Abstract
The effect of genetically modified (GM) plants on environment is now major concern worldwide. The plant roots of rhizosphere soil interact with variety of bacteria which could be influenced by the transgene in GM plants. The antibiotic resistance genes in GM plants may be transferred to soil microbes. In this study we have examined the effect of overexpression of salinity tolerant pea DNA helicase 45 (PDH45) gene on microbes and enzymatic activities in the rhizosphere soil of transgenic rice IR64 in presence and absence of salt stress in two different rhizospheric soils (New Delhi and Odisha, India). The diversity of the microbial community and soil enzymes viz., dehydrogenase, alkaline phosphatase, urease and nitrate reductase was assessed. The results revealed that there was no significant effect of transgene expression on rhizosphere soil of the rice plants. The isolated bacteria were phenotyped both in absence and presence of salt and no significant changes were found in their phenotypic characters as well as in their population. Overall, the overexpression of PDH45 in rice did not cause detectable changes in the microbial population, soil enzymatic activities and functional diversity of the rhizosphere soil microbial community.
Introduction
The soil salinity has become a major problem affecting plant productivity worldwide. Moreover, a rise in sea levels due to global warming is likely to intensify these problems. Therefore there is no alternative but to produce more saline-tolerant plant varieties, particularly for rice, the staple crop (Oryza sativa L.). Since salinity tolerance is controlled by multiple genes, the overexpression of a single gene resulting in field-level tolerance in rice is limited.Citation1 On the other hand, the overexpression of the salt-inducible pea DNA helicase, PDH45, in tobacco and rice (IR64) resulted in strong NaCl stress tolerant phenotype even when irrigated continuously with 200 mM NaCl, without affecting the yield.Citation2,Citation3 PDH45 is a unique member of this family as it contains DESD and SRT instead of the typical DEAD/H and SAT in motifs V and VI, respectively.Citation4
A specific microbial community is found in the zone around the root (called rhizosphere) that is influenced by the plant. The presence of antibiotic resistance genes in genetically modified (GM) plants as selection markers have raised questions about the possible transfer of these genes to indigenous microbes in the soil. For this reason, environmental risk assessment of GM plants has been mainly focused on possible horizontal gene transfer (HGT) to relative plants or to the soil- and plant-associated microbial communities.Citation5 It has been suggested that the rhizospheres could be altered in response to plant genetic transformation through HGT from GM plants to the indigenous soil microbes.Citation6 The comparative studies assessing differences between the microbial communities living in the rhizospheres of GM and non-GM plants represent a first step in determining if the presence of transgenic material can produce changes in the environment. It is evident that when GM plants were grown in a place for a long time, they change the rhizospheric microbial metabolism, enzymatic status causing negative effects on soil quality, structure and function and affecting the enzyme synthesis in microbes and activity as well as soil processes such as litter decomposition and mineralization.Citation7 There were some transient differences in the rhizospheric microbial community between soils cultivated with transgenic and non-transgenic plants. It has been shown that the Cry1Ab (gene from Bacillus thuringiensis subsp Kurstaki) protein is released in root exudates and binds rapidly onto surface-active particles in soil and become less accessible to microbial degradation but retains the insecticidal activity.Citation8 Therefore, accumulation of these proteins in soil may influence soil biological processes and microbial community composition. Blackwood and Buyer (2004)Citation9 used phospholipid fatty acid (PLFA) profiles and community-level physiological profiles of microbes to determine whether growing Bt corn had any effect on soil microbial communities as compared with the growth of non-Bt corn. They found that the profiles of the microbial communities were heavily affected by soil type, but the effect of expression of the Bt gene in corn was small.
An analysis of the soil enzymatic activity is one of microbiological indicators of soil quality. Enzymes participate in numerous biochemical processes occurring in the soil and they are sensitive to all environmental changes caused by natural and anthropogenic factors.Citation10 Enzymes are secreted by floral and faunal organisms, but most often they are produced by microorganisms in soil. The soil analysis therefore, includes the determination of the activity of intracellular enzymes, enzymes found on the cell surface and free enzymes. Their activity is related to the physical properties of the soil, organic matter content and the mechanism of action. The measurement of enzymatic activity in combination with the count of key microorganisms provides sensitive information about the changes occurring in the soil.Citation11 Some well-developed techniques, such as traditional plate based, most probable number (MPN) and direct microscopic counts, as well as molecular-based procedures and fatty acid analyses, can be used for characterizing soil microorganisms and to evaluate possible influence of transgenic plant on soil ecosystem.Citation12 The alteration of biological properties of the soil have often been proposed as an early and sensitive indicators of soil ecological stress or other environmental changes.Citation13 The assessment of microbial populations in combination with their activity provides more sensitive information than either activity or population analysis alone. The soil microorganisms like bacteria and fungi are the dominant organisms in soil and play central roles in the breakdown of organic matter, mineralization and fixation of nutrient, control of plant disease and amelioration of soil structure.Citation14 The impacts on soil microbial communities are therefore an important aspect of environmental risk assessment especially to monitor transgenic insect-resistant plants.Citation15
In the present study, we used overexpressing PDH45 T1 transgenic rice plants as our experimental system and we evaluated the changes in the rhizospheric soil of two different locations of India (New Delhi and Odisha, India) and soil microbial communities colonizing the rhizosphere of PDH45 transgenic rice plant in comparison to its non transgenic counterpart in the presence and absence of salt. In addition, the effects of PDH45-transgene on rhizospheric soil dehydrogenase activity (DHA), phosphatase, urease and nitrate reductase activities were also evaluated.
