Abstract
Abiotic stress and light induce anthocyanin accumulation in Arabidopsis. Here, we demonstrate that mesophyll-localized phytochromes regulate nitrogen-, phosphate- and cold-induced anthocyanin accumulation in shoots of Arabidopsis. Whereas ecotype-dependent differences result in distinct total levels of anthocyanin accumulation in response to light, cold, or nutrient-deficient treatments, phytochromes generally gate light- and/or stress-induced anthocyanin accumulation in shoots, as plants depleted of mesophyll-localized phytochromes lack or have highly attenuated induction of anthocyanins. Observed interactions between light and stress were found to be wavelength dependent, with red and far-red light stimulating higher total levels of anthocyanin accumulation under cold temperatures, especially in response to nitrogen limitation, whereas blue light did not. The roots of plants depleted of mesophyll-localized phytochromes still respond to nutrient deficiency as determined by elongation of primary roots and root hair elongation when plants are grown under nitrogen- or phosphate-limited conditions. Plants which are constitutively deficient in photoreceptors in both shoots and roots, i.e., phy or cry mutants, exhibit defects in light- and stress-induced anthocyanin accumulation and defects in root development. Taken together, these results suggest that the response to nutrient limitation in roots and shoots is under distinct control by spatial-specific pools of phytochromes in Arabidopsis.
Introduction
Anthocyanins are water-soluble pigments derived from flavonoids, which serve vital roles during plant development and in response to environmental stresses. The accumulation of anthocyanins occurs in specific tissues at discrete developmental stages and is strictly regulated.Citation1 Among the environmental factors that regulate this process are light, temperature, nutrients, and stress.Citation2 The synthesis and accumulation of anthocyanins at distinct times and in distinct tissues can impact specific physiological processes in plants. For example, light-induced accumulation of these compounds has been implicated in protection of cells against damaging light exposure.Citation3
Light regulation of anthocyanin biosynthesis and accumulation in Arabidopsis involves phytochromes and cryptochromes.Citation4-Citation9 Phytochromes and cryptochromes that are localized in specific tissues and/or organs are able to mediate distinct photoresponses.Citation10-Citation13 Anthocyanin levels can also be induced by stress in plants. Low temperature can result in the induction of anthocyanins in Arabidopsis, which is light dependent.Citation14,Citation15 Photoreceptor-dependent LONG HYPOCOTYL 5 (HY5), which is a member of the bZIP factor family,Citation16 and HY5-HOMOLOG (HYH),Citation17 have roles in low temperature-induced, light-dependent anthocyanin accumulation.Citation15 Prior results showed that HY5 and HYH were required for light-induced anthocyanin accumulation,Citation17,Citation18 thus these factors may serve as central regulators for light- and low temperature-induced anthocyanin accumulation.Citation15 Nutrient deficiency can also result in increased anthocyanin accumulation. Some plants grown under phosphate-deficient conditionsCitation19-Citation24 or nitrogen-deficient conditionsCitation25-Citation28 accumulate significantly higher levels of anthocyanins than when seedlings are grown in nutrient-replete conditions. Notably, low temperature and nitrogen deficiency interact, resulting in an increased anthocyanin accumulation under white light than either stress alone.Citation29,Citation30
The roles of specific and/or spatially distinct photoreceptor pools in the cross-talk between plant responses to light and nutrient deficiency have not been fully investigated. We investigated the roles of phytochromes in stress- and light-induced accumulation of anthocyanins in Arabidopsis under distinct wavelengths of light. We explore the functional specialization of spatially-localized pools of phytochromes in regulating a plant’s ability to response to nitrogen-limitation, phosphate-limitation, and/or low temperature in the stress-related induction of anthocyanin accumulation.
Materials and Methods
Plant materials and light sources
Mesophyll phytochrome-deficient CAB3::pBVR1 and CAB3::pBVR2 transgenic plant lines were previously isolated.Citation13 The remaining Arabidopsis ecotypes and genotypes utilized include the following wild-type (WT), mutants and T-DNA insertion lines obtained from the Arabidopsis Biological Resource Center: No-0 WT, Col-0 WT, phyA (SALK_014575),Citation31,Citation32 phyB (SALK_ 022035),Citation31,Citation32 cry1–401Citation33 and cry2–1.Citation34 Continuous blue (Bc), continuous red (Rc) and continuous far-red (FRc) light sources were described previously.Citation13 FR fluence rates were measured with a StellarNet EPP2000 spectroradiometer (Apogee Instruments, Logan, UT). All other light fluence rates were measured using a Li-Cor light meter (model LI-250, Li-Cor, Lincoln, NE) with an attached Li-Cor quantum sensor (model LI-190SA).
