Abstract
The limb is one of the premier models for studying how a simple embryonic anlage develops into complex three-dimensional form. One of the key issues in the limb field has been to determine how the limb becomes patterned along its proximal (shoulder/hip) to distal (digits) axis. For decades it has been known that the apical ectodermal ridge (AER) plays a crucial role in distal outgrowth and patterning of the vertebrate embryonic limb. Most studies have explored the relationship between the AER and the progressive assignment of cell fates to mesenchyme along the proximal to distal (PD) axis. Comparatively few, however, have examined the additional role of the AER to regulate distal outgrowth of the limb and how this growth may also influence pattern along the PD axis. Here, I will review key studies that explore the role of growth in limb development. In particular, I will focus on a recent flurry of papers that examine the role of the Wnt/planar cell polarity (PCP) pathway in regulating directed growth of the limb mesenchyme. Finally, I will discuss a potential mechanism that relates the AER to the Wnt/PCP pathway and how directed growth can play a role in shaping the limb along the PD axis.
Introduction
The embryonic limb has historically been a key model to understand the mechanisms whereby an organ develops in three dimensions. One of the key experiments in this field that has guided much subsequent research was reported by John Saunders in 1948.Citation1 He demonstrated that removal of a ridge of ectodermal tissue at the distal margin of the limb that he termed the apical ectodermal ridge (AER) results in distal truncations of the limb skeleton. He found that early removal of the AER right after its formation results in development of only the most proximal portion of the stylopodal element (i.e., humerus), whereas removal at increasingly later stages in development allows outgrowth of the more distal elements in a progressive fashion.Citation1 Three major conclusions can be made from this experiment. First, the AER is required for outgrowth and patterning along the proximodistal (PD) axis of the limb. Second, because the ridge is of ectodermal origin, it must exert its effects on the mesenchymal skeletal precursors via a signal. Finally, distal pattern is established as a function of time.
In the ensuing decades, the mechanism(s) whereby the AER establishes distal pattern over time has been the focus of intense investigation. From these studies, several models have been proposed. Perhaps the most influential, the progress zone model, posits that cells within signaling range of the AER form a so-called progress zone.Citation2 As long as cells remain within this zone, they can progress to more distal limb fates, whereas those that are pushed out (via proliferation) at earlier times take on proximal fates. Hence, according to this model, the mechanism whereby the AER establishes a proximodistal pattern as a function of time is that limb mesenchyme cells progress from proximal to distal fates as they spend time in the presence of signals from the overlying AER. In this model, growth is non-directional and only plays a passive role, forcing cells to exit the PZ so that fates can be determined along the PD axis.
A second prominent hypothesis is the pre-specification model, which proposes that each of the PD elements [i.e., stylopod (humerus/femur), zeugopod (radius ulna/tibia fibula) and auto-pod (wrist, ankle digits)] exist as three pre-specified packets of cells in the distal limb.Citation3 The role of the AER in this model is to prevent apoptosis in the distal-most mesenchyme, so that these packets can expand in proximal to distal sequence. Similar to the PZ model, the role of growth in this paradigm is passive, and it is not clear by what mechanism expansion of the pre-specified zones occurs, or whether the AER plays any role in this expansion. Recently, both the progress zone and pre-specification models have been called into question, because they are not consistent with current genetic and molecular data.Citation4
Within the past decade, several groups have explored a two-signal hypothesis for proximodistally patterning the limb.Citation4–Citation8 The model postulates that in the very early limb, the mesenchymal population of cells is small enough that it is simultaneously exposed to retinoic acid (RA) signals from the flank and Wnt and Fgf signals from the AER. This cocktail of secreted factors keeps the mesenchyme in a naïve state, such that the cells can become any of the three PD elements of the limb.Citation5 As cells grow outward, the AER is pushed away from the flank, producing a proximal population that continues to be exposed to RA from the flank and a distal population that remains exposed to signals from the AER. Under the influence of RA, the proximal mesenchyme takes on a stylopod fate, whereas the distal population exposed to Wnts and Fgfs takes on a zeugopod and then autopod fate.Citation5,Citation8 This model provides an excellent molecular framework to understand how fates are assigned as a function of time along the proximal-to-distal axis. Clearly growth plays an important role in this model (i.e., it is critical that the AER and a population of mesenchymal cells grow away from the flank), but mechanisms of distal outgrowth are not explored.
As just outlined, many of the key experiments have investigated the relationship between the AER and proximal-to-distal patterning. Relatively few, however, have investigated the other critical function of the AER to regulate distal outgrowth. Here, I will review past work where researchers directly examine the relationship of growth in limb development. I will then discuss recent work that considers the role of the Wnt/PCP pathway in regulating directed outgrowth of the limb mesenchyme. Finally, I will relate AER function to the activation of the Wnt/PCP pathway and how the two function together to shape the limb along the PD axis over time.