Results
Analysis of PDH45 T1 transgenic plants
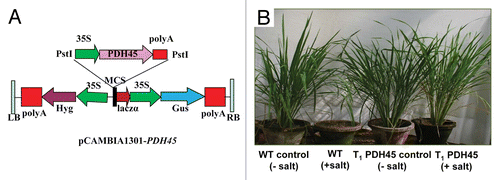
The PDH45 transgenic plants has been analyzed and described earlier.Citation2 Briefly, the PDH45 overexpressing transgenics rice plants (T1) performed well in water irrigated as well as in salt stress (200 mM NaCl) conditions. All the plants set viable seeds in both the conditions without yield loss (). Furthermore, the T1 transgenic plants showed significantly higher levels of leaf chlorophyll, nutrient content, net photosynthetic rate and phenotypic expressions and yield when irrigated with 200 mM NaCl as compared with the WT plants.Citation2
Physico-chemical properties of the experimental soil
The physico-chemical properties of the rhizospheric soil of New Delhi, India and Odisha, India used in pots of WT as well as transgenic plants were lateritic were (). The soil type of New Delhi was sandy clay loam having sand 62%, slit 21%, clay 17%, bulk density of 1.43 g cm3, particle density of 2.67 g cm3. The soil pH was 8.2 and 9.1 in absence and presence of salt, respectively. The soil of Odisha was alluvial sandy loam having sand 71%, slit 18%, clay 11%, bulk density of 1.75 g cm3, particle density of 2.97 g cm3. The pH of the soil in absence and presence of salt was 6.53 and 7.1, respectively. The soil analysis of two soils showed organic carbon content of 0.45% and 0.42% available nitrogen 247 and 212 kg/ha, available phosphorus 48 and 22.02 kg/ha, available potassium 285, 102.06 kg/ha, available calcium 253 and 128.9 kg/ha, available magnesium 198 and 208 kg/ha and available sulfur 18.6 and 12.3 kg/ha in absence as well as presence of salt. The available sodium content was 20 and 18 Kg/ha in absence of salt and 27 and 25 kg/ha in presence of salt, both in case of the WT and the PDH45 transgenic rice rhizosphere.
Table 1. Physico-chemical properties of soil
Population of bacteria and nematodes in rhizosphere soil of WT and PDH45 transgenic T1 plants
Five types of different bacterial colonies were isolated from the rhizospheric soil (New Delhi, India) of WT control and WT salt and six different bacterial colonies were isolated from PDH 45 transgenic control and PDH 45 transgenic salt treated pots on nutrient agar medium. The colonies differed in morphology (circular, low convex, convex, flat, plicate), color (brown, fluorescent, off white or white), size (ranged from 0.60–1.00 mm) and texture (gummy, not gummy, mucoid) (). Seven different types of bacterial colonies were isolated from the rhizospheric soil (Odisha) of WT control and WT salt pots. Six different types of bacterial colonies were isolated from rhizospheric soil of PDH45 transgenic control and PDH 45 transgenic salt treated plants (). and summarizes the morphological feature assessed for the isolated colonies. The population dynamics of rhizospheric bacteria were found 51, 55 and 49, 53 × 105 cfu/g in WT and 48, 52 and 48, 50 × 105 cfu/g in PDH45 transgenic plants in absence and presence of salt respectively, (). The population of nematodes was 911.3, 845 and 917.4, 853 in each pot (app. 8 kg soil) in case of WT and 915.2, 842 and 920.3, 847 in PDH45 transgenic in the absence and presence of salt, respectively ().