Plant growth media and growth conditions
Basal medium (Basal) consisted of 1X Murashige and Skoog Basal Salt Mixture (PhytoTechnology Laboratories®, KS) with 0.9% (w/v) Phytoblend agar (Caisson Laboratories, UT) and 1% (w/v) Sucrose, adjusted to pH 5.7 with NaOH. For N limitation (-N), media contained 1X Murashige and Skoog Modified Basal Salt Mixture without Nitrogen, Phosphorous, and Potassium (PhytoTechnology Laboratories®, KS) with 0.17 g/L of K2HPO4, 0.9% (w/v) Phytoblend and 1% (w/v) Sucrose, adjusted to pH 5.7 with HCl. For P limitation (-P), media contained 1X Murashige and Skoog Modified Basal Salt Mixture without Nitrogen, Phosphorous, and Potassium with 1.65 g/L of NH4NO3, 1.9 g/L of KNO3, 0.9% (w/v) Phytoblend and 1% (w/v) Sucrose, adjusted to pH 5.7 with NaOH.
Seeds were sterilized and planted as previously published.Citation13 Sterilized seeds were planted in 100- x 20-mm petri dishes on Basal, -N and -P media and treated with a R pulse at ~75 µmol m−2 s−1 for 5 min prior to imbibition. Imbibing seeds were cold-stratified at 4 °C for 3 d in darkness. Plates were grown under FRc illumination of 5 µmol m−2 s−1, Rc illumination of 50 µmol m−2 s−1, Bc illumination of 25 µmol m−2 s−1or in darkness for 4 d at 22 °C or 10 d at 12 °C.
Quantification of Anthocyanin Levels
Anthocyanins were extracted from seedlings using 1% (v/v) HCl in methanol as describedCitation35 with prior detailed modifications.Citation6 Two-tailed, unpaired Mann-Whitney U-test analyses were performed to compare the means of anthocyanin contents per mg of fresh weight of transgenic or mutant seedlings relative to cognate WT seedlings and respective nutrient limitations.
Analysis of Root Growth
Seedlings were grown vertically in square 10- x 10-cm petri plates on Basal, -N, or -P media containing 1% (w/v) Phytoblend agar (Caisson Laboratories, UT) under monochromatic light conditions as described previously.Citation10 Plates containing 7-d-old seedlings were scanned to obtain root images. To quantify root lengths, the roots of scanned seedlings were measured using Image J software (NIH).
Results
Abiotic stress results in a mesophyll phytochrome-dependent enhancement in the accumulation of anthocyanins
Under FRc light, No-0 and Col-0 WT ecotypes exhibited an induction in anthocyanin accumulation under limiting nitrogen and phosphate conditions at 22 °C (, representative seedling images and pigment quantification). Lines in which mesophyll-specific inactivation of phytochromes is achieved by expression of a gene encoding a phytochrome chromophore-degrading enzyme biliverdin reductase (BVR), i.e., CAB3::pBVR lines (expression in plastids under control of the CAB3 promoter), or a phyA mutant showed greatly reduced levels of light-induced anthocyanin accumulation (). These lines also exhibited dampened induction responses to nitrogen-limited and phosphate-limited growth compared with WT (P < 0.001, ). Cold treatment (i.e., 12 °C) generally resulted in a greater accumulation of anthocyanins than at ambient temperature (i.e., 22 °C) under nutrient-deficient conditions for the WT ecotypes (; ). Cold induction of anthocyanins on phosphate-limited medium was ecotype specific, with an increased response for No-0 WT that was 4.3-fold higher than on basal medium, whereas there was essentially no response to phosphate-limitation in the cold for Col-0 (). However, this nutrient limitation-induced anthocyanin accumulation response is phytochrome-dependent as CAB3::pBVR and phyA lines largely lacked anthocyanin accumulation under these nutrient-deficient conditions ().
Figure 1. Development and anthocyanin content of wild-type and phytochrome-deficient seedlings under continuous far-red light. No-0 wild-type (No-0 WT), CAB3::pBVR1 (CAB-1), CAB3::pBVR2 (CAB-2), Col-0 wild-type (Col-0 WT), and phyA (Salk_014575) seedlings were grown at 22 °C for 4 d or 12 °C for 10 d on basal (B), nitrogen-limited (-N), or phosphate-limited (-P) Phytoblend medium with 1% sucrose under continuous FR (FRc) illumination of 5 μmol m-2 s-1. (A, B) Images of seedlings. Scale bar represents 1 cm. (C, D) Anthocyanin content. Bars represent the mean (± SD) of at least 3 independent measurements. Numbers above each bar represent the percent of anthocyanins accumulated relative to WT grown on the same medium. Fold-increase values for anthocyanin levels for seedlings grown on nutrient-deficient medium relative to growth of the same line on basal medium are shown below the bar graph. Two-tailed, unpaired Mann-Whitney U-test analyses were performed to compare the means of anthocyanin contents per mg of fresh weight for a particular line on basal medium relative to respective nutrient limitations. Letters above bars: a, P < 0.001; b, P < 0.01; c, P ≤ 0.05.