The Proliferation Gradient Model
One of the first studies to focus on the role of growth in limb development was a computational model conceived by Ede and Law (1969).Citation9 One of the basic tenets of this paradigm was that proliferation of the limb mesenchyme is anisotropic (i.e., rates of mitoses differ depending on position within the limb; in this case, it was proposed that there was a gradient of mitotic rates increasing as cells neared the apical ectodermal ridge). Based on this parameter, this computer generated model predicted that growth of the limb would proceed distally in a fashion similar to what one observes in normal limb growth in vivo.Citation9 Subsequent, elegant mathematical models confirmed that anisotropic gradients of proliferation in the distal limb are sufficient to describe distal outgrowth of the limb.Citation10,Citation11 Several early empirical studies demonstrated that Fgfs can function as mitogens to the limb mesenchyme, giving credence to proliferation gradient models.Citation12–Citation14 However, these earlier studies have now been countered by careful spatial analyses of mitoses in mouse and chick limb buds that show that mitotic cells are uniformly distributed throughout the mesenchyme.Citation15–Citation18 In addition, Sun et al. have shown that limb buds of mice lacking the major source of Fgf signals in the AER (Fgf4 and Fgf8), although significantly smaller than wild-type, exhibit mitotic indices that are indistinguishable from wild-type mice.Citation16 Hence, Fgf signals from the AER do not appear to significantly influence growth rates of the limb mesenchyme. Interestingly, Fgf4/Fgf8 double mutant limbs also do not initially manifest any increase in cell death in the mesenchyme. Hence Fgf4/Fgf8 mutants exhibit a significantly smaller limb bud for reasons other than simple proliferation and cell survival.Citation16
Perhaps the most convincing study to demonstrate that distally biased growth in the limb is not based on a proliferation gradient is a recent, elegant study performed by Boehm et al., who revisited the proliferation gradient model.Citation19 Using state of the art equipment and methodologies, the authors generated a high resolution, 3D proliferation map of the developing limb. These data showed that while there is an increased rate of proliferation in the distal-most mesenchyme (2X), this is not sufficient to account for empirically determined distal outgrowth of the limb.Citation19 Hence, there must be mechanisms other than proliferation that are responsible for distally oriented growth.
Directed Growth of the Mesenchyme
What might be possible mechanisms that underlie polarized growth of the limb mesenchyme? As early as the 1970s, it was demonstrated that there was an underlying polarity to limb mesenchyme cells in that they exhibited distally oriented Golgi bodies.Citation20 Although it was then argued that the polarized localization of Golgi might be important for distal secretion of extracellular matrix for elongating cartilaginous elements, it is now realized that Golgi are localized at the leading edge of motile cellsCitation21,Citation22 and could therefore underlie polarized growth.
Subsequent work by Li and Muneoka demonstrated evidence for polarized growth by showing that labeled limb mesenchyme cells grow in linear fashion toward the AER.Citation23 When the authors removed a central portion of the AER and labeled mesenchyme in the subadjacent mesoderm, the cells redirected their growth toward residual AER, again suggesting that the ridge is a source of a chemoattractive signal. The authors went on to show that beads soaked in Fgf4 protein (one of several Fgf signals secreted from the AER) and grafted some distance from the AER were alone sufficient to redirect growth of adjacent mesenchyme toward the bead.Citation23 Genetic evidence in the mouse corroborates these findings. Mouse chimeras composed of mutant cells that lack the ability to respond to Fgf signals (FgfR1-/- or Shp2-/- cells) and wild-type cells, exhibit only wild-type cells in the distal limb mesenchyme.Citation24
Two additional groups have recently reinvestigated the possibility of Fgfs regulating directed growth of the mesenchyme. Wyngaarden et al., have shown that at the stage of limb initiation in chick embryos, Fgf does not appear to play any clear role in chemotactically attracting mesenchyme cells.Citation25 Gros et al. transplanted Fgf-soaked beads in older limb buds and then employed sophisticated imaging techniques to observe the behavior of nearby mesenchyme cells.Citation26 The authors demonstrated that while Fgfs do not influence directed migration or oriented cell divisions of adjacent mesenchyme cells, they do elicit an increase in velocity of random cell movement. This effect escalates as cells near the source of Fgf, which creates an overall net movement toward the bead (see ref. Citation26 and below). The basis for this discrepancy may be due to the fact that canonical Fgf signaling appears to become active in the limb mesenchyme after limb initiation stages.Citation27,Citation28
The Role of Wnt5a/PCP Signaling in Directed Outgrowth of the Limb
In addition to Fgf signals, it is possible that other molecules secreted from the AER or those that are activated downstream regulate directed growth of the mesenchyme. Some potential candidates are components of the Wnt/PCP signaling pathway, which have been shown in a variety of contexts to regulate oriented cell divisions and directed migration of cells in the morphogenesis of many tissues (reviewed in refs. Citation29, Citation30). Mouse mutants lacking core components of this pathway exhibit a phenotype characterized by craniorachischisis, shortening of the head-to-tail axis and randomized orientation of stereociliary bundles in the cochlea.Citation31–Citation36 They exhibit, however, very modest (if any) limb defects. Given the phenotypic overlap that exists between mutants lacking various PCP core proteins and the fact that none dramatically affect distal outgrowth and patterning of the limb, it could be surmised that Wnt/PCP signaling does not play a significant role in directed growth of the limb mesenchyme.