Table 2. Colony characteristics of different isolates of rhizospheric soil (New Delhi, India) of pots
Table 3. Colony characteristics of different isolates of rhizospheric soil (Odisha, India) of pots
Table 4. Population of nematodes and bacteria in rhizospheric soil of different pots
DHA, alkaline phosphatase, urease and nitrate reductase activity in rhizosphere soil
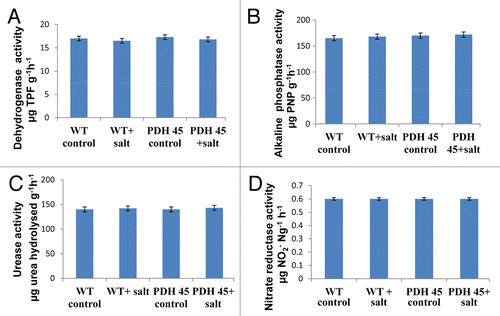
The effect of transgenic rice on rhizospheric soil enzymes, DHA, alkaline phosphatase, urease and nitrate reductase, enzyme assay were performed. On the basis of above enzymatic assay it was found that PDH 45 transgenic rice plant did not show a significant difference when compared with its WT counterpart both in the presence and absence of salt. In the absence of salt, PDH45 T1 transgenic and WT rice showed DHA activity values of 20.2 ± 1.5 and 20.1 ± 1.5 μg TPF g−1 soil h−1, in New Delhi and 16.5 ± 1.4 and 17.0 ± 1.6 μg TPF g−1 soil h−1in Odisha, soil alkaline phosphatase activity were 170.4 ± 10.5 and 170.4 ± 10.5 µg pNP g−1 soil h−1 in New Delhi and 160 ± 11.4 and 163 ± 11.5 µg pNP g−1 soil h−1 in Odisha soil, urease activity as 154.7 ± 10.2 and 154.2 ± 10.2 µg urea hydrolysed g−1h−1 in New Delhi soil and 140 ± 11.1 and 142 ± 11.3 µg urea hydrolysed g−1h−1 in Odisha soil. The nitrate reductase activity were 0.5 ± 0.01 and 0.5 ± 0.01 µg NO2- Ng−1 h−1 in New Delhi soil and 0.7 ± 0.01 and 0.7 ± 0.01µg NO2− Ng−1 h−1 in Odisha soil, respectively ( and ). A slight difference in the enzymatic activities of salt treated soil were observed. In the presence of salt, values of DHA were 21.1 ± 1.5 and 21.1 ± 1.5 μg TPF g−1 soil h−1 in New Delhi and 16 ± 1.4 and 16.5 ± 1.4 μg TPF g−1 soil h−1 in Odisha soil. Alkaline phosphatase level was 165.1 ± 10.5 and 166.9 ± 10.5 µg pNP g−1 soil h−1 in New Delhi and 163 ± 10.4 and 160 ± 10.4 µg pNP g−1 soil h−1 in Odisha soil, urease activity as 154.2 ± 10.2 and 154.7 ± 10.2 µg urea hydrolysed g−1h−1 in New Delhi soil and 140 ± 10.4 µg urea hydrolysed g−1h−1 in Odisha soil. The nitrate reductase activity as 0.5 ± 0.01 and 0.5 ± 0.01 µg NO2− Ng−1 h−1 and 0.7 ± 0.01 µg NO2− Ng−1 h−1 in WT and PDH45 transgenic rice plants, respectively ( and ).
Figure 2. Enzyme activity assay of soils (New Delhi, India). Variations of dehydrogenase (A) acid phosphatase (B), urease (C) and nitrate reductae (D) activities in: (1) WT control (−salt); (2) WT+salt; (3) T1-PDH 45-salt; (4) T1-PDH 45+salt (200 mM NaCl). Data are significantly not different at p < 0.05, n = 3).
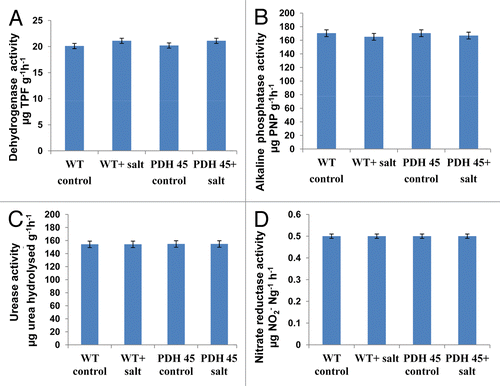
Figure 3. Enzyme activity assay of soils (Odisha, India).Variations of dehydrogenase (A) acid phosphatase (B), urease (C) and nitrate reductae (D) activities in: (1) WT control (−salt); (2) T+salt; (3) T1-PDH 45-salt; (4) T1-PDH 45+salt (200 mM NaCl). Data are significantly not different at p < 0.05, n = 3).
Discussion
In the present study, we tried to analyze the effect of salt stress tolerant PDH45 transgenic plants on the rhizosphere soil to assess its potential as a candidate for field trials. Our results on PDH45 transgenic rice showed that introduction of transgenic plants did not alter the physico-chemical properties of rhizosphere soil, enzymatic activity and also the population of soil microflora.