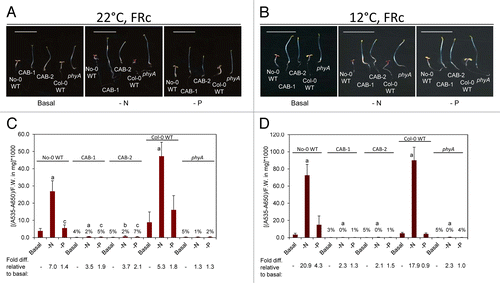
Table 1. Fold-difference values for anthocyanin levels determined for seedlings grown at 12 °C vs. 22 °C under different light conditions. No-0 wild-type (No-0 WT), CAB3::pBVR1, CAB3::pBVR2, Col-0 wild-type (Col-0 WT), phyA, phyB, cry1, and cry2 seedlings were grown on basal (B), nitrogen-limited (-N), or phosphate-limited (-P) medium at 12 °C or 22 °C under continuous far-red (FRc), red (Rc) or blue (Bc) illumination. Fold-difference values for anthocyanin content for seedlings grown at 12 °C relative to 22 °C are indicated (based on averages for 3 independent experiments)
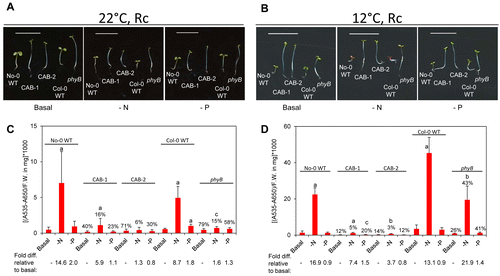
Similar responses were noted for an induction of anthocyanin under nutrient-deficient conditions under Rc at 22 °C (). CAB3::pBVR lines exhibited a more severe phenotype than a phyB mutant, especially for phosphate-limited conditions (). When grown under Rc at lower temperature, the WT ecotypes showed a greater accumulation of anthocyanins than at ambient temperature (, ). At cold temperatures, the defect of CAB3::pBVR lines was more severe than the phyB mutant under all conditions, i.e., basal, nitrogen-limited or phosphate- limited media (). The latter result suggests a role for phytochromes other than phyB in the induction of anthocyanins under Rc at 12 °C.
Figure 2. Development and anthocyanin content of wild-type and phytochrome-deficient seedlings under continuous red light. No-0 wild-type (No-0 WT), CAB3::pBVR1 (CAB-1), CAB3::pBVR2 (CAB-2), Col-0 wild-type (Col-0 WT), and phyB (Salk_022035) seedlings were grown at 22 °C for 4 d or 12 °C for 10 d on basal (B), nitrogen-limited (-N), or phosphate-limited (-P) Phytoblend medium with 1% sucrose under continuous R (Rc) illumination of 50 μmol m-2 s-1. (A, B) Images of seedlings. Scale bar represents 1 cm. (C, D) Anthocyanin content. Bars represent the mean (± SD) of at least 3 independent measurements. Numbers above each bar represent the percent of anthocyanins accumulated relative to WT grown on the same medium. Fold-increase values for anthocyanin levels for seedlings grown on nutrient-deficient medium relative to growth of the same line on basal medium are shown below the bar graph. Two-tailed, unpaired Mann-Whitney U-test analyses were performed to compare the means of anthocyanin contents per mg of fresh weight for a particular line on basal medium relative to respective nutrient limitations. Letters above bars: a, P < 0.001; b, P < 0.01; c, P ≤ 0.05.
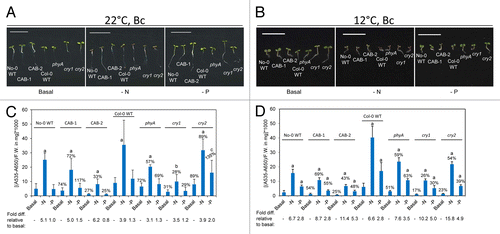
Under Bc, the CAB3::pBVR2 line and cry1 mutant exhibited the most severe defects in the nutrient deficiency-dependent induction of anthocyanins (). Whereas nearly all phytochrome and cryptochrome deficient lines exhibited lower levels of anthocyanin than their WT parents at ambient temperatures, the CAB3::pBVR1 and cry2 mutant lines accumulated more anthocyanins than their cognate parents on phosphate-limited medium at 22 °C (). However, at 12 °C all of the photoreceptor deficient mutants exhibited reduced anthocyanin accumulation on all growth media. Different from Rc- and FRc-dependent growth, under Bc there was virtually no low temperature-dependent induction of anthocyanin accumulation for WT lines (; ).