Although not considered to be part of the Wnt/PCP core, the secreted ligand Wnt5a has long been known to signal via β-catenin-independent pathways.Citation37 Given that mutations in the zebrafish ortholog Wnt5 result in convergence and extension defects, some have proposed that it signals through the PCP pathway.Citation29,Citation30,Citation37 Further support of this hypothesis came from work in the mouse demonstrating that Wnt5a genetically interacts with the PCP core protein Vangl2 in neural tube closure and in cochlear development.Citation38 In addition to these defects, it has been shown that Wnt5a mutants do exhibit axis truncations reminiscent of PCP core mutants; however, they also exhibit significant shortening of the limbs, face and genitals,Citation39 none of which have been reported for PCP core mutants, suggesting that Wnt5a may signal via PCP-independent pathways.Citation40
To elucidate the pathway through which Wnt5a signals, much work has been done to identify downstream signaling components. Minami and colleagues have demonstrated that Wnt5a biochemically interacts with Ror receptors,Citation41 members of a family of orphan receptor tyrosine kinases that are most closely related to MuSK and the Trk family of neurotrophin receptors.Citation42 Genetically, Ror2 mouse mutants exhibit a phenotype reminiscent of that of Wnt5a mutants (i.e., shortening of body axis, limbs, face, genitals), only less severe.Citation41 Ror1/Ror2 double mutants show an exacerbated phenotype relative to the Ror2 single mutants but that is still less severe than Wnt5a mutants.Citation43 Further evidence demonstrating that Wnt5a signals through Ror receptors comes from humans. Individuals with recessive Robinow syndrome (RRS) characterized by mesomelic limb shortening, dwarfism, shortened genitalia and brachydactyly are homozygous for null alleles in the Ror2 gene.Citation44–Citation46 Individuals that are heterozygous for C-terminal truncations of Ror2 exhibit a dominant disorder called Brachydactyly type B (BDB1), manifested by a shortening of the phalanges.Citation47,Citation48 Recently, it was shown that a dominantly heritable disorder that is indistinguishable from RRS was due to mutations in conserved cysteines of the Wnt5a gene.Citation49 Hence, these data strongly corroborate the mouse studies that demonstrate that Wnt5a signals through Ror receptors and that Wnt5a/Ror signaling is crucial for limb outgrowth. These data, however, do not directly show that Wnt5a/Ror signaling is part of the Wnt/PCP pathway.
Two very recent studies link the two phenotypically disparate Wnt5a/Ror2 and the Wnt/PCP pathways together. Wang et al., have shown that Vangl2Lp/Lp (looptail allele) mutants lack second phalangeal elements, similar to the clinical manifestations of human BDB1 patients.Citation50 They further show that reducing the dosage of Wnt5a by half in a Vangl2Lp/Lp mutant background results in an exacerbated limb defect characterized by mesomelic shortening that is reminiscent of RRS.Citation50 These data, therefore, demonstrate robust genetic interactions that link Wnt5a/Ror2 signaling to the Wnt/PCP pathway in the limb. A second study reported by Gao et al. provides the strongest evidence to date that Wnt5a signals via the PCP pathway in the limb.Citation51 The authors first show genetic interactions between Ror2 and Vangl2. Double mutants exhibit a limb phenotype that very closely resembles that of a Wnt5a mutant. They further show that Wnt5a, Ror2 and Vangl2 associate biochemically (via co-IP and FRET analyses). The importance of this biochemical interaction is that the three must form a ternary complex for Vangl2 to be phosphorylated on critical serine and threonine residues (via the recruitment of CKI; see ). Phosphorylation of Vangl2 is required for it to be polarized to the proximal membrane of limb mesenchyme cells. Further, mutant alleles of Wnt5a and Ror2 identical to those that result in BDB1 and RRS in humans preclude Vangl2 phosphorylation in cell culture assays. The authors go on to show that phosphorylation of critical serine and threonine residues in Vangl2 are functionally important. Vangl2 alleles where these serines and threonines are replaced with alanines cannot rescue axis defects in zebrafish Vangl2 (trilobite) mutants. While phosphorylation of Vangl2 is critical for its asymmetric localization and PCP activity, it is not the only important issue. It appears that a gradient of Wnt5a/Ror2/Vangl2 ternary complex activity is critical for polarized localization of Vangl2, because over-expression of the Wnt5a ligand in chicken limb buds results in ubiquitous high levels of Vangl2 phosphorylation but precludes its asymmetric localization. Hence, the endogenous Wnt5a gradient that exists in the distal limb of vertebrate limbs is likely crucial for polarized growth of the mesenchyme. Taken together, all of these data argue strongly in favor of Wnt5a, Ror2 and Vangl2 as signaling via the Wnt/PCP pathway to regulate distal growth of the limb.
Wnt5a Signals are Necessary and Sufficient for Directed Outgrowth of the Mesenchyme
Two recent papers have examined the role of Wnt5a signaling in directed growth of the limb. Wyngaarden et al., use transgenic nuclear and membrane labels to examine the behavior of lateral plate mesodermal (LPM) cells at the level of the forelimb during limb bud initiation of cultured mouse embryos.Citation25 They observe that the general polarity bias of lateral plate cells (as indicated by the long axis of the cell) is parallel to growth along the embryonic axis [anteroposterior (AP) axis]. They also observe that cell movements and cell divisions are oriented along this axis. Interestingly, at the level of the forelimbs (and presumably the hindlimbs), LPM cells are polarized orthogonal to the AP axis and parallel to the nascent proximodistal (PD) axis of the initiating limb. Furthermore, these cells migrate and proliferate distally as well. In Wnt5a mutants, however, observable polarity of the LPM cells is lost, and the orientation of mitoses is randomized. Distally directed cell movements are maintained in Wnt5a mutants; however, there is a sharp reduction in their velocity.
These studies nicely complement work performed by Gros et al. (2010), who also examine the role of polarized growth in limb morphogenesis but at later stages after the limb bud has initiated.Citation26 They demonstrate that the limb mesenchyme in chick and mouse, rather than being a disorganized mass of cells, is polarized with the long axis of these cells, being oriented toward the axis of outgrowth. They also show that mitoses are distally oriented. Further, mesenchyme moves distally in directed fashion with robust velocity (distance traveled per unit time), efficiency (tendency to move in a linear trajectory) and coherence (tendency of neighboring cells to move in similar trajectories). In Wnt5a mutants, all of these processes are disrupted. Cells tend to become rounded, losing their axis of asymmetry and directionality of cell division. Cell movements, although distally directed, decrease in velocity, coherence and efficiency. To determine whether Wnt5a accomplishes its manifold influences on the limb mesenchyme through the Wnt/PCP pathway, the authors treat cultured chick embryos with the JNK inhibitor SP600125. The behavior of the mesenchyme in these embryos phenocopies that of Wnt5a mutants, providing additional evidence that Wnt5a signals through the PCP pathway.
