Abstract
Auxin's capacity to regulate aspects of plant development has been well characterized in model plant systems. In contrast, orchids have received considerably less attention, but the realization that many orchid species are endangered has led to culture-based propagation studies which have unveiled some functions for auxin in this system. This mini-review summarizes the many auxin-mediated developmental responses in orchids that are consistent with model systems; however, it also brings to the forefront auxin responses that are unique to orchid development, namely protocorm formation and ovary/ovule maturation. With regard to shoot establishment, we also assess auxin's involvement in orchid germination, PLB formation, and somatic embryogenesis. Further, it makes evident that auxin flow during germination of the undifferentiated, but mature, orchid embryo mirrors late embryogenesis of typical angiosperms. Also discussed is the use of orchid protocorms in future phytohormone studies to better understand the mechanisms behind meristem formation and organogenesis.
Abbreviations
| PAT | = | polar auxin transport |
| PLB | = | protocorm-like body |
| BA | = | benzyladenine |
| TZD | = | thidiazuron |
| BAP | = | 6-Benzylaminopurine |
| IBA | = | indolbutyric acid |
| KN | = | kinetin |
| NAA | = | 1-naphthalenacetic acid |
| 2,4-D | = | 2,4-dichlorophenoxyacetic acid |
| IAA | = | indolacetic acid |
| NPA | = | 1-n-naphthylphthalamic acid |
| TIBA | = | 2,3,5-triiodobenzoic acid |
| CW | = | coconut water |
| GA | = | gibberellic acid |
| JA | = | jasomonic acid |
Development is regulated by phytohormone-induced responses which ultimately govern form and function throughout the life of the plant. While hormone studies on Arabidopsis have been very revealing, research with non-traditional systems, such as orchids, have also helped to expand our knowledge of phytohormones in plant development. Orchidaceae is one of the largest and most diverse plant familiesCitation1 whose cultivation has been a primary focus of research due to its high commercial valueCitation2 and increased risk for extinction.Citation3 Detailed culture media recipes containing a specific mix of phytohormones have been designed for a variety of explants of different species.Citation4,5 From these tissue culture optimization studies, one can garner some understanding about the phytohormones involved in aspects of orchid development, such as organ initiation,Citation6,7 trichome formation,Citation8 and germination.Citation9,10 However, studies have also revealed hormone regulation of events that are atypical in model systems, including fungal and bacterial assisted germination,Citation11 formation of protocorms,Citation8,12 and delayed ovule maturation.Citation13,14 Although there are a host of physiological and environmental parameters which contribute to these events, the aim of this mini-review is to highlight the regulatory role of auxin during select stages of orchid development.
Auxin has been characterized as a plant morphogen due to its ability to control leaf initiation in the meristem, stimulate root formation, guide tropic responses and organize tissue patterns within the developing embryo.Citation15,16 Indolacetic acid (IAA) is the most common naturally occurring auxin, and synthetic auxins include compounds such as naphthalene-1-acetic acid (NAA) and 2,4-dichlorophenoxyacetic acid (2,4-D). Auxins are primarily synthesized in the shoot apex and move in a polar, basipetal fashion through the stem, and acropetally in the root, with a transition to basipital flow from the root tip, often described as a fountain-like flow.Citation17 Export of auxin from cells is directed via PIN-FORMED (PIN) proteins, whose placement in the membrane is highly regulated by endosomal delivery and selective removal.Citation18,19 With the assistance of additional auxin transporters, including ABCB that works synergistically with PIN and AUX1 which moves HIAA into adjacent cells, PIN arrangement yields a continuous exodus of auxin from one side of the cell, providing directionality to the flow.Citation20,21 Polar auxin transport (PAT) can be interrupted by a number of compounds, many of which seem to interfere with endosomal action and/or inhibit auxin efflux at the membrane.Citation21-23 Examples of the most commonly used inhibitors are 2,3,5-triiodobenzoic acid (TIBA), 1-naphthalenacetic acid (NPA), monensin, and Brefeldin A. PAT inhibitors have been used by many researchers to determine when and where auxin delivery is critical for a particular plant response, such as organ initiation and tropic growth.Citation16,24,25
Auxin Mediates Pollination-induced Floral Modifications and Inflorescence Initiation
Beginning with pollination, auxin plays a critical role in the orchid life cycle. Pollen-born auxin is the initiator of perianth death, stigmatal closure, ovary enlargement and ovule maturation ( ). Several studies have demonstrated that application of NAA to the orchid stigmatic surface mimics the floral senescence response triggered by pollination.Citation26-28 Auxin initiates rapid changes, including elongation of ovary epidermal cells to form trichomes, an increase in ovary diameter, and, depending on the species, initiation of ovule development or maturation of partially developed ovules.Citation13,14,29 Most of these changes are mediated through auxin-stimulated ethylene production.Citation29-31 In fact, a combination of auxin and ethylene are needed for optimal ovary/ovule development. The addition of ethylene antagonists to pollinated or unpollinated, NAA-treated stigmas resulted in partial stigmatal closure and moderate ovary swelling, and only a small amount of perianth senescence, while ethylene alone promoted death of the perianth without ovary enlargement or closure of the stigma.Citation13,14 Moreover, auxin up-regulated the ethylene biosythethic genes in Phalaenopsis, Phal-ACS2 and Phal-ACS3, and auxin-induced ethylene production was secondarily enhanced through ethylene-stimulated Phal-ACS1 expression.Citation30 Independent of ethylene, auxin treatment depressed transcript levels of the Phalaenopsis gene, PeMADS6, whose function is to sustain the life of the floral parts while inhibiting the completion of ovary and ovule maturation after pollination.Citation32 However, both auxin and ethylene response elements have been identified in the promoter region of PeMADS6, implicating both hormones in this pollination-induced shift in gene expression.Citation32 Auxin and ethylene may also mediate biochemical changes that contribute to orchid flower senescence. Pollination stimulated oxidative stress in floral organs, as evidenced by electrolyte leakage and high free radical levels in these tissues.Citation33 The PAT inhibitor, TIBA, and silver nitrate, an ethylene inhibitor, partially prevented this damage, suggesting that auxin-ethylene signaling plays a role in controlling oxidative stress responses during floral senescence.Citation34 Following pollination, an increase in hydrolytic activity produced soluble sugars, amino acids, and phosphorus in senescing petals and sepals.Citation35,36 Inhibitors of auxin and ethylene partially prevented the activity of these enzymes during the death process.Citation35