The literature survey indicates variable effect of transgenic plants on rhizosphere soil. While a few reports have indicated the adverse effects of the transgenic plants, few others have demonstrated no significant difference in composition of the rhizosphere. It has been reported that Bt corn affects the microbial communities, activities of some enzymes and microbe-mediated processes and functions in the soil.Citation12 The study by Aira et al., (2010)Citation16 on the microbial communities in maize rhizosphere states that the plant genotype (su1 and sh2 genes) strongly influence the structure and growth of rhizosphere microbial communities. However, the results presented in this study are consistent with the experiment conducted with Bt rice.Citation17 They did not find any significant differences in dehydrogenase and phosphatase activities, respiration, methanogenesis and in composition of fungal community in rhizosphere soil of transgenic and wild type rice. Oliveira et al., (2008)Citation18 found that the microbial populations of the soil did not affect in presence of Bt maize. Likewise, some studies have proven that the long-term cultivation of transgenic plants did not affect the soil microbial diversity.Citation19 It is interesting to note that in the present study, no significant differences were observed in enzyme activity in soil rhizosphere of PDH45 transgenic and non transgenic (WT) plants of same variety, which also correlates with the experiment on Bt cotton where except for dehydrogenase enzyme, the differences in the activity of alkaline phosphatase, nitrate reductase and urease enzymes between Bt and non Bt plants rhizosphere were statistically non-significant.Citation20 No adverse effect was observed in MCM6 transgenic tobacco cultivation on soil enzymatic activities and rhizosphere microbial communities.Citation21 From the above data, it is clear that the cultivation of transgenic plants in all cases did not affect on soil microbial community and enzymatic activity. It depends on the particular plants, techniques, protein and environmental conditions.Citation22
In this study we have tested the impact of PDH45 transgenic rice plant in two different soil and no significant variations were observed in population of bacteria and nematodes in PDH45 transgenic and non-transgenic rice rhizosphere. The observation of this study correlates to the observation reported earlier by Saxena and Stotzky (2001).Citation7 They observed that transgenic corn crops had no apparent effect on nematodes.
Conclusion
In the present study it was observed that the PDH45 transgenic rice plants had no detectable adverse effects on the soil microbial community composition, physico-chemical properties and enzymatic activities of the soil rhizosphere as compared with their WT counterpart. The rhizospheric soil bacterial populations and enzyme activities revealed minor alterations among transgenic and non-transgenic rice plants and therefore, these studies have shown the possibility that there was no evidence to indicate any adverse effects of transgenic PDH45 on the native soil microflora.
Material and Methods
Plasmid construct and Agrobacterium-mediated transformation of rice
PCR amplified PDH45 ORF (1.2 Kb; amplified using Forward primer: 5′- GAGCTCATGGCGACAACTTCTGTGG-3′ Reverse primer: 5′-GAGCTCGAGTTATATAAGATCACCAATATTC-3′) was cloned in pRT100 using XbaI restriction enzymes. The PDH45 plant expression cassette from pRT100 was then mobilized to binary vector pCAMBIA1301 using restriction enzyme PstI (). Competent strain of Agrobacterium tumefaciens (LBA4404) was transformed with pCAMBIA1301-PDH45 construct as described earlier.Citation23 Scutella derived calli of rice IR64 were used for Agrobacterium-mediated transformation as described.Citation23 Putative T0 transgenic plants were transferred to pots containing vermiculite and were incubated in green house operating at 28°C, 16 h light at 100–125 μ mol m–2 s–1 and 70–75% relative humidity. The plants were regularly irrigated with nutrient medium and were grown to maturity. The transgenic plants were then screened by PCR. The seeds from T0 plants were collected and selected by growing in hygromycin containing medium. The PCR positive T1 transgenic lines were used for Southern blot analysis and the homozygous lines were used for further study.
Experimental site and soil sampling
The pot experiments were conducted in the green house of International Centre for genetic Engineering and Biotechnology, New Delhi using two type of soils (New Delhi, India and Odisha, India) . In triplicates, the T1 transgenic and wild type (WT) rice plants were grown in earthen pots at 28°C, 16 h light at 100–125 μ mol m–2 s–1 and 70–75% relative humidity. The plants were grown for 90 d to maturity and seeds were collected. The tolerance of plants to salinity was tested after 21 d in the presence and absence of salt (200 mM NaCl). Rhizosphere soil samples collected from each pot was transferred to plastic bags and plant debris was removed manually. Soil samples were then powdered and sieved and kept at 4°C, in dark for further analysis.
Physical and chemical properties of rhizosphere soil
The physico-chemical characters of soil and nutrient constituents viz. soil type, pH, electrical conductivity Eh (mS/cm), organic carbon (OC) (%), available nitrogen (AN) (Kg ha−1); available phophorus (AP) (kg/ha), available potassium (AK) (kg/ha), available calcium (ACa) (Kg ha−1), available magnesium (AMg) (Kg ha−1) and available sulfur (AS) (Kg ha−1) and available sodium (ANa) (Kg ha−1) were analyzed in the laboratory following standard methods (as described below) immediately after sample collection.