In addition to showing a requirement for Wnt5a for directed growth of the mesenchyme, both Gros et al. and Wyngaarden et al. show that an ectopic source of Wnt5a is sufficient to redirect nearby mesenchyme from its normal distally oriented course toward the source.Citation25,Citation26
As observed by both Gros et al. and Wyngaarden et al., Wnt5a mutants exhibit weak distally biased cell movements despite the loss of observable cell polarity and oriented cell divisions, suggesting that another underlying pathway is also responsible for directed migration.Citation25,Citation26 Gros et al., therefore, reinvestigated the role of Fgfs in directed growth of the limb mesenchyme, as a previous study showed that it had a chemotactic effect on the limb mesenchyme (see ref. Citation23). The authors examine velocity, coherence and efficiency of limb mesenchyme exposed to MEK1, MAPK or FgfR1 inhibitors or to an ectopic source of Fgf. They show that Fgf/MAPK is necessary and sufficient for increasing the motility of cells but does not have any overall effects on directionality. As cells approach the source of Fgf signals, they become increasingly motile, creating an overall net movement toward the source. Hence, Wnt5a/PCP signaling and Fgf/MAPK signaling provide a robust system to drive distally oriented growth in the limb.
Dynamic Recruitment Shapes the Limb Bud along the PD Axis
The AER recruits cells toward itself by direct and indirect mechanisms. As described above, it is the source of Fgf signals that regulate oriented growth of the mesenchyme in the direction of increasing Fgf concentration.Citation23,Citation26 Second, signals from the AER are necessary to induce a gradient of Wnt5a expression in the distal mesenchyme (see refs. Citation52, Citation53 and J.R.B., unpublished), which, in turn, regulates growth in the direction of increasing Wnt5a concentration.Citation25,Citation26,Citation51 Given that the AER elicits growth of the mesenchyme toward itself, it seems logical that the dimensions of the AER would, in turn, dictate the shape of the mesenchyme that it recruits. Interestingly, at pre-AER stages, Fgf8 is expressed in a domain that is very thick along the dorsoventral (DV) axisCitation26 yet short along the AP axis (J.R.B, unpublished). Hence, the pre-AER approaches a circle in shape (), which would be predicted to recruit a cylindrical mass of mesenchyme that prefigures the formation of the humerus (). Over time, the AER, via the activity of the Fgf/Shh/Gremlin signaling loop, extends its length along the AP axis.Citation54,Citation55 Through the action of Engrailed1 and Dkk1, the AER thins along the DV axis.Citation56 These gradual changes in the shape of the AER could underlie the progressive changes in shape of the limb skeleton along the PD axis ( and C). Based on this hypothesis, it would be predicted that mutant embryos exhibiting defects in the shape of the AER would exhibit corresponding defects in the shape of the limb bud and, ultimately, the limb skeleton. Consistent with this hypothesis, Gli3 mutants that possess an AER that is significantly longer along the AP axis than wild-type controls also possess an extra wide limb paddle, which results in polydactyly.Citation54 Conversely, Wnt3n/c; RARCre mutants possess an anteroposteriorly shortened AER and exhibit ulnar hemimelia and severe oligodactyly.Citation53 Mutants where the AER fails to thin along the DV axis also exhibit shortened, thickened elementsCitation56 or dorsoventrally layered digits.Citation57,Citation58 Both manifestations may be the result of an AER that is thickened abnormally along the dorsoventral axis, which recruits an extra thick population of mesenchyme. Taken together, these data argue that shape of the AER dictates the shape of the limb bud, which, in turn, is critical for limb pattern ().
Coordinating Growth and Patterning along the PD Axis of the Limb
Although the changing dimensions of the AER may play a role in fashioning the limb bud so that appropriate elements (i.e., stylopod, zeugopod, autopod) can form in proximal to distal sequence in the limb, it is clearly not the only important mechanism for generating PD pattern. For example, while disruption of PCP signaling severely disrupts growth along the PD axis, recognizable proximal to distal pattern is nevertheless maintained,Citation39,Citation41,Citation43,Citation50 demonstrating that other mechanisms must be responsible for generating this pattern. Perhaps a residual PD pattern in Wnt5a mutants could be established due to normal AER morphogenesis (see ref. Citation39) coupled with underlying (albeit weak), distally oriented growth regulated by Fgf/MEK1 signaling (see ref. Citation26 and ). However, it is clear that in addition to polarized growth, signals emanating from the AER and the flank are critical for determining appropriate fates of cells along the proximal to distal axis.Citation5–Citation8,Citation59 Yet, it is likely that this two-signal mechanism is also dependent on distally oriented growth in order to separate the proximal and distal signaling centers, so that the different fates can be assigned along the PD axis. Hence, directed growth and patterning are likely inseparable processes in normal limb development.
Fgf signaling is a central component of regulating both distally oriented growth and regulating proximal-to-distal fate decisions (). When Fgf signaling from the AER is disrupted, outgrowth and patterning of the limb are completely abrogatedCitation16,Citation60 (). In mutants where Wnt5a/PCP signaling is disrupted, Fgf signaling can partially compensate in regulating distally oriented growth as well as participate in distally patterning the limb (). Hence, the limb is patterned properly along the PD axis but is dramatically shortened, as is the case in various PCP mutants.Citation39,Citation41,Citation43,Citation50
Concluding Remarks
Elucidating the role of Wnt/PCP signaling in polarized growth has been a significant breakthrough in understanding the genetic basis of morphogenesis. The recent discovery that Wn5a/Ror2 signaling is a core module of the PCP pathway in the limb and regulates directed migration and oriented cell divisions has major implications in understanding the mechanisms whereby simultaneous growth and patterning of the limb are accomplished. That activation of the PCP pathway in the limb ultimately depends on signals from the AER sheds light on a novel paradigm: a signaling center that induces PCP activity can dictate the shape of an anlage along the axis of outgrowth. That Fgf and Wnt5a expression overlaps at other loci that undergo out growth (tail bud, branchial arches, etc.) suggests that this may be an important mechanism for shaping organs throughout the embryo. Furthermore, mechanisms responsible for regulating the dimensions of these signaling centers (i.e., the AER) have perhaps played an important evolutionary role in generating morphological variations that exist between species.