Table 1. Developmental responses to auxin alone or in conjunction with other phytohormones
Auxin also appears to play a role in floral bud initiation, but reports of its action are not entirely consistent between studies conducted with different orchid species ( ). The cytokinin, benzyladenine (BA), promoted inflorescence initiation in select orchid species,Citation37,38 however in Dendrobium, IAA countered the inductive effect of BA on flowering.Citation39 In addition, IAA suppressed flowering apex initiation in Aranda, but once inflorescence growth commenced, IAA treatment had no impact on floral bud initiation.Citation40 Surprisingly, endogenous IAA levels increased in response to TDZ-induced floral bud formation from Dendrobium cultured shoot tips, suggesting a role for auxin in the conversion of a vegetative shoot into a floral shoot.Citation41 The impact of auxin flow to the site of bud initiation is not clear from the literature. TIBA inhibited inflorescence formation in Cymbidium,Citation42 while the application of auxin antagonists stimulated floral initiation in Aranda,Citation43 but the buds failed to develop into a mature inflorescence. Although most research suggests an inhibitory effect of auxin on flowering, there are a few studies that indicate a possible inductive role for this hormone; thus continued research in this area is needed to determine the precise role of auxin in this important developmental stage of the orchid life cycle.
Polar Auxin Transport Affects Protocorm and Meristem Formation
PAT and establishment of shoot/root poles
In most angiosperms, embryonic meristems are set up during the heart stage of embryogenesis, leading to clear tissue patterning for shoot/root axis development. An auxin organizing center is located near the anterior end of the embryo, and from this region, auxin flows to the basal pole and works to establish the hypophysis, which is the founder cell for the root meristem.Citation44 PAT also occurs from the hypophysis and from the auxin organizing center to the site of cotyledon initiation ().Citation44 In orchids, embryogenesis terminates at a stage comparable to the globular embryo of typical angiosperms, which is before early meristems are established.Citation45 The mature orchid seed is “dust-like” in size and weight, lacks a primordial shoot/root axis, and the protoderm is the only distinctive primary meristem.Citation46-48 Upon germination, embryo cells divide to form a protocorm, which is a tuberous, sphere or oblong structure that is several times the dimensions of the original embryo, and comprised of undifferentiated cells.Citation45, Citation49-51 Approximately two to three weeks later, a meristem forms with evidence of a first leaf.Citation45, Citation49-51 Thus, early germination of the orchid seed parallels the shoot meristem establishment events observed during mid/late embryogenesis in a typical angiosperm. Similar to embryonic meristem formation in Arabidopsis, orchid protocorms may also require an auxin organizing center, post-embryogenesis, which can orchestrate shoot development through PAT ( ). This is consistent with studies where orchid protocorms that were treated with PAT inhibitors (TIBA and monensin) exhibited reduced first leaf formation.Citation8 Moreover, work done to selectively kill an increasingly larger proportion of the embryonic posterior cells of Spathloglottis orchid seed demonstrated that when 50–75% of the posterior orchid seed were deemed dead, seedlings still developed; but death in excess of 75% resulted in non-viable seed.Citation52 Demise of this anterior region may have eliminated the putative auxin organizing center and prevented germination of the orchid seed. Embryonic PAT is also important for development of the root meristem in Arabidopsis embryogenesis, such that after the hypophysis divides, cytokinins accumulate in the apical cell formed from the division, which is designated as the root meristem quiescent center progenitor.Citation44 Orchid seedlings generate a root meristem and complete seedling establishment late in germination, often not until 30–90 d in culture ( ). A hypophysis may develop during orchid embryogenesis and remain quiescent until needed or it may become established later in the germination process. The inconsequentiality of living cells in the posterior end, for germination and seedling establishment,Citation52 suggests that either the hypophysis is not set up during embryogenesis or that orchid embryonic cells exhibit flexibility, such that later in development the more anterior cells can take on the functionality of the hypophysis. The exact role of auxin in orchid root meristem initiation has not been studied.
Figure 1. (A) A model for the comparison of developmental timing and PAT during embryogenesis and germination of a standard angiosperm, Arabidopsis, and a typical orchid. A. Arabidopsis establishes a clear primordial shoot and root during embryogenesis which can rapidly produce a radicle and young leaves during germination. (B) Embryos of orchid seed arrest development at a stage comparable to the globular stage of Arabidopsis embryogenesis. Early stages of germination in orchid are similar to late embryogenesis of Arabidopsis with the formation of a shoot, but root formation is considerably later in orchid germination. Shifts in auxin flow from an auxin organizing center plays a role in embryogenesis of Arabidopsis and regulates germination of orchid seeds.