Figure 3. Development and anthocyanin content of wild-type, phytochrome-deficient and cryptochrome mutant seedlings under continuous blue light. No-0 wild-type (No-0 WT), CAB3::pBVR1 (CAB-1), CAB3::pBVR2 (CAB-2), Col-0 wild-type (Col-0 WT), phyA, cry1, and cry2 seedlings were grown at 22 °C for 4 d or 12 °C for 10 d on basal (B), nitrogen-limited (-N), or phosphate-limited (-P) Phytoblend medium with 1% sucrose under continuous B (Bc) illumination of 25 μmol m-2 s-1. (A, B) Images of seedlings. Scale bar represents 1 cm. (C, D) Anthocyanin content. Bars represent the mean (± SD) of at least 3 independent measurements. Numbers above each bar represent the percent of anthocyanins accumulated relative to WT grown on the same medium. Fold-increase values for anthocyanin levels for seedlings grown on nutrient-deficient medium relative to growth of the same line on basal medium are shown below the bar graph. Two-tailed, unpaired Mann-Whitney U-test analyses were performed to compare the means of anthocyanin contents per mg of fresh weight for a particular line on basal medium relative to respective nutrient limitations. Letters above bars: a, P < 0.001; b, P < 0.01; c, P ≤ 0.05.
Nutrient deficiencies are sensed and regulated by distinct photoreceptor pools in seedlings
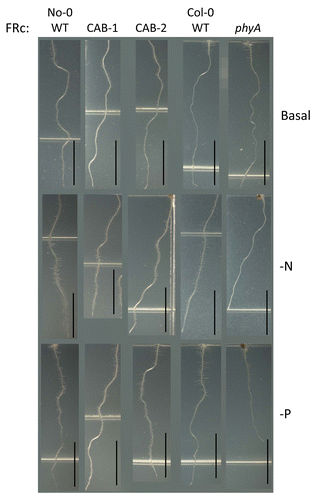
Under FRc, No-0 and Col-0 WT seedlings exhibited significantly longer primary roots on -N medium (), a response previously noted for Arabidopsis grown under white light.Citation36,Citation37 CAB3::pBVR lines and phyA primary roots were significantly shorter on -N and -P than on basal medium (). No-0 WT, CAB3::pBVR lines, and Col-0 WT seedlings exhibited longer root hairs under FRc on nitrogen- and phosphate-limited growth compared with growth on basal medium (). Notably, a phyA mutant, which lacks phyA in both shoots and roots, exhibited a shorter primary root on basal medium (P = 0.017, ) and lacked the elongated root hairs observed under nutrient deficiency (). The shorter primary root for phyA mutant under FRc has previously observed.Citation10,Citation38,Citation39
Figure 4. Light-dependent primary root lengths of wild-type, phytochrome chromophore–deficient, phytochrome apoprotein and cryptochrome apoprotein mutants. No-0 wild-type (No-0 WT), CAB3::pBVR1 (CAB-1), CAB3::pBVR2 (CAB-2), Col-0 wild-type (Col-0 WT), phyA, phyB, cry1, and cry2 seedlings were grown vertically on basal (B), nitrogen-limited (-N), or phosphate-limited (-P) Phytoblend medium containing 1% (w/v) Suc for 7 d at 22 °C under (A) far-red continuous (FRc) light of 5 µmol m−2 s−1, (B) red continuous (Rc) light of 50 µmol m−2 s−1, or (C) blue continuous (Bc) light of 25 µmol m−2 s−1. Bars represent the mean (± S.D.) of primary root lengths in mm (n = 5 to 10 for 2 independent biological experiments). For statistical significance tests comparisons were made for a particular line on basal medium relative to the respective nutrient limitations: a, P < 0.001; b, P < 0.01; c, P ≤ 0.05.
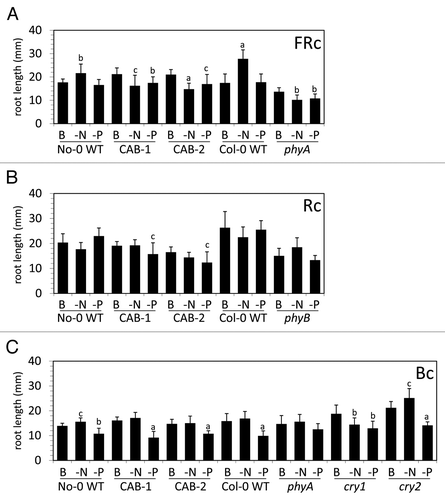
Figure 5. Root development of wild-type and phytochrome-deficient seedlings under continuous far-red light. No-0 wild-type (No-0 WT), CAB3::pBVR1 (CAB-1), CAB3::pBVR2 (CAB-2), Col-0 wild-type (Col-0 WT), and phyA seedlings were grown vertically at 22 °C for 7 d on basal, nitrogen-limited (-N), or phosphate-limited (-P) Phytoblend medium with 1% Suc under far-red continuous (FRc) illumination of 5 µmol m-2 s-1. Representative images of seedlings from 2 independent biological replicates are shown. Scale bars represent 0.5 cm.