Abbreviations
| AER | = | apical ectodermal ridge |
| AP | = | anteroposterior |
| BDB1 | = | brachydactyly type B |
| DV | = | dorsoventral |
| FGF | = | fibroblast growth factor |
| LPM | = | lateral plate mesoderm |
| PCP | = | planar cell polarity |
| PD | = | proximodistal |
| PZ | = | progress zone |
| RA | = | retinoic acid |
| RRS | = | recessive Robinow syndrome |
Figures and Tables
Figure 1 Wnt5a signals through a Ror2/Vangl2 receptor complex. (A) In the absence of a Wnt5a signal, Ror2 and Vangl2 are inactive. Vangl2 is unphosphorylated. (B) In the presence of Wnt5a, a ternary complex with Ror2 and Vangl2 forms, which recruits CKiδ that, in turn, phosphorylates critical serine and threonine residues in Vangl2. Phosphorylated Vangl2 is critical for asymmetric localization of Vangl2 and could underlie polarized growth. See reference Citation51.
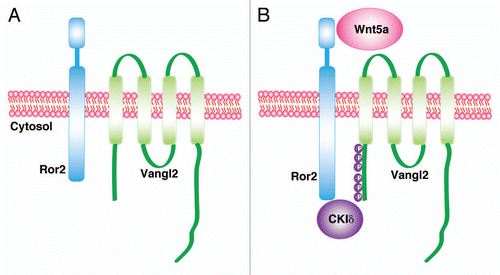
Figure 2 The dimensions of the AER change dramatically over time and underlie morphogenetic changes in the limb bud. (A) The pre-AER (orange), as indicated through Fgf8 expression, is short along the anteroposterior (AP) axis [anterior (a), left; posterior (p), right] and thick along the dorsoventral (DV) axis [dorsal (d), up; ventral (v), down], approaching a circular shape. Fgf8 emanating from the pre-AER elicits directed growth of the adjacent mesenchyme through the induction of Wnt5a/PCP signaling and through canonical Fgf/MAPK signaling. The “circular” AER recruits a cylindrical mass of mesenchyme that prefigures the shape of the stylopod. (B) Over time, the AER lengthens along the AP axis and thins along the DV, recruiting an oval solid mass of mesenchyme that presages the zeugopod. (C) At late stages, the AER extends dramatically along the AP axis and thins along the DV axis, recruiting a thin paddle of mesenchyme that prefigures the autopod. a, anterior; p, posterior; d, dorsal; v, ventral with respect to the limb.
![Figure 2 The dimensions of the AER change dramatically over time and underlie morphogenetic changes in the limb bud. (A) The pre-AER (orange), as indicated through Fgf8 expression, is short along the anteroposterior (AP) axis [anterior (a), left; posterior (p), right] and thick along the dorsoventral (DV) axis [dorsal (d), up; ventral (v), down], approaching a circular shape. Fgf8 emanating from the pre-AER elicits directed growth of the adjacent mesenchyme through the induction of Wnt5a/PCP signaling and through canonical Fgf/MAPK signaling. The “circular” AER recruits a cylindrical mass of mesenchyme that prefigures the shape of the stylopod. (B) Over time, the AER lengthens along the AP axis and thins along the DV, recruiting an oval solid mass of mesenchyme that presages the zeugopod. (C) At late stages, the AER extends dramatically along the AP axis and thins along the DV axis, recruiting a thin paddle of mesenchyme that prefigures the autopod. a, anterior; p, posterior; d, dorsal; v, ventral with respect to the limb.](/cms/asset/0d457c87-89dd-4610-a8e0-95d4f8761f6b/kogg_a_10919049_f0002.gif)
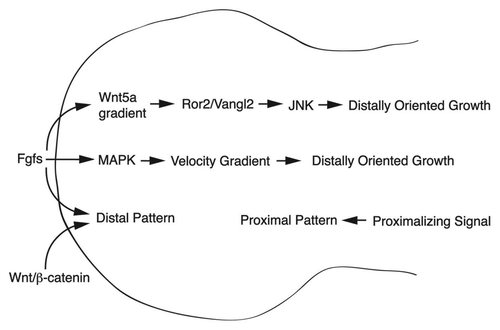
Figure 3 Coordination of growth and patterning in the limb. Fgfs that emanate from the AER play a central role in growth and patterning of the limb. First, they elicit a gradient of cell migration velocity (cells closest to the source of Fgf migrate with higher velocity than those further away), which generates a net distal bias in growth.Citation26 Fgfs (and perhaps other signals from the AER )Citation52,Citation53 are required for a gradient of Wnt5a expression in the distal limb, which is critical for a graded Vangl2 phosphorylation. A gradient of Vangl2 activity appears to underlie distally oriented growth. Fgf and canonical Wnt signals from the AER are required for the establishment of distal fates along the PD axis. According to this model, loss of Fgf signaling from the AER would disrupt both outgrowth and patterning of the limb. The loss of Wnt5a/PCP signaling would result in a severe disruption in outgrowth; however, some residual outgrowth and relatively normal patterning would occur.