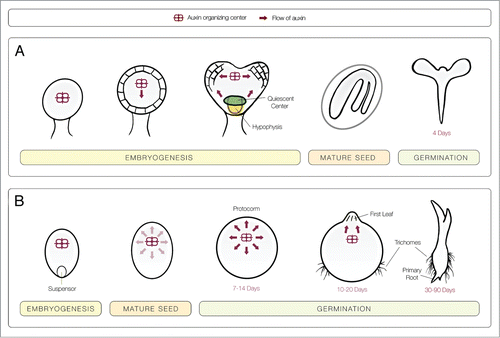
Germination: Protocorm and early shoot
Studies have indicated that auxin influences protocorm development. In select orchid species, exogenous auxin application increased protocorm DNA content,Citation53 diameters,Citation8 morphology,Citation54 and numbers during germination.Citation12 In vivo, orchid seed depend upon a fungal symbiont for germination. Interestingly, there is evidence that IAA from auxin-producing, orchid-associated bacteria enhance the mycorrhiza-assisted germination,Citation11 however there is no study that indicates that the fungal seed symbiont produces auxin. With regard to a direct in vitro effect, a few studies have indicated that low concentrations of auxin alone can promote germination.Citation9,55 In contrast, others claimed that high auxin levels inhibit germination,Citation54,55 but a consensus of researchers have indicated that a combination of auxin and cytokinins were most effective at promoting germination of orchid seed ( ).Citation9, Citation54-57 Additionally, most of the literature is nebulous as to how researchers designate the germinated state, whether as first leaf formation or as protocorm enlargement. It is likely that those who define it as protocorm expansion claim that auxin induces germination, while those who require first leaf formation report a delay. This interpretation of the germination studies is consistent with the finding that exogenous auxin promotes a protocorm state and causes young seedlings leaves to fuse;Citation8 a response very reminiscent of the Arabidopsis auxin transport double mutants (pin1 and pid), which generated fleshy, fused cotyledons during embryo development.Citation58 Similar to what is observed during Arabidopsis embryogenesis, it is likely that redistribution of auxin and the establishment of appropriate auxin gradients is also responsible for shoot formation during orchid seed germination ( ).
Protocorm like-bodies (PLBs)
The maintenance of a protocorm stage of development by disrupting auxin gradients is further supported by studies on the formation of protocorm-like bodies (PLBs), whereby exogenous auxin application promoted an undifferentiated state; but reduction or removal of auxin from the culture media resulted in somatic embryos which formed shoots ( ). Traditionally, researchers have designated any protocorm-shaped, culture-derived, ectopic formation as a PLB, if it is capable of producing a shoot. In addition, some researchers have likened PLBs to somatic embryos, since their development is consistent with in vivo orchid seed development and they possess hydroxyproline-rich glycoproteins (HRGPs) in the wall that are common to seed embryos.Citation46 Callus, which is used as starting tissue for the production of PLBs, can be auxin-induced from an array of orchid explants, including shoot tips, protocorms, rhizomes, root tips and leaves ( ). Further, various orchid explants have been reported to generate PLBs in response to auxin and cytokinin, similar to protocorm formation during seed germination, described above, but the optimal recipe differs for each species ( ). In some studies, hormone application was not used in the final steps of PLB generation. For example, in Dendrobium, NAA plus BAP generated callus, which when subcultured on hormone-free media formed PLBs.Citation59 Similarly, in Oncidium and Cymbidium, 2,4-D and TDZ treatment generated embryonic callus, which initiated PLBs after subculture on hormone-free media.Citation60,61 However, root-derived callus from Oncidium generated PLBs that completed somatic embryogenesis on low levels of NAA with higher TDZ concentrations.Citation60 Alternatively, in Spathoglottis, a lower ratio of cytokinins (BAP):NAA promoted PLB formation from seedling nodal explants, but a higher ratio induced shoot formation.Citation62,63 Researchers have also demonstrated that auxin can inhibit cytokinin-induced direct somatic embryogenesis in Phalaenopsis and Oncidium species.Citation64,65 Overall, these findings suggest that high exogenous auxin levels promote and/or maintain undifferentiated growth, while hormone-depleted media or lower levels of auxin in conjunction with cytokinins typically produce PLBs from callus. This conclusion further supports the premise that, in vivo, uniform auxin distribution helps to maintain a dedifferentiated protocorm until auxin is redirected to appropriate locations and is present in precise concentrations to help stimulate differentiation ( ).
Auxin Regulates the Formation of Vegetative Structures
Shoot induction and growth
Researchers have made significant strides in characterizing the antagonistic relationship between auxin and cytokinin during shoot formation.Citation66,67,44 In many orchid species, the formation of shoots from the nodes of a stem or stem-like tissue, such as rhizomes or inflorescence stalks, is a very common in vitro response to exogenously applied cytokinin and auxin ( ). In fact, many culture recipes call for a high cytokinin-to-auxin ratio,Citation54,68 which mirrors the requirements of orchid seed germination and somatic embryogenesis, as previously described. An orchid root tip culture that produced PLBs and subsequent shoots without the addition of exogenous hormones, shifted its endogenous hormone levels to one with a high cytokinin-to-auxin ratio, suggesting that this ratio is also needed for in vivo orchid shoot formation.Citation69 Then again, two studies demonstrated that seedling nodal explants can generate new shoots with auxin application alone.Citation8,63 Thus the effects of auxin on the establishment of shoot formation is likely species, tissue, and developmentally specific; and in certain cases it may not require the presence of cytokinin to elicit this developmental response. The lone effect of auxin on shoot growth was also evident when researchers co-cultured Cattleya seedlings with an auxin-producing bacterial species isolated from Cymbidium shoots.Citation70 The endophyte-inoculated Cattleya seedlings exhibited an increase in leaf number and, in some cases, an increase in shoot dry weight. In addition, some orchid tissue culture studies reported a general increase in shoot growth in response to auxin alone or in conjunction with cytokinins ( ).