Under Rc, only CAB3::pBVR seedlings exhibited a shorter primary root on -P relative to basal (). No-0 WT seedlings and CAB3::pBVR lines exhibited increased root hair length under nitrogen- and phosphate-limited growth compared with growth on basal medium in Rc (). The Col-0 WT line exhibited elongated root hairs most notably on phosphate-limited medium compared with basal (). The phenotype of a phyB mutant was distinct in that the root hairs did not significantly elongate in response to phosphate limitation relative to growth on basal medium (). The primary root of phyB was also shorter than WT on basal medium under Rc (P < 0.0001, ), a phenotype previously observed for RcCitation10,Citation39 and white light.Citation40
Figure 6. Root development of wild-type and phytochrome-deficient seedlings under continuous red light. No-0 wild-type (No-0 WT), CAB3::pBVR1 (CAB-1), CAB3::pBVR2 (CAB-2), Col-0 wild-type (Col-0 WT), and phyB seedlings were grown vertically at 22 °C for 7 d on basal, nitrogen-limited (-N), or phosphate-limited (-P) Phytoblend medium with 1% sucrose under red continuous (Rc) illumination of 50 µmol m-2 s-1. Representative images of seedlings from 2 independent biological replicates are shown. Scale bars represent 0.5 cm.

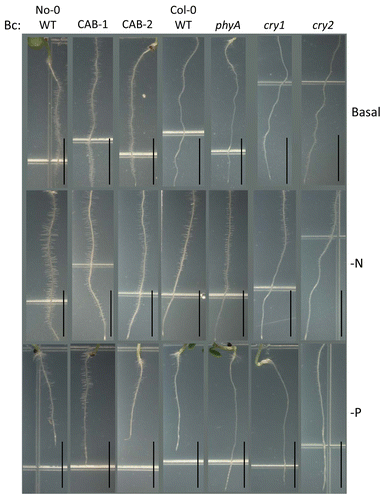
Under Bc, No-0 WT exhibited a longer primary root length on -N medium (), which was similar to the phenotype under FRc (). The roots of all lines, except the phyA mutant, were shorter on -P than on basal medium (). All lines exhibited longer root hairs on N-limited media under Bc illumination (). Under phosphate-limitation, phyA and cry1 mutants did not exhibit stimulation of root hairs as did the other lines under Bc ().
Figure 7. Root development of wild-type, phytochrome-deficient and cryptochrome mutant seedlings under continuous blue light. No-0 wild-type (No-0 WT), CAB3::pBVR1 (CAB-1), CAB3::pBVR2 (CAB-2), Col-0 wild-type (Col-0 WT), phyA, cry1, and cry2 seedlings were grown vertically at 22 °C for 7 d on basal, nitrogen-limited (-N), or phosphate-limited (-P) Phytoblend medium with 1% sucrose under blue continuous (Bc) illumination of 25 µmol m-2 s-1. Representative images of seedlings from 2 independent biological replicates are shown. Scale bars represent 0.5 cm.
Discussion
Mesophyll-localized phytochromes are required for nitrogen or phosphate limitation-induced accumulation of anthocyanins under FR and R illumination as CAB3::pBVR lines are equivalent to phyA mutant under FR () and similarly deficient in anthocyanin accumulation under Rc (). Similar phenotypes were previously observed on replete medium at ambient temperatures.Citation13,Citation41 The role of mesophyll-localized phyA, phyB and light-stable phytochromes in addition to phyB are shown here to be important additionally for regulating the induction of anthocyanins by low temperature and nutrient limitation (–; ). The regulatory roles of phytochromes in mediating an increased accumulation of anthocyanins are observed at both ambient and cold temperatures under R and FR light (–), but not for B (). Notably, though defective relative to cognate WT lines under B, all of the transgenic and mutant lines still accumulate significant levels of anthocyanins under Bc (), which provides evidence that the severe defects observed under FRc () and Rc () are due to specific disruptions in phytochrome-dependent signaling rather than a complete inability of these lines altogether to accumulate anthocyanins. The lack of an apparent low-temperature induction of anthocyanins under B suggests that low-temperature, light-dependent induction of anthocyanins is regulated by phytochrome perception of R and FR.
