, Stortelers C, Bouwman P, van Rooijen MA, Pradere JP, et al. Autotaxin, a secreted lysophospholipase D, is essential for blood vessel formation during development. Mol Cell Biol 2006; 26:5015 - 5022
- Tanaka M, Okudaira S, Kishi Y, Ohkawa R, Iseki S, Ota M, et al. Autotaxin stabilizes blood vessels and is required for embryonic vasculature by producing lysophosphatidic acid. J Biol Chem 2006; 281:25822 - 25830
- Rivera-Lopez CM, Tucker AL, Lynch KR. Lysophosphatidic acid (LPA) and angiogenesis. Angiogenesis 2008; 11:301 - 310
- Fredholm BB, Arslan G, Halldner L, Kull B, Schulte G, Wasserman W. Structure and function of adenosine receptors and their genes. Naunyn-Schmied Arch Pharmacol 2000; 362:364 - 374
- Dusseau JW, Hutchins PM, Malbasa DS. Stimulation of angiogenesis by adenosine on the chick chorioallantoic membrane. Circ Res 2986; 59:163 - 170
- Adair TH. Growth regulation of the vascular system: an emerging role for adenosine. Am J Physiol Regul Integr Comp Physiol 2005; 289:283 - 296
- Marshall JM. Roles of adenosine and nitric oxide in skeletal muscle in acute and chronic hypoxia. Adv Exp Med Biol 2001; 502:349 - 363
- Cronstein BN. Adenosine receptors and wound healing. Scientific World Journal 2004; 4:1 - 8
- Ziada AM, Hudlicka O, Tyler KR, Wright AJ. The effect of long-term vasodilation on capillary growth and performance in rabbit heart and skeletal muscle. Cardiovasc Res 1984; 18:724 - 732
- Feoktistov I, Goldstein AE, Ryzhov S, Zeng D, Belardinelli L, Boyno-Yasenetckaya T, et al. Differential expression of adensonie receptors in human endoethelial cells: role of A(2B) receptors in angiogenic factor regulation. Circ Res 2002; 90:531 - 538
- Pinhal-Enfield G, Ramanathan M, Hasko G, Vogel SN, Salzman AL, Boons GJ, et al. An angiogenic switch in macrophages involving synergy between toll-like receptors 2, 4, 7 and 9 and adenosine A(2A) receptors. Am J Pathol 2003; 163:711 - 721
- Clark AN, Youkey R, Liu XP, Jia LG, Blatt R, Day YJ, et al. A(1) adenosine receptor activation promotes angiogenesis and release of VEGF from monocytes. Circ Res 2007; 101:1130 - 1138
- Fredholm BB, Ijzerman AP, Jacobson KA, Klotz KN, Linden J. International Union of Pharmacology XXV. Nomenclature and classification of adenosine receptors. Pharma Rev 2001; 53:527 - 552
- Fan TP, Yeh JC, Leung KW, Yue P, Wong R. Angiogenesis: from plants to blood vessels. Trends Pharmacol Sci 2006; 27:297 - 309
- Newman DJ, Cragg GM, Snader KM. Natural products as sources of new drugs over the period 1981–2002. J Nat Prod 2003; 66:1022 - 1037
- Wainwright M. Miracle Cure: The Story of Penicillin and the Golden Age of Antibiotics 1990; Oxford, UK Blackwell
- Newman DJ, Cragg GM, Snader KM. The influence of natural products upon drug discovery. Nat Prod Rep 2000; 17:215 - 234
- Sneader W. Drug Protoypes and their Exploitation 1996; Chichester, UK Wiley
- Mann J. The Elusive Magic Bullet: The Search for the Perfect Drug 1999; Oxford, UK Oxford University Press 39 - 78
- Crowley BM, Boger DL. Total synthesis and evaluation of [psi[CH2NH]Tpg(4)]vancomycin aglycon: Reengineering vancomycin for dual D-Ala-D-Ala and D-Ala-D-Lac binding. J Am Chem Soc 2006; 128:2885 - 2892
- Wang S, Zheng Z, Weng Y, Yu Y, Zhang D, Fan W, Dai R, Hu Z. Angiogenesis and antiangiogenesis activity of Chinese medicinal herbal extracts. Life Sci 2004; 74:2467 - 2478
- Renaud S, de Lorgeril M. Wine, alcohol, platelets, and the French paradox for coronary heart disease. Lancet 1992; 339:1523 - 1526
- Law M, Wald N. Why heart disease mortality is low in France: The time lag explanation. BMJ 1999; 318:1471 - 1476
- Das DK, Maulik N. Resveratrol in cardioprotection: a therapeutic promise of alternative medicine. Mol Interv 2006; 6:36 - 47