In Arabidopsis, site directed auxin has been shown to play a role in organ initiation from the meristem, whether it be vegetativeCitation16 or floral.Citation71 Further, it has been determined that boundary formation between leaf or floral organ cell initials and the meristem cells occurs through auxin flow coupled with specified gene expression.Citation16,71 For example, down-regulation of CUC and STM expression at the location of primordium initiation and up-regulation of CUC at the site of leaf demarcation designates the site for organ establishment.Citation71 The effect of PAT inhibitors on orchid first leaf development from protocorms,Citation8 confirms the need for appropriate auxin placement/concentration and suggests that the protocorm is functioning like a meristem whereby leaf initiation is also regulated by auxin-controlled gene expression. Thus the orchid protocorm may be a simple and efficient system to study gene expression during meristem establishment and leaf initiation.
Root induction and growth
The promotive effect of auxin on new root formation has been well-studied in model plant systems.Citation72 Likewise, techniques for the production of plantlets from various orchid explants include rooting on auxin-containing media ( ). Root formation occurred in response to most auxins, but IBA or IAA was especially effective in many orchid species.Citation7, Citation73-75 Moreover, high endogenous cytokinin to auxin ratios in root tips may suppress lateral root formation in Aranda and Vanda species, since removal of the root tips spurred lateral root formation.Citation76 Researchers have also documented that auxin is not only involved in initiation, but also enhanced root growth.Citation77,78 Further, orchid roots are typically inhabited by fungalCitation79 and bacterialCitation80,81 symbionts that synthesize IAA. In epiphytes, auxin-producing rhizobacteria are located in the protective velamen layer.Citation81 When provided with l-tryptophan, which is a common root exudate and an auxin precursor, these endophytic isolates generate copious amounts of IAA.Citation80 Researchers have clearly demonstrated that the IAA produced by these microorganisms is biologically active, promoting both root growth and seed germination.Citation79-81
Rhizogenesis
Rhizome formation is of special interest to orchid biologists, since they are commonly used for propagation in the orchid industry. Auxin-promoted rhizogenesis, both with and without cytokinins, has been documented in germinating seeds, seedlings and adult plants ( ). Protocorms branch into rhizome-like structures during germination in species such as Cymbidium and Geodorum, a growth response which was enhanced with auxin application.Citation82,83 In addition, NAA and IAA helped to sustain rhizome growth in Spathoglottis,Citation84 and in Cymbidium Citation85 auxins induced rhizome growth while inhibiting shoot production. Further, researchers have reported auxin-stimulated production of rhizomes from seedling leaf axils,Citation8 and in adult plants, rhizogenesis was promoted by topical application of NAA on psuedobulbs of Cymbidium.Citation85 In Geodorum, the direction of rhizome growth was differentially affected by cytokinins and auxins. BAP caused a diagravitropic, or horizontal growth-response, while NAA induced positive gravitropic growth.Citation86
Trichome formation
Hair formation in Arabidopsis is a hormone-regulated process, in which root hairs are generated in response to auxin and ethylene, and the trichomes of stems and leaves are promoted by GA and JA.Citation87,88 Similar to the response seen in Arabidopsis roots, orchid ovaries produced epidermal hairs with stigmatic NAA application, suggesting that hair formation is auxin and/or ethylene induced.Citation13,14 During standard orchid seedling establishment, trichomes are produced from protocorm epidermal cells several days to weeks before first root formation ( ). Some researchers speculate that these hairs are essential for early water uptakeCitation89 and/or they play a role in setting up the fungal symbiotic relationship which is necessary for in vivo germination.Citation90 Precocious hair formation occurred in Spathoglottis seedlings when young protocorms were sub-cultured onto IAA or 2,4-D.Citation8 In addition, hairs were initiated from leaves and rhizomes when 30-d seedlings were sub-cultured on auxin-containing media.Citation8 Similarly, Orchis seed cultured on high levels of IAA had enhanced growth of protocorm epidermal hairs, but as kinetin levels were increased, trichome numbers decreased.Citation54 In the same study, GA also enhanced epidermal hair formation on Orchis protocorms.Citation54 Interestingly, the auxin response would suggest a root-like nature to the protocorm, but the GA response in Orchis suggests that the protocorm is responding like a stem. The few studies conducted on hair initiation in orchid systems make it difficult to surmise a conclusive statement about the precise role of these hormones. Although auxin is a clear contender, it is also possible that GA, ethylene and/or cytokinins may also regulate trichome formation in certain orchid species and tissue types.