Although lines exhibiting a depletion of mesophyll-localized phytochromes were largely impaired in nutrient-deficient and cold-induced stimulation of anthocyanin levels, the roots of these lines, which still accumulate root-localized phytochromes,Citation10 exhibit near wild-type responses to nutrient starvation, with the exception of a lack of root elongation in response to nitrogen limitation (-). In contrast, phy mutants, which exhibit phytochrome deficiencies in all tissues including roots, largely lack nutrient limitation-induced changes in root elongation and root hair morphology (-). These results suggest that photoreceptors in the roots themselves are important for responding to nutrient limitation, which is sensed in the root, but mesophyll-localized phytochromes primarily regulate nutrient limitation-induced anthocyanin accumulation in shoots.
Our results provide evidence for a role of phytochromes in mesophyll cells in gating the response of plants to nutrient limitation- and low temperature-induced anthocyanin induction, and roles for root-localized photoreceptors in local root responses to nutrient deficiency. These results support a central role for phytochromes in coordinating cross-talk between light and stress signals.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
This work was supported by the National Science Foundation (grant no. MCB-0919100 to B.L.M.). We thank Dr Robert Larkin for providing cry1 and cry2 seeds.
References
- Tonelli C, Dolfini S, Ronchi A, Consonni G, Gavazzi G. Light inducibility and tissue specificity of the R gene family in maize. Genetica 1994; 94:225 - 34; http://dx.doi.org/10.1007/BF01443436
- Rabino I, Mancinelli AL. Light, temperature, and anthocyanin production. Plant Physiol 1986; 81:922 - 4; http://dx.doi.org/10.1104/pp.81.3.922; PMID: 16664926
- Drumm-Herrel H, Mohr H. Photosensitivity of seedlings differing in their potential to synthesize anthocyanin. Physiol Plant 1985; 64:60 - 6; http://dx.doi.org/10.1111/j.1399-3054.1985.tb01213.x
- Ahmad M, Lin C, Cashmore AR. Mutations throughout an Arabidopsis blue-light photoreceptor impair blue-light-responsive anthocyanin accumulation and inhibition of hypocotyl elongation. Plant J 1995; 8:653 - 8; http://dx.doi.org/10.1046/j.1365-313X.1995.08050653.x; PMID: 8528277
- Ahmad M, Cashmore AR. The blue-light receptor cryptochrome 1 shows functional dependence on phytochrome A or phytochrome B in Arabidopsis thaliana.. Plant J 1997; 11:421 - 7; http://dx.doi.org/10.1046/j.1365-313X.1997.11030421.x; PMID: 9107032
- Montgomery BL, Yeh KC, Crepeau MW, Lagarias JC. Modification of distinct aspects of photomorphogenesis via targeted expression of mammalian biliverdin reductase in transgenic Arabidopsis plants. Plant Physiol 1999; 121:629 - 39; http://dx.doi.org/10.1104/pp.121.2.629; PMID: 10517855
- Ahmad M, Jarillo JA, Smirnova O, Cashmore AR. Cryptochrome blue-light photoreceptors of Arabidopsis implicated in phototropism. Nature 1998; 392:720 - 3; http://dx.doi.org/10.1038/33701; PMID: 9565033
- Fox AR, Soto GC, Jones AM, Casal JJ, Muschietti JP, Mazzella MA. cry1 and GPA1 signaling genetically interact in hook opening and anthocyanin synthesis in Arabidopsis. Plant Mol Biol 2012; 80:315 - 24; http://dx.doi.org/10.1007/s11103-012-9950-x; PMID: 22855128
- Neff MM, Chory J. Genetic interactions between phytochrome A, phytochrome B, and cryptochrome 1 during Arabidopsis development. Plant Physiol 1998; 118:27 - 35; http://dx.doi.org/10.1104/pp.118.1.27; PMID: 9733523
- Costigan SE, Warnasooriya SN, Humphries BA, Montgomery BL. Root-localized phytochrome chromophore synthesis is required for photoregulation of root elongation and impacts root sensitivity to jasmonic acid in Arabidopsis. Plant Physiol 2011; 157:1138 - 50; http://dx.doi.org/10.1104/pp.111.184689; PMID: 21875894