Acknowledgments
I thank Michael Stark, Marc Hansen and Aaron Smith for assistance with the manuscript. J.R.B. is supported by a grant from the NIH (HD060087).
References
- Saunders JW Jr. The proximo-distal sequence of origin of the parts of the chick wing and the role of the ectoderm. J Exp Zool 1948; 108:363 - 403; PMID: 18882505; http://dx.doi.org/10.1002/jez.1401080304
- Summerbell D, Lewis JH, Wolpert L. Positional information in chick limb morphogenesis. Nature 1973; 244:492 - 496; PMID: 4621272; http://dx.doi.org/10.1038/244492a0
- Dudley AT, Ros MA, Tabin CJ. A re-examination of proximodistal patterning during vertebrate limb development. Nature 2002; 418:539 - 544; PMID: 12152081; http://dx.doi.org/10.1038/nature00945
- Tabin C, Wolpert L. Rethinking the proximodistal axis of the vertebrate limb in the molecular era. Genes Dev 2007; 21:1433 - 1442; PMID: 17575045; http://dx.doi.org/10.1101/gad.1547407
- Cooper KL, Hu JK, ten Berge D, Fernandez-Teran M, Ros MA, Tabin CJ. Initiation of proximal-distal patterning in the vertebrate limb by signals and growth. Science 2011; 332:1083 - 1086; PMID: 21617075; http://dx.doi.org/10.1126/science.1199499
- Mariani FV, Ahn CP, Martin GR. Genetic evidence that FGFs have an instructive role in limb proximal-distal patterning. Nature 2008; 453:401 - 415; PMID: 18449196; http://dx.doi.org/10.1038/nature06876
- Mercader N, Leonardo E, Piedra ME, Martinez AC, Ros MA, Torres M. Opposing RA and FGF signals control proximodistal vertebrate limb development through regulation of Meis genes. Development 2000; 127:3961 - 3970; PMID: 10952894
- Roselló-Diez A, Ros MA, Torres M. Diffusible signals, not autonomous mechanisms, determine the main proximodistal limb subdivision. Science 2011; 332:1086 - 1088; PMID: 21617076; http://dx.doi.org/10.1126/science.1199489
- Ede DA, Law JT. Computer simulation of vertebrate limb morphogenesis. Nature 1969; 221:244 - 248; PMID: 5812579; http://dx.doi.org/10.1038/221244a0
- Dillon R, Othmer HG. A mathematical model for outgrowth and spatial patterning of the vertebrate limb bud. J Theor Biol 1999; 197:295 - 330; PMID: 10089144; http://dx.doi.org/10.1006/jtbi.1998.0876
- Morishita Y, Iwasa Y. Growth based morphogenesis of vertebrate limb bud. Bull Math Biol 2008; 70:1957 - 1978; PMID: 18668295; http://dx.doi.org/10.1007/s11538008-9334-1
- Hornbruch A, Wolpert L. Cell division in the early growth and morphogenesis of the chick limb. Nature 1970; 226:764 - 766; PMID: 5443258; http://dx.doi.org/10.1038/226764a0
- Niswander L, Martin GR. FGF-4 and BMP-2 have opposite effects on limb growth. Nature 1993; 361:68 - 71; PMID: 8421496; http://dx.doi.org/10.1038/361068a0
- Reiter RS, Solursh M. Mitogenic property of the apical ectodermal ridge. Dev Biol 1982; 93:28 - 35; PMID: 7128937; http://dx.doi.org/10.1016/0012-1606(82)90235-4
- Fernández-Teran MA, Hinchliffe JR, Ros MA. Birth and death of cells in limb development: a mapping study. Dev Dyn 2006; 235:2521 - 2537; PMID: 16881063; http://dx.doi.org/10.1002/dvdy.20916
- Sun X, Mariani FV, Martin GR. Functions of FGF signalling from the apical ectodermal ridge in limb development. Nature 2002; 418:501 - 508; PMID: 12152071; http://dx.doi.org/10.1038/nature00902
- Lu P, Yu Y, Perdue Y, Werb Z. The apical ectodermal ridge is a timer for generating distal limb progenitors. Development 2008; 135:1395 - 1405; PMID: 18359901; http://dx.doi.org/10.1242/dev.018945
- Yu K, Ornitz DM. FGF signaling regulates mesenchymal differentiation and skeletal patterning along the limb bud proximodistal axis. Development 2008; 135:483 - 491; PMID: 18094024; http://dx.doi.org/10.1242/dev.013268
- Boehm B, Westerberg H, Lesnicar-Pucko G, Raja S, Rautschka M, Cotterell J, et al. The role of spatially controlled cell proliferation in limb bud morphogenesis. PLoS Biol 2010; 8:e1000420; PMID: 20644711; http://dx.doi.org/10.1371/journal.pbio.1000420
- Holmes LB, Trelstad RL. Patterns of cell polarity in the developing mouse limb. Dev Biol 1977; 59:164 - 173; PMID: 892225; http://dx.doi.org/10.1016/0012-1606(77)90251-2
- Mellor H. Cell motility: Golgi signalling shapes up to ship out. Curr Biol 2004; 14:R434 - R435; PMID: 15182693; http://dx.doi.org/10.1016/j.cub.2004.05.038
- Nabi IR. The polarization of the motile cell. J Cell Sci 1999; 112:1803 - 1811; PMID: 10341200
- Li S, Muneoka K. Cell migration and chick limb development: chemotactic action of FGF-4 and the AER. Dev Biol 1999; 211:335 - 347; PMID: 10395792; http://dx.doi.org/10.1006/dbio.1999.9317