Conclusion and Forward Look
True to its function in other plant systems, auxin seems to orchestrate dramatic morphogenic responses during orchid development. From pollination to organogenesis, auxin, and its interplay with other phytohormones, dictates key events in cell signaling that lead to appropriate developmental responses. The pathways connecting the auxin-stimulated outcome with the presence or flow of this hormone are yet to be fully elucidated. However, certain aspects of orchid development are beautifully simple and may provide a unique opportunity to further study these auxin-regulated mechanisms. For example, hair initiation, meristem establishment, first leaf formation, or primary root development can be readily studied in culture as these structures emerge from a mass of undifferentiated cells, the protocorm. Such a culture system provides an efficient means to manipulate hormone levels, both through biosynthesis and transport. Moreover, ovule development, which is inducible by auxin, can be more easily controlled and studied in orchids than other plant systems. The interactions of microorganisms with orchids and the role of auxin in this relationship are very interesting. Further attention needs to be given to this symbiotic relationship and whether the orchid plant is truly dependent on these endophytes for events like seed germination and root development, or if the auxin produced by the microorganisms only augments endogenous auxin produced by orchid tissues. Past tissue culture studies with auxin and other supplements has certainly provided a start to our understanding of this hormone in orchid development, but future studies are needed to more completely connect the molecular biology with these morphological changes that play such a fundamental role in orchid growth and maturation.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
The authors would like to express their gratitude to Drs Jay Jones and Todd Lorenz for consultation and support in the writing of this manuscript. We would also like to thank Dr. Frantisek Baluska for the invitation to write this review.
Funding
This work was financially supported by the USDA (NIFA award number 2011–38422–30942).
References
- Cribb PJ, Lell SP, Dixon KW, Barrett RL. Chapter One - Orchid conservation: a global perspective. Orchid conservation: Natural History Publications, 2003:1-24.
- Hew CS, Yong JWH. Chapter One - The Relevance of Orchid Physiology to the Industry. The physiology of tropical orchids in relation to the industry: World Scientific, 2004:1-10.
- Swarts ND, Dixon KW. Terrestrial orchid conservation in the age of extinction. Ann Bot 2009; 104:543-56; PMID:19218582; http://dx.doi.org/10.1093/aob/mcp025
- Arditti J. Micropropagation of orchids, Vol. I and II, 2nd ed: Blackwell, 2008.
- Teixeira da Silva JA. Orchids: Advances in tissue culture, genetics, phytochemistry and transgenic biotechnology. Floricult Ornam Biotech 2013; 7:1-52
- Chen T-Y, Chen J-T, Chang W-C. Multiple shoot formation and plant regeneration from stem nodal explants of Phaphiopedilum orchids. In Vitro Cell Dev Biol Plant 2002; 38:595-7; http://dx.doi.org/10.1079/IVP2002332
- Mohanty P, Das MC, Kumaria S, Tandon P. High-efficiency cryopreservation of the medicinal orchid Dendrobium nobile Lindl. Plant Cell Tissue Organ Cult 2012; 109:297-305; http://dx.doi.org/10.1007/s11240-011-0095-4
- Novak SD, Whitehouse GA. Auxin regulates first leaf development and promotes the formation of protocorm trichomes and rhizome-like structures in developing seedlings of Spathoglottis plicata (Orchidaceae). AoB Plants 2013; 5:pls053; http://dx.doi.org/10.1093/aobpla/pls053
- Godo T, Komori M, Nakaoki E, Yukawa T, Miyoshi K. Germination of mature seeds of Calanthe tricarinata Lindl., an endangered terrestrial orchid, by asymbiotic culture in vitro. In Vitro Cell Dev Biol Plant 2010; 46:323-8; http://dx.doi.org/10.1007/s11627-009-9271-1
- De KK, Majumdar S, Sharma R, Sharma B. Green pod culture and rapid micropropagation of Dendrobium chrysanthum Wall.-a horticultural and medicinal orchid. Folia Hortic 2006; 18:81-90
- Wilkinson KG, Dixon KW, Sivasithamparam K, Ghisalberti EL. Effect of IAA on symbiotic germination of an Australian orchid and its production by orchid-associated bacteria. Plant Soil 1994; 159:291-5; http://dx.doi.org/10.1007/BF00009292
- Miyoshi K, Mii M. Phytohormone pre-treatment for the enhancement of seed germination and protocorm formation by the terrestrial orchid, Calanthe discolor (Orchidaceae), in asymbiotic culture. Sci Hortic (Amsterdam) 1995; 63:263-7; http://dx.doi.org/10.1016/0304-4238(95)00813-9
- Zhang XS, O’Neill SD. Ovary and gametophyte development are coordinately regulated by auxin and ethylene following pollination. Plant Cell 1993; 5:403-18; PMID:12271070; http://dx.doi.org/10.1105/tpc.5.4.403
- Tsai W-C, Hsiao Y-Y, Pan Z-J, Kuoh C-S, Chen W-H, Chen H-H. The role of ethylene in orchid ovule development. Plant Sci 2008; 175:98-105; http://dx.doi.org/10.1016/j.plantsci.2008.02.011
- Vanneste S, Friml J. Auxin: a trigger for change in plant development. Cell 2009; 136:1005-16; PMID:19303845; http://dx.doi.org/10.1016/j.cell.2009.03.001