Measurement of soil pH and electrical conductivity of soil
To determine Eh and pH of the soil samples, 50 g of soil was suspended in 100 ml distilled, deionised water and stirred for 1 h at 100 rpm on a rotary shaker. The supernatant of the sample was then collected by centrifuged it at 10,000 g for 5 min. Eh (mS/cm) was recorded through a conductivity meter (Systronics) against 0.01N KCl and pH was measured through a pH meter (Systronics).Citation24
Estimation of available carbon content of soil
One gram of soil sample was transferred to a 500 ml wide mouth flask and 10 ml 1N K2Cr2O7 was added to it and swirled to disperse the soil completely. Then 20 ml H2SO4 was added to it and allowed to stand for 30 min. Then 200 ml water was added followed by 3–4 drops of ferroin indicator. The solution was then titrated against 0.5 N ferrous ammonium sulfate (FAS).Citation25
Estimation of available nitrogen content of soil
Available nitrogen content of the soils was determined by modified Kjeldahl digestion method.Citation26 A twenty gram soil sample was taken in a 800 ml Kjeldahl flask. Ten ml distilled water was added to resuspend the soil. Then, 100 ml 0.32% KMnO4, a few glass beads, 2–3 ml paraffin liquid was added to it. Twenty ml (2%) boric acid mixed with indicator (bromo cresol green: methyl red:: 2:1) in a 250 ml conical flask was placed under receiver tube and was titrated against 0.02 N H2SO4 taken in burette until pink color started appearing.
Determination of available phosphorus in soil
The available phosphorus content of the soil was determined as explained by Page et al., (1982).Citation27 Soil (2.5 g) was taken in a 100 ml conical flask, a pinch of Olsen’s reagent (0.5 M NaHCO3) was added to it and the flask was shaken to thoroughly mix the ingredients. Five ml of filtered solution was taken in a 25 ml volumetric flask and 5 ml ammonium molybdate (0.5 N), 1 ml freshly prepared SnCl2 (10 mM) solutions were added and the volume was made up to 25 ml by addition of distilled water. The optical density (OD) was measured at 660 nm.
Determination of available potassium and sodium in soil
The available potassium content of the soil was determined by method described by Jackson (1973).Citation26 Soil (5 g) was taken in a 250 ml conical flask and 25 ml of 1N CH3COONH4 solution was added to it. The flask was shaken for 30 min, the solution was filtered and the filtrate was diluted to 50 ml with 1N CH3COONH4 solution and the data were measured by flame photometer (Bellstone) and the concentration was calculated by plotting the readings against the standard curve.
Sodium (Na) can be extracted with ammonium acetate solution in the same way as K, subsequently; Na in the extract can be determined by flame photometry. Certain elements, including Na, have the property that, when their salts are introduced into a flame, they emit light with a wavelength (color) specific to the element and of intensity proportional to the concentration.Citation28
Determination of available sulfur in soil
The available sulfur content of the soils was determined by method described by Chesin and Yien (1951).Citation29 Twenty grams of soil sample was taken in a 250 ml conical flask, 100 ml monocalcium phosphate extracting solution (500 mg/l) was added to it and filtered. Ten ml filtrate was taken in a 25 ml volumetric flask, 2.5 ml 25% HNO3, 2 ml acetic phosphoric acid, 0.5 ml BaSO4 solution and 0.2 g of BaCl2 crystals were added to it. Then the volume was made upto 25 ml and the optical density (OD) was measured at 440 mm.
Determination of calcium and magnesium from soil
The available magnesium and calcium contents of the soil were determined by method described by Jodral-segado (2006).Citation30 The homogenized soil sample (300 mg) was placed in a 100-ml volumetric flask and mineralized by addition of 5 ml of concentrated HNO3 and heated at 90°C for 45 min in a sand mineralization block. Five ml of 4:1 mixture of HNO3 and HClO4 was added to the solution and the heating was continued at 130°C for an additional 2 h until the sample was completely mineralized. Then the mixture was cooled and the resulting solution diluted to 25 ml with ultra pure water. A second dilution was prepared by taking different aliquots of the previous dissolution and diluting them with ultra pure water. In order to avoid the phosphate interference, 0.2 ml of LaCl3 solution (10 mM) was added as a matrix modifier.Citation31 Calcium and magnesium determinations were performed by direct aspiration into the flame atomic absorption spectrophotometer (Perkin-Elmer 1100B double beam atomic absorption spectrophotometer, Perkin-Elmer Corp). The presence of matrix interference was observed only for calcium. Therefore, for this element the samples were analyzed by the standard addition method. All magnesium determinations were performed by the linear calibration method.Citation28
Isolation of rhizospheric bacteria
To obtain the isolated bacteria, serial dilution (10−4 dilution) prepared from 1 g soil sample was plated on nutrient agar media. Dissimilar colonies obtained on plates after an incubation time of 2–5 d at 35 ± 0.1°C were carefully picked. The individual colonies were checked for homogeneity under phase contrast microscope and the pure cultures were maintained both on slants of nutrient medium at 4 ± 0.1°C as well as with 15% glycerine and lyophilized powder at −80 ± 0.1°C. The identification of the rhizospheric bacteria was performed as described earlier by Khan (2006).Citation32 The populations of total bacteria from each pot were counted as colony forming units (cfu/g dr. soil).