- Wang XB, Huang J, Zou JG, Su EB, Shan QJ, Yang ZJ, Cao KJ. Effects of resveratrol on number and activity of endothelial progenitor cells from human peripheral blood. Clin Exp Pharmacol Physiol 2007; 34:1109 - 1115
- Fukuda S, Kaga S, Zhan L, Bagchi D, Das DK, Bertelli A, et al. Resveratrol ameliorates myocardial damage by inducing vascular endothelial growth factor-angiogenesis and tyrosine kinase receptor Flk-1. Cell Biochem Biophys 2006; 44:43 - 49
- Wallerath T, Deckert G, Ternes T, Anderson H, Huige L, Witte K, et al. Resveratrol, a polyphenolic phytoalexin present in red wine, enhances expression and activity of endothelial nitric oxide synthase. Circ 2002; 106:1652 - 1658
- Hsieh TC, Juan G, Darzynkiewicz Z, Wu JM. Resveratrol increases nitric oxide synhase, induces accumulation of p53 and p21, and suppresses cultured bovine pulmonary artery endothelial cell proliferation by perturbing progression through S to G2. Cancer Res 1999; 59:2596 - 2601
- Bertelli AAE, Giovannini L, Giannessi D, Miglior M, Bernini W, Fregoni M, et al. Antiplatelet activity of synthetic and natural resveratrol in red wine. Int J Tissue React 1995; 17:1 - 3
- Sengupta S, Toh SA, Sellers LA, Skepper JN, Koolwijk P, Leung HW, et al. Modulating angiogenesis: the yin and the yang in ginseng. Circ 2004; 110:1219 - 1225
- De Smet PA. Herbal remedies. N Engl J Med 2002; 347:2046 - 2056
- Yun T, Lee Y, Lee YH, Kim SI, Yun HY. Anticarcinogenic effect of Panax ginseng C.A. Meyer and identification of active compounds. J Korean Med Sci 2001; 16:6 - 18
- Duda RB, Zhong Y, Navas V, Li MZ, Toy BR, Alavarez JG. American ginseng and breast cancer therapeutic agents synergistically inhibit MCF-7 breast cancer cell growth. J Surg Oncol 1999; 72:230 - 239
- Leung KW, Cheng YK, Mak NK, Chan KKC, Fan TPD, Wong RNS. Signaling pathway of ginsenoside-Rg1 leading to nitric oxide production in endothelial cells. FEBS Letters 2006; 580:3211 - 3216
- Yue P, Wong D, Ha WY, Fung MC, Mak NK, Yeung HW, et al. Elucidation of the mechanisms underlying the angiogenic effects of ginsenoside Rg1 in vivo and in vitro. Angiogenesis 2005; 8:205 - 216
- Kang SY, Schini-Kerth VB, Kim ND. Ginsenosides of the protopanaxatriol group cause endothelium-dependent relaxation in the rat aorta. Life Sciences 1995; 56:1577 - 1586
- Huang YC, Chen CT, Chen SC, Lai PH, Liang HC, Chang Y, et al. A natural compound (ginsenoside Re) isolated from Panax ginseng as a novel angiogenic agent for tissue regeneration. Pharma Res 2005; 22:636 - 646
- Koedam Koedam JA, Smink JJ, van Buul-Offers SC. Glucocorticoids inhibit vascular endothelial growth factor expression in growth plate chondrocytes. Mol Cell Endocrinol 2002; 197:35 - 44
- Schiffelers RM, Banciu M, Metselaar JM, Storm G. Therapeutic application of long-circulating liposomal gluocorticoids in auto-immune diseases and cancer. J Liposome Res 2006; 16:185 - 194
- Lee YJ, Chung E, Lee KY, Lee YH, Huh B, Lee SK. Ginsenoside-Rg1, one of the major active molecules from Panax ginseng, is a functional ligand of glucocorticoid receptor. Mol Cell Endocrinol 1997; 133:135 - 140
- Kiran MS, Sameer Kumar VB, Viji RI, Sherin GT, Rajasekharan KN, Sudhakaran PR. Opposing effects of curcuminoids on serum stimulated and unstimulated angiogenic response. J Cell Physiol 2007; 215:251 - 264
- Srimal RC, Dhawan BN. Pharmacology of dieruloyl methane (curcumin), a non-steroidal anti-inflammatory agent. J Pharm Pharmacol 1973; 25:447 - 452
- Satoskar RR, Shah SJ, Shenoy SG. Evaluation of anti-inflammatory property of curcumin (diferuloyl methane) in patients with postoperative inflammation. Int J Clin Pharma Ther Tox 1986; 24:651 - 654
- Kuttan R, Bhanumathy P, Nirmala K, George MC. Potential anticancer activity of turmeric (Curcuma longa). Cancer Letter 1985; 129:197 - 202