- Reinhardt D, Mandel T, Kuhlemeier C. Auxin regulates the initiation and radial position of plant lateral organs. Plant Cell 2000; 12:507-18; PMID:10760240; http://dx.doi.org/10.1105/tpc.12.4.507
- Swarup R, Bennett M. Auxin transport: the fountain of life in plants? Dev Cell 2003; 5:824-6; PMID:14667404; http://dx.doi.org/10.1016/S1534-5807(03)00370-8
- Baluska F, Schlicht M, Volkmann D, Mancuso S. Vesicular secretion of auxin: Evidences and implications. Plant Signal Behav 2008; 3:254-6; PMID:19704646; http://dx.doi.org/10.4161/psb.3.4.5183
- Dhonukshe P, Huang F, Galvan-Ampudia CS, Mähönen AP, Kleine-Vehn J, Xu J, Quint A, Prasad K, Friml J, Scheres B, et al. Plasma membrane-bound AGC3 kinases phosphorylate PIN auxin carriers at TPRXS(N/S) motifs to direct apical PIN recycling. Development 2010; 137:3245-55; PMID:20823065; http://dx.doi.org/10.1242/dev.052456
- Titapiwatanakun B, Blakeslee JJ, Bandyopadhyay A, Yang H, Mravec J, Sauer M, Cheng Y, Adamec J, Nagashima A, Geisler M, et al. ABCB19/PGP19 stabilises PIN1 in membrane microdomains in Arabidopsis. Plant J 2009; 57:27-44; PMID:18774968; http://dx.doi.org/10.1111/j.1365-313X.2008.03668.x
- Blakeslee JJ, Peer WA, Murphy AS. Auxin transport. Curr Opin Plant Biol 2005; 8:494-500; PMID:16054428; http://dx.doi.org/10.1016/j.pbi.2005.07.014
- Friml J, Palme K. Polar auxin transport–old questions and new concepts? Plant Mol Biol 2002; 49:273-84; PMID:12036254; http://dx.doi.org/10.1023/A:1015248926412
- Muday GK, DeLong A. Polar auxin transport: controlling where and how much. Trends Plant Sci 2001; 6:535-42; PMID:11701382; http://dx.doi.org/10.1016/S1360-1385(01)02101-X
- Scanlon MJ. The polar auxin transport inhibitor N-1-naphthylphthalamic acid disrupts leaf initiation, KNOX protein regulation, and formation of leaf margins in maize. Plant Physiol 2003; 133:597-605; PMID:14500790; http://dx.doi.org/10.1104/pp.103.026880
- Friml J, Wiśniewska J, Benková E, Mendgen K, Palme K. Lateral relocation of auxin efflux regulator PIN3 mediates tropism in Arabidopsis. Nature 2002; 415:806-9; PMID:11845211; http://dx.doi.org/10.1038/415806a
- Arditti J, Knauft RL. The effects of auxin, actinomycin D, ethionine and puromycin on post-pollination behavior in Cymbidium (Orchidaceae) flowers. Am J Bot 1969; 56:620-8; http://dx.doi.org/10.2307/2440436
- Arditti J, Jeffrey DC, Flick BH. Postpollination phenomena in orchid flowers. III. Effects and interactions of auxin, kinetin or gibberellin. New Phytol 1971; 70:1125-41; http://dx.doi.org/10.1111/j.1469-8137.1971.tb04595.x
- Ketsa S, Wisutiamonkul A, van Doorn WG. Auxin is required for pollination-induced ovary growth in Dendrobium orchids. Funct Plant Biol 2006; 33:887-92; http://dx.doi.org/10.1071/FP06034
- Ketsa S, Rugkong A. Ethylene production, senescence and ethylene sensitivity of Dendrobium ‘Pompadour’ flowers following pollination. J Hortic Sci Biotechnol 2000; 75:149-53
- Bui AQ, O’Neill SD. Three 1-aminocyclopropane-1-carboxylate synthase genes regulated by primary and secondary pollination signals in orchid flowers. Plant Physiol 1998; 116:419-28; PMID:9449850; http://dx.doi.org/10.1104/pp.116.1.419
- Burg SP, Dijkman MJ. Ethylene and auxin participation in pollen induced fading of vanda orchid blossoms. Plant Physiol 1967; 42:1648-50; PMID:16656700; http://dx.doi.org/10.1104/pp.42.11.1648
- Tsai WC, Lee PF, Chen HI, Hsiao YY, Wei WJ, Pan ZJ, Chuang MH, Kuoh CS, Chen WH, Chen HH. PeMADS6, a GLOBOSA/PISTILLATA-like gene in Phalaenopsis equestris involved in petaloid formation, and correlated with flower longevity and ovary development. Plant Cell Physiol 2005; 46:1125-39; PMID:15890679; http://dx.doi.org/10.1093/pcp/pci125
- Attri LK, Nayyar H, Bhanwra RK, Pehwal A. Pollination-induced oxidative stress in floral organs of Cymbidium pendulum (Roxb.) Sw. and Cymbidium aloifolium (L.) Sw. (Orchidaceae): a biochemical investigation. Sci Hortic (Amsterdam) 2008; 116:311-7; http://dx.doi.org/10.1016/j.scienta.2008.01.009
- Attri LK, Nayyar H, Bhanwra RK, Vij SP. Pollination-induced floral senescence in orchids: status of oxidative stress. Russ J Plant Physiol 2008; 55:821-8; http://dx.doi.org/10.1134/S1021443708060125
- Attri LK, Nayyar H, Bhanwra RK, Vij SP. Post-pollination biochemical changes in floral organs of Rhynchostylis retusa (L.) Bl. and Aerides multiflora Roxb. (Orchidaceae). J Plant Biol 2007; 50:548-56; http://dx.doi.org/10.1007/BF03030708
- Hew CS, Tan SC, Chin TY, Ong TK. Influence of ethylene on enzyme activities and mobilization of materials in pollinated Arachnis orchid flowers. J Plant Growth Regul 1989; 8:121-30; http://dx.doi.org/10.1007/BF02025279
- Blanchard MG, Runkle ES. Benzyladenine promotes flowering in Doritaenopsis and Phalaenopsis orchids. J Plant Growth Regul 2008; 27:141-50; http://dx.doi.org/10.1007/s00344-008-9040-0
- Goh CJ. Hormonal regulation of flowering in a sympodial orchid hybrid Dendrobium louisae. New Phytol 1979; 82:375-80; http://dx.doi.org/10.1111/j.1469-8137.1979.tb02663.x
- Goh CJ, Yang AL. Effects of growth regulators and decapitation on flowering of Dendrobium orchid hybrids. Plant Sci Lett 1978; 12:287-92; http://dx.doi.org/10.1016/0304-4211(78)90080-9
- Goh CJ, Seetoh HC. Apical control of flowering in an orchid hybrid Aranda Deborah. [abstract]. Ann Bot (Lond) 1973; 37:112-9; http://aob.oxfordjournals.org/content/37/1/113.