Morphological and staining characteristics of the bacteria
For assessment of morphological characteristics (colony form, color, elevation, margin, size and consistency shape, size, motility and Gram’s status), the individual bacterial isolate were observed under phase contrast light microscope (100× objective).Citation33 Overnight grown bacterial cultures were smeared on glass slides and observed under microscope. Gram staining and spore stain (malachite green) of the bacterial culture was performed as described earlier.Citation33
Isolation of nematodes
The nematodes were extracted from PDH45 transgenic and non transgenic rice plants rhizosphere soil (250 g) by the Baermann technique.Citation34 Nematodes samples from PDH45 transgenic and non-transgenic (WT) rice plants in water suspension were uniformly placed over tissue paper supported by screen in a petriplates, which were filled with water. After placing the sample, petriplates were covered with respective cap and incubated at 24 ± 2°C for 36 h. The nematodes present in soil were counted by transferring them to flat bottom partitioned counting dishes (36 squares) where the number of nematodes in 10 random squares were counted by observing under stereoscopic dissecting microscope (Nikon, 10–100× zoom). The averaged value of the count thus obtained was multiplied by 36 to get the total count of the nematodes.
Determination of soil dehydrogenase activity (DHA) activity
The soil dehydrogenase activity was evaluated as described earlier.Citation35 Five grams of soil suspended in 5 ml of a TTC (Triphenyl Tetrazolium Chloride) solution (5 g TTC in 0.2 M Tris-HCl buffer, pH 7.4) was incubated at 37°C for 12 h. To stop the reaction, two drops of concentrated sulfuric acid were added followed by addition of toluene (5 ml). In order to extract TPF (Triphenylformazan), the mixture was shaken at 250 rpm for 30 min, followed by a 5 min centrifuging at 4,500 g. The color intensity of the supernatant thus obtained was measured at 492 nm by UV-Vis spectrophotometer (UV-1201, Shimadzu Corp).
Soil alkaline phosphatase activity
The alkaline phosphatase activity was measured by incubating 1 g soil with extraction solution (0.25 ml of toluene, 4 ml methylumbelliferone (MUB) buffer (pH 11), 1 ml p- nitrophenolphosphate solution (in MUB buffer)) for 1 h. Following the incubation, 1 ml of CaCl2 (0.5 M) and 4 ml of NaOH (0.5 M) were added to the flask and the contents were further incubated at 37°C for 1 h. The suspension was filtered (Whatmann no. 2 filter paper) and the absorbance of the filtrate was measured at 400 nm.Citation36
Soil urease activity
The urease activity in the soil was measured by assessing its ability to hydrolyse urea in reaction mixture.Citation37 Five grams of soil was mixed with 5 ml of urea solution (10 mg urea/ml) and incubated for 5 h at 37°C. The mixture was further mixed with 50 ml 2 M KCl-PMA (phenyl-mercury acetate) for 1 h under shaking, followed by filtration. Two ml from the filtrate was mixed with 10 ml 2 M KC1-PMA and 30 ml coloring reagent (25 ml 2.5% diacetylmonoxime (DAM) + 10 ml of 0.25% thiosemicarbazide (TSC) in 500 ml acid reagent) and incubated in 65°C for 30 min and kept in ice cold water for 15 min. The absorbance of the reaction mixture was measured at 527 nm.
Nitrate reductase activity
The nitrate reductase activity was measured as previously described.Citation38 Briefly, 10 ml of substrate solution (4 ml of 0.19 mM 2,4 dinitrophenol, 1 ml 25 mM KNO3, 5 ml distilled water) was added to 5 g soil sample. The mixture was incubated at 37°C for 24 h while the control sample was immediately frozen at −20°C. After incubation, 10 ml of 4M KCl was added to the samples and control and filtered immediately. To 5 ml of the filtrate, 3 ml of 0.19 M ammonium chloride buffer and 2 ml of color reagent (viologen dyes) was added. The absorbance of the reaction mixture was measured at 520 nm.
Statistical analyses
All the experiments were performed using three biological and three technical replicates. The data was analyzed statistically and standard error was calculated. Analysis of variance (ANOVA) was performed on the data using SPSS (10.0 Inc) to determine least significant difference (LSD) for significant data to identify difference in the mean of the treatment.