- Sharma OP. Antioxidant activity of curcumin and related compounds. Biochem Pharma 1976; 25:1811 - 1812
- Toda S, Miyase T, Arichi H, Tanizawa H, Takino Y. Natural antioxidants III. Antioxidative components isolated from rhizome of Curcuma longa L. Chem Pharma Bulletin 1985; 33:1725 - 1728
- Negi PS, Jayaprakasha GK, Jagan Mohan Rao L, Sakariah KK. Antibacterial activity of turmeric oil: a byproduct from curcumin manufacture. J Agi Food Chem 1999; 47:4297 - 4300
- Yang S, Graham J, Kahn JW, Schwartz EA, Gerritsen ME. Functional roles of PECAM-1 (CD31) and VE Cadherin (CD144) in tube assembly and lumen formation in three dimensional collagen rats. Am J Pathol 1999; 155:887 - 895
- Kraling BM, Razon MJ, Boon LM, Zurakowski D, Seachord C, Darveau RP, et al. E-selectin is present in proliferating endothelial cells in human hemangiomas. Am J Pathol 1996; 148:1181 - 1191
- Wahlstrom BO, Blennow G. A study on the fate of curcumin in the rat. Acta Pharmacologica et Toxicologica 1978; 43:86 - 92
- Holder GM, Plummer JL, Ryan AJ. The metabolism and excretion of curcumin (1,7-bis-(4-hydroxy-3methosyphenyl)-1,6-hepadine-3,5-dione) in the rat. Xenobiotica 1978; 8:761 - 776
- Sidhu GS, Mani H, Gaddipati JP, Singh AK, Seth P, Banaudha KK, et al. Curcumin enhances wound healing in streptozotocin induced diabetic rats and genetically diabetic mice. Wound Repair Regen 1999; 7:362 - 374
- Sidhu GS, Singh AK, Thaloor D, Banaudha KK, Patnaik GK, Srimal RC, et al. Enhancement of wound healing by curcumin in animals. Wound Rep Reg 1998; 6:167 - 178
- Jagetia GC, Rajanikant GK. Role of curcumin, a naturally occurring phenolic compound of turmeric in accelerating the repair of excision wound, in mice whole-body exposed to various doses of γ-radiation. J Surg Res 2004; 120:127 - 138
- Gopinath D, Rafiuddin Ahmed M, Gomathi K, Chitra K, Sehgal PK, et al. Dermal would healing processes with curcumin incorporated collagen films. Biomaterials 2004; 25:1911 - 1917
- Karsan A, Pollet I, Yu LR, Chan KC, Conrads TP, Lucas DA, Andersen R, Veenstra T. Quantitative proteomic analysis of sokotrasterol sulfate-stimulated primary human endothelial cells. Mol Cellular Proteomics 2005; 4:191 - 204
- Murphy S, Larrivee B, Pollet I, Craig KS, Williams DE, Huang XH, et al. Identification of sokotrasterol as a novel proangiogenic steroid. Circ Res 2006; 99:257 - 265
- Brennen WN, Cooper CR, Capitosti S, Brown ML, Sikes RA. Thalidomide and analogues: current proposed mechanisms and therapeutic usage. Clin Prostate Cancer 2004; 3:54 - 61
- Capitosti SM, Hansen TP, Brown ML. Thalidomide analogues demonstrate dual inhibition of both angiogenesis and prostate cancer. Bioorg Med Chem 2004; 12:327 - 336
- van de Weert M, Hennink WE, Jiskoot W. Protein instability in poly(lactic-co-glycolic acid) microparticles. Pharm Res 2000; 17:1159 - 1167
- Fu K, Klibanov AM, Langer R. Protein stability in controlled-release systems. Nat Biotechnol 2000; 18:24 - 25
- Rothen-Weinhold A, Oudry N, Schwach-Abdellaoui K, Frutiger-Hughes S, Hughes GJ, Jeannerat D, et al. Formation of peptide impurities in polyester matrices during implant manufacturing. Eur J Pharm Biopharm 2000; 49:253 - 257
- Wieghaus KA, Capitosti SM, Anderson CR, Blackman BR, Price RJ, Brown ML, et al. Small molecule inducers of angiogenesis for tissue engineering. Tissue Eng 2006; 12:1903 - 1913
- Wieghaus KA, Gianchandani EP, Papin JA, Brown ML, Botchwey EA. Mechanistic interrogation of phthalimide neovascular factor 1 (PNF1) using network analysis tools. Tissue Eng 2007; 13:2561 - 2575
- Wieghaus KA, Gianchandani EP, Paige MA, Brown ML, Papin JA, Botchwey EA. Novel pathway compendium analysis eludicates mechanism of pro-angiogenic synthetic small molecule with in vivo validation. Bioinformatics 2008; 24:2384 - 2390