- de Melo Ferreira W, Barbante Kerbauy G, Elizabeth Kraus J, Pescador R, Mamoru Suzuki R. Thidiazuron influences the endogenous levels of cytokinins and IAA during the flowering of isolated shoots of Dendrobium. J Plant Physiol 2006; 163:1126-34; PMID:17032618; http://dx.doi.org/10.1016/j.jplph.2005.07.012
- Kostenyuk I, Oh BJ, So IS. Induction of early flowering in Cymbidium niveo marginatum Mak. in vitro. Plant Cell Rep 1999; 19:1-5; http://dx.doi.org/10.1007/s002990050701
- Goh CJ. Regulation of floral initiation and development in an orchid hybrid Aranda Deborah. [abstract]. Ann Bot (Lond) 1977; 41:763-9; http://aob.oxfordjournals.org/content/41/4/763.
- Su YH, Liu YB, Zhang XS. Auxin-cytokinin interaction regulates meristem development. Mol Plant 2011; 4:616-25; PMID:21357646; http://dx.doi.org/10.1093/mp/ssr007
- Vinogradova T, Andronova EV. Chapter Four - Development of orchid seeds and seedlings. Orchid Biology: Reviews and Perspectives VIII: Kluwer Academic Publishers, 2002:167-234.
- Lee YI, Hsu ST, Yeung EC. Orchid protocorm-like bodies are somatic embryos. Am J Bot 2013; 100:2121-31; PMID:24136821; http://dx.doi.org/10.3732/ajb.1300193
- Arditti J, Ghani AKA. Tansley review, 110. Numerical and physical properties of orchid seeds and their biological implications. New Phytol 2000; 145:367-421; http://dx.doi.org/10.1046/j.1469-8137.2000.00587.x
- Yam TW, Yeung EC, Ye X-L, Zee S-Y, Arditti J. Chapter Six - Orchid Embryos. Orchid biology: reviews and perspectives VIII. Kluwer Academic Publishers, 2002; 287-384.
- Dutra D, Kane ME, Richardson L. Asymbiotic seed germination and in vitro seedling development of Cyrtopodium punctatum: a propagation protocol for an endangered Florida native orchid. Plant Cell Tissue Organ Cult 2009; 96:235-43; http://dx.doi.org/10.1007/s11240-008-9480-z
- Yamazaki J, Miyoshi K. In vitro asymbiotic germination of immature seed and formation of protocorm by Cephalanthera falcata (Orchidaceae). Ann Bot 2006; 98:1197-206; PMID:17071633; http://dx.doi.org/10.1093/aob/mcl223
- Dutra D, Johnson TR, Kauth PJ, Stewart SL, Kane ME, Richardson L. Asymbiotic seed germination, in vitro seedling development, and greenhouse acclimatization of the threatened terrestrial orchid Bletia purpurea. Plant Cell Tissue Organ Cult 2008; 94:11-21; http://dx.doi.org/10.1007/s11240-008-9382-0
- Novak SD, Pardiwala RS, Gray BL. A study of NaOCl-induced necrosis indicates that only half of the embryo is required for seedling establishment in Spathoglottis plicata. Lindleyana 2008; 21:32-8
- Lim WL, Loh CS. Endopolyploidy in Vanda Miss Joaquim (Orchidaceae). New Phytol 2003; 159:279-87; http://dx.doi.org/10.1046/j.1469-8137.2003.00797.x
- Hadley G, Harvais G. The effect of certain growth substances on asymbiotic germination and development of Orchis purpurella. New Phytol 1968; 67:441-5; http://dx.doi.org/10.1111/j.1469-8137.1968.tb06393.x
- Sharma SK, Tandon P. Influence of growth regulators on asymbiotic germination and early seedling development of Coelogyne punctulata Lindl. Biology, Conservation, and culture of orchids. Papers presented at a national seminar organized by the Orchid Society of India, Panjab University, 1986:441-51.