- Endo M, Mochizuki N, Suzuki T, Nagatani A. CRYPTOCHROME2 in vascular bundles regulates flowering in Arabidopsis. Plant Cell 2007; 19:84 - 93; http://dx.doi.org/10.1105/tpc.106.048157; PMID: 17259260
- Endo M, Nakamura S, Araki T, Mochizuki N, Nagatani A. Phytochrome B in the mesophyll delays flowering by suppressing FLOWERING LOCUS T expression in Arabidopsis vascular bundles. Plant Cell 2005; 17:1941 - 52; http://dx.doi.org/10.1105/tpc.105.032342; PMID: 15965119
- Warnasooriya SN, Montgomery BL. Detection of spatial-specific phytochrome responses using targeted expression of biliverdin reductase in Arabidopsis. Plant Physiol 2009; 149:424 - 33; http://dx.doi.org/10.1104/pp.108.127050; PMID: 18971430
- Leyva A, Jarillo JA, Salinas J, Martinez-Zapater JM. Low temperature induces the accumulation of Phenylalanine Ammonia-Lyase and Chalcone Synthase mRNAs of Arabidopsis thaliana in a light-dependent manner. Plant Physiol 1995; 108:39 - 46; PMID: 12228452
- Zhang Y, Zheng S, Liu Z, Wang L, Bi Y. Both HY5 and HYH are necessary regulators for low temperature-induced anthocyanin accumulation in Arabidopsis seedlings. J Plant Physiol 2011; 168:367 - 74; http://dx.doi.org/10.1016/j.jplph.2010.07.025; PMID: 20932601
- Oyama T, Shimura Y, Okada K. The Arabidopsis HY5 gene encodes a bZIP protein that regulates stimulus-induced development of root and hypocotyl. Genes Dev 1997; 11:2983 - 95; http://dx.doi.org/10.1101/gad.11.22.2983; PMID: 9367981
- Holm M, Ma L-G, Qu L-J, Deng X-W. Two interacting bZIP proteins are direct targets of COP1-mediated control of light-dependent gene expression in Arabidopsis. Genes Dev 2002; 16:1247 - 59; http://dx.doi.org/10.1101/gad.969702; PMID: 12023303
- Vandenbussche F, Habricot Y, Condiff AS, Maldiney R, Van der Straeten D, Ahmad M. HY5 is a point of convergence between cryptochrome and cytokinin signalling pathways in Arabidopsis thaliana.. Plant J 2007; 49:428 - 41; http://dx.doi.org/10.1111/j.1365-313X.2006.02973.x; PMID: 17217468
- Atkinson D. Some general effects of phosphorus deficiency on growth and development. New Phytol 1973; 72:101 - 11; http://dx.doi.org/10.1111/j.1469-8137.1973.tb02014.x
- Rubio V, Linhares F, Solano R, Martín AC, Iglesias J, Leyva A, Paz-Ares J. A conserved MYB transcription factor involved in phosphate starvation signaling both in vascular plants and in unicellular algae. Genes Dev 2001; 15:2122 - 33; http://dx.doi.org/10.1101/gad.204401; PMID: 11511543
- Nilsson L, Müller R, Nielsen TH. Increased expression of the MYB-related transcription factor, PHR1, leads to enhanced phosphate uptake in Arabidopsis thaliana.. Plant Cell Environ 2007; 30:1499 - 512; http://dx.doi.org/10.1111/j.1365-3040.2007.01734.x; PMID: 17927693
- Narise T, Kobayashi K, Baba S, Shimojima M, Masuda S, Fukaki H, Ohta H. Involvement of auxin signaling mediated by IAA14 and ARF7/19 in membrane lipid remodeling during phosphate starvation. Plant Mol Biol 2010; 72:533 - 44; http://dx.doi.org/10.1007/s11103-009-9589-4; PMID: 20043234
- Sánchez-Calderón L, López-Bucio J, Chacón-López A, Gutiérrez-Ortega A, Hernández-Abreu E, Herrera-Estrella L. Characterization of low phosphorus insensitive mutants reveals a crosstalk between low phosphorus-induced determinate root development and the activation of genes involved in the adaptation of Arabidopsis to phosphorus deficiency. Plant Physiol 2006; 140:879 - 89; http://dx.doi.org/10.1104/pp.105.073825; PMID: 16443695
- Jain A, Cao A, Karthikeyan AS, Baldwin JC, Raghothama KG. Phosphate deficiency suppresses expression of light-regulated psbO and psbP genes encoding extrinsic proteins of oxygen-evolving complex of PSII. Curr Sci 2005; 89:1592 - 6
- Bongue-Bartelsman M, Phillips DA. Nitrogen stress regulates gene expression of enzymes in the flavonoid biosynthetic pathway of tomato. Plant Physiol Biochem 1995; 33:539 - 46
- Wang X, Bian Y, Cheng K, Zou H, Sun SS-M, He J-X. A comprehensive differential proteomic study of nitrate deprivation in Arabidopsis reveals complex regulatory networks of plant nitrogen responses. J Proteome Res 2012; 11:2301 - 15; http://dx.doi.org/10.1021/pr2010764; PMID: 22329444