- Wieghaus KA, Nickerson MM, Petrie Aronin CE, Sefcik LS, Paige M, Price RJ, Brown ML, Botchwey EA. Expansion of microvascular networks in vivo by phthalimide neovascular factor 1 (PNF1). Biomaterials 2008; 29:4698 - 4708
- Sanchez T, Estrada-Hernandez T, Paik JH, et al. Phosphorylation and action of the immunomodulator FTY720 inhibits vascular endothelial cell growth factor-induced vascular permeability. J Biol Chem 2003; 278:47281 - 47290
- Butler J, Lana D, Round O, LaMontagne K. Functional characterization of sphingosine 1-phosphate receptor agonist in human endothelial cells. Prostaglandins Other Lipid Med 2004; 73:29 - 45
- Schmid G, Guba M, Ischenko I, Papyan A, Joka M, Schrepfer S, et al. The immunosuppressant FTY720 inhibits tumor angiogenesis via the sphingosine 1-phosphate receptor 1. J Cell Biochem 2007; 101:259 - 270
- Whetzel AM, Bolick DT, Srinivasan S, Macdonald TL, Morris MA, Ley K, Hedrick CC. Sphingosine-1 phosphate prevents monocyte/endothelial interactions in type 1 diabetic NOD mice through activation of the S1P1 receptor. Circ Res 2006; 99:731 - 739
- Osada M, Yatomi Y, Ohmori T, Ikeda H, Ozaki Y. Enhancement of sphingosine 1-phosphate-induced migration of vascular endothelial cells and smooth muscle cells by an EDG-5 antagonist. Biochem Biophys Res Commun 2002; 299:483 - 487
- Inokia I, Takuwad N, Sugimotoa N, Yoshiokaa K, Takatac S, Kanekob S, et al. Negative regulation of endothelial morphogenesis and angiogenesis by S1P2 receptor. Biochem Biophys Res Commun 2006; 346:293 - 300
- Yan G, Chen S, You B, Sun J. Activation of sphingosine kinase-1 mediates induction of endothelial cell proliferation and angiogenesis by epoxyeicosatrienoic acids. Cardiovasc Res 2008; 78:308 - 314
- Donati C, Cencetti F, Nincheri P, Bernacchioni C, Brunelli S, Clementi E, et al. Sphingosine 1-Phosphate Mediates Proliferation and Survival of Mesoangioblasts. Stem Cells 2007; 25:1713 - 1719
- Salomone S, Potts EM, Tyndall S, Ip PC, Chun J, Brinkmann V, Waeber C. Analysis of sphingosine 1-phosphate receptors involved in constriction of isolated cerebral arteries with receptor null mice and pharmacological tools. Br J Pharmacol 2008; 153:140 - 147
- Sanna MG, Wang SK, Gonzalez-Cabrera PJ, Don A, Marsolais D, Matheu MP, et al. Enhancement of capillary leakage and restoration of lymphocyte egress by a chiral S1P1 antagonist in vivo. Nat Chem Biol 2006; 2:434 - 441
- Qian L, Xu Y, Simper T, Jiang G, Aoki J, Umezu-Goto M, et al. Phosphorothioate analogues of alkyl lysophosphatidic acid as LPA3 receptor-selective agonists. Chem Med Chem 2006; 1:376 - 383
- Chen J, Chen Y, Zhu W, Han Y, Han B, Xu R, et al. Specific LPA receptor subtype mediation of LPA-induced hypertrophy of cardiac myocytes and involvement of Akt and NFkappaB signal pathways. J Cell Biochem 2008; 103:1718 - 1731
- Heise CE, Santos WL, Schreihofer AM, Heasley BH, Mukhin YV, Macdonald TL, Lynch KR. Activity of 2-substituted lysophosphatidic acid (LPA) analogs at LPA receptors: discovery of a LPA1/LPA3 receptor antagonist. Mol Pharmacol 2001; 60:1173 - 1180
- Xu YJ, Tappia PS, Goyal RK, Dhalla NS. Mechanisms of the lysophosphatidic acid-induced increase in [Ca(2+)](i) in skeletal muscle cells. J Cell Mol Med 2008; 12:942 - 954
- Jeon ES, Moon HJ, Lee MJ, Song HY, Kim YM, Cho M, et al. Cancer-derived lysophosphatidic acid stimulates differentiation of human mesenchymal stem cells to myofibroblastlike cells. Stem Cells 2008; 26:789 - 797
- Gustin C, Van Steenbrugge M, Raes M. Lysosphosphatidic acid (LPA) modulates monocyte migration directly and via LPA-stimulated endothelial cells. Am J Physiol Cell Physiol 2008; [Epub ahead of print]
- Yin F, Watsky MA. LPA and S1P increase corneal epithelial and endothelial cell transcellular resistance. Invest Ophthalmol Vis Sci 2005; 46:1927 - 1933