Acknowledgments
We thank Dr Renu Tuteja (ICGEB, New Delhi, India) and Dr Tushar Kanti Dangar (Central Rice Research Institute, Cuttack, Odisha, India) for their helpful comments/corrections. Work on signal transduction, abiotic stress tolerance and plant microbes interactions in NT’s laboratory are partially supported by Department of Science and Technology (DST) and Department of Biotechnology (DBT), Government of India.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- Fukuda A, Nakamura A, Tagiri A, Tanaka H, Miyao A, Hirochika H, et al. Function, intracellular localization and the importance in salt tolerance of a vacuolar Na(+)/H(+) antiporter from rice. Plant Cell Physiol 2004; 45:146 - 59; http://dx.doi.org/10.1093/pcp/pch014; PMID: 14988485
- Sahoo RK, Gill SS, Tuteja N. Pea DNA helicase 45 promotes salinity stress tolerance in IR64 rice with improved yield. Plant Signal Behav 2012; 7:1042 - 6; http://dx.doi.org/10.4161/psb.20915; PMID: 22827940
- Sanan-Mishra N, Pham XH, Sopory SK, Tuteja N. Pea DNA helicase 45 overexpression in tobacco confers high salinity tolerance without affecting yield. Proc Natl Acad Sci USA 2005; 102:509 - 14; http://dx.doi.org/10.1073/pnas.0406485102; PMID: 15630095
- Pham XH, Reddy MK, Ehtesham NZ, Matta B, Tuteja N. A DNA helicase from Pisum sativum is homologous to translation initiation factor and stimulates topoisomerase I activity. Plant J 2000; 24:219 - 29; http://dx.doi.org/10.1046/j.1365-313x.2000.00869.x; PMID: 11069696
- Lee YE, Yang SH, Bae TW, Kang HG, Lim PO, Lee HY. Effects of field-grown genetically modified Zoysia grass on bacterial community structure. J Microbiol Biotechnol 2011; 21:333 - 40; PMID: 21532315
- Lynch JM, Benedetti A, Insam H, Nuti MP, Smalla K, Torsvik V, et al. Microbial diversity in soil: ecological theories, the contribution of molecular techniques and the impact of transgenic plants and transgenic microorganisms. Biol Fertil Soils 2004; 40:363 - 85; http://dx.doi.org/10.1007/s00374-004-0784-9
- Saxena D, Stotsky G. Bacillus thuringiensis (Bt) toxin released from root exudates and biomass of Bt corn has no apparent effect on earthworms, nematodes, protozoa, bacteria, and fungi in soil. Soil Biol Biochem 2001; 33:1225 - 30; http://dx.doi.org/10.1016/S0038-0717(01)00027-X
- Vettori C, Paffetti D, Saxena D. Persistence of toxins and cells of Bacillus thuringiensis subsp. Kurstaki introduced in sprays to Sardinia soils. Soil Biol Biochem 2003; 35:1635 - 42; http://dx.doi.org/10.1016/j.soilbio.2003.08.009
- Blackwood CB, Buyer JS. Soil microbial communities associated with Bt and non-Bt corn in three soils. J Environ Qual 2004; 33:832 - 6; http://dx.doi.org/10.2134/jeq2004.0832; PMID: 15224917
- Trasar-Capeda C, Lieros MC, Seoane S, Gil-Sotres F. Limitations of soil enzymes as indicators of soil pollution. Soil Biol Biochem 2000; 32:1867 - 75; http://dx.doi.org/10.1016/S0038-0717(00)00160-7
- Brookes PC. The use of microbial parameters in monitoring soil pollution by heavy metal. Biol Fertil Soils 1995; 19:269 - 79; http://dx.doi.org/10.1007/BF00336094
- Icoz I, Saxena D, Andow DA, Zwahlen C, Stotzky G. Microbial populations and enzyme activities in soil in situ under transgenic corn expressing cry proteins from Bacillus thuringiensis.. J Environ Qual 2008; 37:647 - 62; http://dx.doi.org/10.2134/jeq2007.0352; PMID: 18396552
- Oliveira A, Pampulha ME. Effects of long-term heavy metal contamination on soil microbial characteristics. J Biosci Bioeng 2006; 102:157 - 61; http://dx.doi.org/10.1263/jbb.102.157; PMID: 17046527
- Bruinsma M, Kowalchuk GA, van Veen JA. Effects of genetically modified plants on microbial communities and processes in soil. Biol Fertil Soils 2003; 37:329 - 37
- Andow DA, Zwahlen C. Assessing environmental risks of transgenic plants. Ecol Lett 2006; 9:196 - 214; http://dx.doi.org/10.1111/j.1461-0248.2005.00846.x; PMID: 16958885
- Aira M, Brandon MG, Lazcano C, Baath E, Dominguez J. Plant genotype strongly modifies the structure and growth of maize rhizosphere microbial communities. Soil Biol Biochem 2010; 42:2276 - 81; http://dx.doi.org/10.1016/j.soilbio.2010.08.029