- Saxton TM, Ciruna BG, Holmyard D, Kulkarni S, Harpal K, Rossant J, et al. The SH2 tyrosine phosphatase shp2 is required for mammalian limb development. Nat Genet 2000; 24:420 - 423; PMID: 10742110; http://dx.doi.org/10.1038/74279
- Wyngaarden LA, Vogeli KM, Ciruna BG, Wells M, Hadjantonakis AK, Hopyan S. Oriented cell motility and division underlie early limb bud morphogenesis. Development 2010; 137:2551 - 2558; PMID: 20554720; http://dx.doi.org/10.1242/dev.046987
- Gros J, Hu JK, Vinegoni C, Feruglio PF, Weissleder R, Tabin CJ. Wnt5A/JNK and FGF/MAPK pathways regulate the cellular events shaping the vertebrate limb bud. Curr Biol 2010; 20:1993 - 2002; PMID: 21055947; http://dx.doi.org/10.1016/j.cub.2010.09.063
- Corson LB, Yamanaka Y, Lai KM, Rossant J. Spatial and temporal patterns of ERK signaling during mouse embryogenesis. Development 2003; 130:4527 - 4537; PMID: 12925581; http://dx.doi.org/10.1242/dev.00669
- Lewandoski M, Mackem S. Developmental biology: extending the limb and body with vectors and scalars. Curr Biol 2011; 21:R34 - R36; PMID: 21215936; http://dx.doi.org/10.1016/j.cub.2010.11.023
- Roszko I, Sawada A, Solnica-Krezel L. Regulation of convergence and extension movements during vertebrate gastrulation by the Wnt/PCP pathway. Semin Cell Dev Biol 2009; 20:986 - 997; PMID: 19761865; http://dx.doi.org/10.1016/j.semcdb.2009.09.004
- Tada M, Kai M. Noncanonical Wnt/PCP signaling during vertebrate gastrulation. Zebrafish 2009; 6:29 - 40; PMID: 19292674; http://dx.doi.org/10.1089/zeb.2008.0566
- Curtin JA, Quint E, Tsipouri V, Arkell RM, Cattanach B, Copp AJ, et al. Mutation of Celsr1 disrupts planar polarity of inner ear hair cells and causes severe neural tube defects in the mouse. Curr Biol 2003; 13:1129 - 1133; PMID: 12842012; http://dx.doi.org/10.1016/S0960-9822(03)00374-9
- Montcouquiol M, Rachel RA, Lanford PJ, Copeland NG, Jenkins NA, Kelley MW. Identification of Vangl2 and Scrb1 as planar polarity genes in mammals. Nature 2003; 423:173 - 177; PMID: 12724779; http://dx.doi.org/10.1038/nature01618
- Strong LC, Hollander WF. Hereditary loop-tail in the house mouse accompanied by inperforate vagina and with lethal craniorachischisis when homozygous. J Hered 1949; 40:329 - 334
- Wang J, Hamblet NS, Mark S, Dickinson ME, Brinkman BC, Segil N, et al. Dishevelled genes mediate a conserved mammalian PCP pathway to regulate convergent extension during neurulation. Development 2006; 133:1767 - 1778; PMID: 16571627; http://dx.doi.org/10.1242/dev.02347
- Yu H, Smallwood PM, Wang Y, Vidaltamayo R, Reed R, Nathans J. Frizzled 1 and frizzled 2 genes function in palate, ventricular septum and neural tube closure: general implications for tissue fusion processes. Development 2010; 137:3707 - 3717; PMID: 20940229; http://dx.doi.org/10.1242/dev.052001
- Wang Y, Guo N, Nathans J. The role of Frizzled3 and Frizzled6 in neural tube closure and in the planar polarity of inner-ear sensory hair cells. J Neurosci 2006; 26:2147 - 2156; PMID: 16495441; http://dx.doi.org/10.1523/JNEUROSCI.4698-05.2005
- Veeman MT, Axelrod JD, Moon RT. A second canon. Functions and mechanisms of beta-catenin-independent Wnt signaling. Dev Cell 2003; 5:367 - 377; PMID: 12967557; http://dx.doi.org/10.1016/S1534-5807(03)00266-1
- Qian D, Jones C, Rzadzinska A, Mark S, Zhang X, Steel KP, et al. Wnt5a functions in planar cell polarity regulation in mice. Dev Biol 2007; 306:121 - 133; PMID: 17433286; http://dx.doi.org/10.1016/j.ydbio.2007.03.011
- Yamaguchi TP, Bradley A, McMahon AP, Jones SA. Wnt5a pathway underlies outgrowth of multiple structures in the vertebrate embryo. Development 1999; 126:1211 - 1223; PMID: 10021340
- Barrow JR. Wnt/PCP signaling: a veritable polar star in establishing patterns of polarity in embryonic tissues. Semin Cell Dev Biol 2006; 17:185 - 193; PMID: 16765615; http://dx.doi.org/10.1016/j.semcdb.2006.04.002
- Oishi I, Suzuki H, Onishi N, Takada R, Kani S, Ohkawara B, et al. The receptor tyrosine kinase Ror2 is involved in non-canonical Wnt5a/JNK signaling pathway. Genes Cells 2003; 8:645 - 654; PMID: 12839624; http://dx.doi.org/10.1046/j.1365-2443.2003.00662.x
- Minami Y, Oishi I, Endo M, Nishita M. Ror-family receptor tyrosine kinases in noncanonical Wnt signaling: their implications in developmental morphogenesis and human diseases. Dev Dyn 2010; 239:1 - 15; PMID: 19530173