- Feyissa DN, Løvdal T, Olsen KM, Slimestad R, Lillo C. The endogenous GL3, but not EGL3, gene is necessary for anthocyanin accumulation as induced by nitrogen depletion in Arabidopsis rosette stage leaves. Planta 2009; 230:747 - 54; http://dx.doi.org/10.1007/s00425-009-0978-3; PMID: 19621239
- Peng M, Hudson D, Schofield A, Tsao R, Yang R, Gu H, Bi Y-M, Rothstein SJ. Adaptation of Arabidopsis to nitrogen limitation involves induction of anthocyanin synthesis which is controlled by the NLA gene. J Exp Bot 2008; 59:2933 - 44; http://dx.doi.org/10.1093/jxb/ern148; PMID: 18552353
- Olsen KM, Slimestad R, Lea US, Brede C, Løvdal T, Ruoff P, Verheul M, Lillo C. Temperature and nitrogen effects on regulators and products of the flavonoid pathway: experimental and kinetic model studies. Plant Cell Environ 2009; 32:286 - 99; http://dx.doi.org/10.1111/j.1365-3040.2008.01920.x; PMID: 19054348
- Olsen KM, Lea US, Slimestad R, Verheul M, Lillo C. Differential expression of four Arabidopsis PAL genes; PAL1 and PAL2 have functional specialization in abiotic environmental-triggered flavonoid synthesis. J Plant Physiol 2008; 165:1491 - 9; http://dx.doi.org/10.1016/j.jplph.2007.11.005; PMID: 18242769
- Ruckle ME, DeMarco SM, Larkin RM. Plastid signals remodel light signaling networks and are essential for efficient chloroplast biogenesis in Arabidopsis. Plant Cell 2007; 19:3944 - 60; http://dx.doi.org/10.1105/tpc.107.054312; PMID: 18065688
- Mayfield JD, Folta KM, Paul A-L, Ferl RJ. The 14-3-3 Proteins μ and υ influence transition to flowering and early phytochrome response. Plant Physiol 2007; 145:1692 - 702; http://dx.doi.org/10.1104/pp.107.108654; PMID: 17951453
- Ahmad M, Cashmore AR. HY4 gene of A. thaliana encodes a protein with characteristics of a blue-light photoreceptor. Nature 1993; 366:162 - 6; http://dx.doi.org/10.1038/366162a0; PMID: 8232555
- Guo H, Yang H, Mockler TC, Lin C. Regulation of flowering time by Arabidopsis photoreceptors. Science 1998; 279:1360 - 3; http://dx.doi.org/10.1126/science.279.5355.1360; PMID: 9478898
- Feinbaum RL, Ausubel FM. Transcriptional regulation of the Arabidopsis thaliana chalcone synthase gene. Mol Cell Biol 1988; 8:1985 - 92; PMID: 3386631
- Remans T, Nacry P, Pervent M, Girin T, Tillard P, Lepetit M, Gojon A. A central role for the nitrate transporter NRT2.1 in the integrated morphological and physiological responses of the root system to nitrogen limitation in Arabidopsis. Plant Physiol 2006; 140:909 - 21; http://dx.doi.org/10.1104/pp.105.075721; PMID: 16415211
- Sánchez-Calderón L, López-Bucio J, Chacón-López A, Cruz-Ramírez A, Nieto-Jacobo F, Dubrovsky JG, Herrera-Estrella L. Phosphate starvation induces a determinate developmental program in the roots of Arabidopsis thaliana.. Plant Cell Physiol 2005; 46:174 - 84; http://dx.doi.org/10.1093/pcp/pci011; PMID: 15659445
- Kurata T, Yamamoto KT. Light-stimulated root elongation in Arabidopsis thaliana. J Plant Physiol 1997; 151:346 - 51; http://dx.doi.org/10.1016/S0176-1617(97)80263-5
- Shin DH, Cho M-H, Kim T-L, Yoo J, Kim J-I, Han Y-J, Song P-S, Jeon J-S, Bhoo SH, Hahn T-R. A small GTPase activator protein interacts with cytoplasmic phytochromes in regulating root development. J Biol Chem 2010; 285:32151 - 9; http://dx.doi.org/10.1074/jbc.M110.133710; PMID: 20551316
- Reed JW, Nagpal P, Poole DS, Furuya M, Chory J. Mutations in the gene for the red/far-red light receptor phytochrome B alter cell elongation and physiological responses throughout Arabidopsis development. Plant Cell 1993; 5:147 - 57; PMID: 8453299
- Warnasooriya SN, Porter KJ, Montgomery BL. Tissue- and isoform-specific phytochrome regulation of light-dependent anthocyanin accumulation in Arabidopsis thaliana.. Plant Signal Behav 2011; 6:624 - 31; http://dx.doi.org/10.4161/psb.6.5.15084; PMID: 21455024