- Liu W, Lu HH, Wu W, Wei QK, Chen YX, Thies JE. Transgenic Bt rice does not affect enzyme activities and microbial composition in the rhizosphere during crop development. Soil Biol Biochem 2008; 40:475 - 86; http://dx.doi.org/10.1016/j.soilbio.2007.09.017
- Oliveira AP, Pampulha ME, Bennett JP. A two-year field study with transgenic Bacillus thuringiensis maize: effects on soil microorganisms. Sci Total Environ 2008; 405:351 - 7; http://dx.doi.org/10.1016/j.scitotenv.2008.05.046; PMID: 18656246
- Li XG, Liu BA, Cui JJ, Liu DD, Ding SA, Gilna B, et al. No evidence of persistent effects of continuously planted transgenic insect-resistant cotton on soil microorganisms. Plant Soil 2011; 339:247 - 57; http://dx.doi.org/10.1007/s11104-010-0572-2
- Mina U, Chaudhary A, Kamra A. Effect of Bt cotton on enzymes activity and microorganisms in rhizosphere. J Agric Sci 2011; 3:1 - 9
- Chaudhry V, Dang HQ, Tran NQ, Mishra A, Chauhan PS, Gill SS, et al. Impact of salinity-tolerant MCM6 transgenic tobacco on soil enzymatic activities and the functional diversity of rhizosphere microbial communities. Res Microbiol 2012; 163:511 - 7; http://dx.doi.org/10.1016/j.resmic.2012.08.004; PMID: 22989673
- Brusetti L, Francia P, Bertolini C, Pagliuca A, Borin S, Sorlini C, et al. Bacterial communities associated with the rhizosphere of transgenic Bt 176 maize (Zea mays) and its non transgenic counterpart. Plant Soil 2005; 266:11 - 21; http://dx.doi.org/10.1007/s11104-005-5399-x
- Sahoo RK, Tuteja N. Development of Agrobacterium-mediated transformation technology for mature seed-derived callus tissues of indica rice cultivar IR64. GM Crops Food 2012; 3:123 - 8; PMID: 22538224
- Gupta PK. Methods in environmental analysis water, soil and air. Agrobios, India 2004; pp 242-245.
- Walkley A, Black IA. An examination of Degtjareff method for determining soil organic matter and a proposed modification of the chromic acid titration method. Soil Sci 1934; 37:29 - 38; http://dx.doi.org/10.1097/00010694-193401000-00003
- Jackson ML. Soil. Chem Anal 1958; 214 - 21
- Page AL. Methods of soil analysis, Agron. 9, Part 2: Chemical and mineralogical properties, 2nd ed., Am. Soc. Agron., Madison, WI, USA. 1982.
- Richards LA. Diagnosis and improvement of saline and alkali soils. USDA Agriculture Handbook 60, Washington D.C. 1954.
- Chesnin L, Yien C. Turbidimetric determination of available sulphate. Proc- Soil Sci Soc Am 1951; 15:149 - 51; http://dx.doi.org/10.2136/sssaj1951.036159950015000C0032x
- Jodral-segado AM, Navarro-alarco N, Serrana DL, Lopez-martinez MC. Calcium and magnesium levels in agricultural soil and sewage sludge in an industrial area from Southeastern Spain: relationship with plant (Saccharum officinarum) disposition. Soil & Sediment Contamination 2006; 15:367 - 77; http://dx.doi.org/10.1080/15320380600751736
- Moreno-Torres R, Navarro M, Ruiz-Lopez MD, Artacho R, Lopez MC. Comparison of wet and dry mineralization procedures for determining calcium and phosphorus in cow’s milk. Aust J Dairy Technol 2000; 55:23 - 7
- Khan MS. Screening of free-living rhizospheric bacteria for their multiple plant growth promoting activities. Microbiol 2006; 163:173 - 81
- Collee J, Miles RS. Tests for identification of bacteria. In: College, J.R., Duguid, J.P., Fraser, A.G., Marmion, B.P. (eds). Mackie and McCartney’s Practical Medical Microbiology. 13th ed. Churchill Livingstone/Longman, London. 1989; 141-160.
- Van Gundy SD. Nematodes. In: Page AL, Miller RH, Keeney DR. (Eds). Methods of Soil Analysis. Part 2. American Society of Agronomy, Madison, WI, 1982; 1124-128.
- Min H, Ye YF, Chen ZY, Wu WX, Yufeng D. Effects of butachlor on microbial populations and enzyme activities in paddy soil. J Environ Sci Health B 2001; 36:581 - 95; http://dx.doi.org/10.1081/PFC-100106187; PMID: 11599722
- Tabatabai MA, Bremner JM. Use of p-nitrophenyl phosphate for assay of soil phosphatase activity. Soil Biol Biochem 1969; 1:301 - 7; http://dx.doi.org/10.1016/0038-0717(69)90012-1
- Tabatabai MA. Soil enzymes. In: Weaver RW, Angel JS, Bottomley PS. (Eds). Methods of soil analysis. Part 2. Microbial and biochemical properties. Soil Science Society of America, Madison, 1994. 775–833.
- Abdellmagid HM, Tabatabai MA. Nitrate reductase activity of soils. Soil Biol Biochem 1987; 19:421 - 27; http://dx.doi.org/10.1016/0038-0717(87)90033-2