- Nomi M, Oishi I, Kani S, Suzuki H, Matsuda T, Yoda A, et al. Loss of mRor1 enhances the heart and skeletal abnormalities in mRor2-deficient mice: redundant and pleiotropic functions of mRor1 and mRor2 receptor tyrosine kinases. Mol Cell Biol 2001; 21:832 - 935; PMID: 11713269; http://dx.doi.org/10.1128/MCB.21.24.8329-8335.2001
- Afzal AR, Jeffery S. One gene, two phenotypes: ROR2 mutations in autosomal recessive Robinow syndrome and autosomal dominant brachydactyly type B. Hum Mutat 2003; 22:1 - 11; PMID: 12815588; http://dx.doi.org/10.1002/humu.10233
- Afzal AR, Rajab A, Fenske CD, Oldridge M, Elanko N, Ternes-Pereira E, et al. Recessive Robinow syndrome, allelic to dominant brachydactyly type B, is caused by mutation of ROR2. Nat Genet 2000; 25:419 - 422; PMID: 10932186; http://dx.doi.org/10.1038/78107
- van Bokhoven H, Celli J, Kayserili H, van Beusekom E, Balci S, Brussel W, et al. Mutation of the gene encoding the ROR2 tyrosine kinase causes autosomal recessive Robinow syndrome. Nat Genet 2000; 25:423 - 426; PMID: 10932187; http://dx.doi.org/10.1038/78113
- Oldridge M, Fortuna AM, Maringa M, Propping P, Mansour S, Pollitt C, et al. Dominant mutations in ROR2, encoding an orphan receptor tyrosine kinase, cause brachydactyly type B. Nat Genet 2000; 24:275 - 278; PMID: 10700182; http://dx.doi.org/10.1038/73495
- Schwabe GC, Tinschert S, Buschow C, Meinecke P, Wolff G, Gillessen-Kaesbach G, et al. Distinct mutations in the receptor tyrosine kinase gene ROR2 cause brachydactyly type B. Am J Hum Genet 2000; 67:822 - 831; PMID: 10986040; http://dx.doi.org/10.1086/303084
- Person AD, Beiraghi S, Sieben CM, Hermanson S, Neumann AN, Robu ME, et al. WNT5A mutations in patients with autosomal dominant Robinow syndrome. Dev Dyn 2010; 239:327 - 337; PMID: 19918918
- Wang B, Sinha T, Jiao K, Serra R, Wang J. Disruption of PCP signaling causes limb morphogenesis and skeletal defects and may underlie Robinow syndrome and brachydactyly type B. Hum Mol Genet 2011; 20:271 - 285; PMID: 20962035; http://dx.doi.org/10.1093/hmg/ddq462
- Gao B, Song H, Bishop K, Elliot G, Garrett L, English MA, et al. Wnt signaling gradients establish planar cell polarity by inducing Vangl2 phosphorylation through Ror2. Dev Cell 2011; 20:163 - 176; PMID: 21316585; http://dx.doi.org/10.1016/j.devcel.2011.01.001
- Kawakami Y, Wada N, Nishimatsu SI, Ishikawa T, Noji S, Nohno T. Involvement of Wnt-5a in chondrogenic pattern formation in the chick limb bud. Dev Growth Differ 1999; 41:29 - 40; PMID: 10445500; http://dx.doi.org/10.1046/j.1440-169x.1999.00402.x
- Barrow JR, Thomas KR, Boussadia-Zahui O, Moore R, Kemler R, Capecchi MR, et al. Ectodermal Wnt3/betacatenin signaling is required for the establishment and maintenance of the apical ectodermal ridge. Genes Dev 2003; 17:394 - 409; PMID: 12569130; http://dx.doi.org/10.1101/gad.1044903
- Litingtung Y, Dahn RD, Li Y, Fallon JF, Chiang C. Shh and Gli3 are dispensable for limb skeleton formation but regulate digit number and identity. Nature 2002; 418:979 - 983; PMID: 12198547; http://dx.doi.org/10.1038/nature01033
- te Welscher P, Zuniga A, Kuijper S, Drenth T, Goedemans HJ, Meijlink F, et al. Progression of vertebrate limb development through SHH-mediated counteraction of GLI3. Science 2002; 298:827 - 830; PMID: 12215652; http://dx.doi.org/10.1126/science.1075620
- Adamska M, MacDonald BT, Sarmast ZH, Oliver ER, Meisler MH. En1 and Wnt7a interact with Dkk1 during limb development in the mouse. Dev Biol 2004; 272:134 - 144; PMID: 15242796; http://dx.doi.org/10.1016/j.ydbio.2004.04.026
- Loomis CA, Harris E, Michaud J, Wurst W, Hanks M, Joyner AL. The mouse Engrailed-1 gene and ventral limb patterning. Nature 1996; 382:360 - 363; PMID: 8684466; http://dx.doi.org/10.1038/382360a0
- Niswander L, Tickle C, Vogel A, Booth I, Martin GR. FGF-4 replaces the apical ectodermal ridge and directs outgrowth and patterning of the limb. Cell 1993; 75:579 - 587; PMID: 8221896; http://dx.doi.org/10.1016/0092-8674(93)90391-3
- Yashiro K, Zhao X, Uehara M, Yamashita K, Nishijima M, Nishino J, et al. Regulation of retinoic acid distribution is required for proximodistal patterning and outgrowth of the developing mouse limb. Dev Cell 2004; 6:411 - 422; PMID: 15030763; http://dx.doi.org/10.1016/S1534-5807(04)00062-0
- Boulet AM, Moon AM, Arenkiel BR, Capecchi MR. The roles of Fgf4 and Fgf8 in limb bud initiation and outgrowth. Dev Biol 2004; 273:361 - 372; PMID: 15328019; http://dx.doi.org/10.1016/j.ydbio.2004.06.012