- Deb CR, Pongener A. Asymbiotic seed germination and in vitro seedling development of Cymbidium aloifolium (L.) Sw.: a multipurpose orchid. J Plant Biochem Biotechnol 2011; 20:90-5; http://dx.doi.org/10.1007/s13562-010-0031-4
- Hadley G. The interaction of kinetin, auxin and other factors in the development of north temperate orchids. New Phytol 1970; 69:549-55; http://dx.doi.org/10.1111/j.1469-8137.1970.tb07607.x
- Furutani M, Vernoux T, Traas J, Kato T, Tasaka M, Aida M. PIN-FORMED1 and PINOID regulate boundary formation and cotyledon development in Arabidopsis embryogenesis. Development 2004; 131:5021-30; PMID:15371311; http://dx.doi.org/10.1242/dev.01388
- Roy J, Banerjee N. Induction of callus and plant regeneration from shoot tip explants of Dendrobium fimbriatum Lindl Var. oculatum Hk. f. Sci Hortic (Amsterdam) 2003; 97:333-40; http://dx.doi.org/10.1016/S0304-4238(02)00156-5
- Chen J, Chang W. Efficient plant regeneration through somatic embryogenesis from callus cultures of Oncidium (Orchidaceae). Plant Sci 2000; 160:87-93; PMID:11164580; http://dx.doi.org/10.1016/S0168-9452(00)00367-8
- Huan LVT, Takamura T, Tanaka M. Callus formation and plant regeneration from callus through somatic embryo structures in Cymbidium orchid. Plant Sci 2004; 166:1443-9; http://dx.doi.org/10.1016/j.plantsci.2004.01.023
- Teng WL, Nicholson L, Teng MC. Micropropagation of Spathoglottis plicata. Plant Cell Rep 1997; 16:831-5; http://dx.doi.org/10.1007/s002990050329
- Hossain MM, Dey R. Multiple regeneration pathways in Spathoglottis plicata Blume — A study in vitro. S Afr J Bot 2013; 85:56-62; http://dx.doi.org/10.1016/j.sajb.2012.12.005
- Kuo HL, Chen JT, Chang WC. Efficient plant regeneration through direct somatic embryogenesis from leaf explants of Phalaenopsis ‘Little Steve’. In Vitro Cell Dev Biol Plant 2005; 41:453-6; http://dx.doi.org/10.1079/IVP2005644
- Chen JT, Chang WC. TIBA affects the induction of direct somatic embryogenesis from leaf explants of Oncidium. Plant Cell Tissue Organ Cult 2004; 79:315-20; http://dx.doi.org/10.1007/s11240-004-4613-5
- Smith RS. The role of auxin transport in plant patterning mechanisms. PLoS Biol 2008; 6:e323; PMID:19090623; http://dx.doi.org/10.1371/journal.pbio.0060323
- Tanaka H, Dhonukshe P, Brewer PB, Friml J. Spatiotemporal asymmetric auxin distribution: a means to coordinate plant development. Cell Mol Life Sci 2006; 63:2738-54; PMID:17013565; http://dx.doi.org/10.1007/s00018-006-6116-5
- Harvais G. An improved culture medium for growing the orchid Cypripedium reginae axenically. Can J Bot 1982; 60:2547-55; http://dx.doi.org/10.1139/b82-309
- Peres LEP, Kerbauy GB. High cytokinin accumulation following root tip excision changes the endogenous auxin-to-cytokinin ratio during root-to-shoot conversion in Catasetum fimbriatum Lindl (Orchidaceae). Plant Cell Rep 1999; 18:1002-6; http://dx.doi.org/10.1007/s002990050698
- Faria DC, Dias ACF, Melo IS, de Carvalho Costa FE. Endophytic bacteria isolated from orchid and their potential to promote plant growth. World J Microbiol Biotechnol 2013; 29:217-21; PMID:23014841; http://dx.doi.org/10.1007/s11274-012-1173-4
- Heisler MG, Ohno C, Das P, Sieber P, Reddy GV, Long JA, Meyerowitz EM. Patterns of auxin transport and gene expression during primordium development revealed by live imaging of the Arabidopsis inflorescence meristem. Curr Biol 2005; 15:1899-911; PMID:16271866; http://dx.doi.org/10.1016/j.cub.2005.09.052
- Aloni R, Aloni E, Langhans M, Ullrich CI. Role of cytokinin and auxin in shaping root architecture: regulating vascular differentiation, lateral root initiation, root apical dominance and root gravitropism. Ann Bot 2006; 97:883-93; PMID:16473866; http://dx.doi.org/10.1093/aob/mcl027
- Panwar D, Ram K, Shekhawat HNS. In vitro propagation of Eulophia nuda Lindl., an endangered orchid. Sci Hortic (Amsterdam) 2012; 139:46-52; http://dx.doi.org/10.1016/j.scienta.2012.01.011
- Mohapatra A, Rout GR. In vitro micropropagation of Geoderum purpureum R. Br Ind J Biotech 2005; 4:568-70
- Malabadi RB, Mulgund GS, Nataraja K. Efficient regeneration of Vanda coerulea, an endangered orchid using thidiazuron. Plant Cell Tissue Organ Cult 2004; 76:289-93; http://dx.doi.org/10.1023/B:TICU.0000009255.69476.b7
- Zhang NG, Yong JWH, Hew CS, Zhou X. The production of cytokinin, abscisic acid and auxin by CAM orchid aerial roots. J Plant Physiol 1995; 147:371-7; http://dx.doi.org/10.1016/S0176-1617(11)82170-X
- Rafique R, Fatima B, Mushtaq S, Iqbal MS, Rasheed M, Ali M, Hasan SZU. Effect of indole-3-butyric acid (IBA) on in vitro root induction in Dendrobium orchid (Dendrobium sabin H.). Afr J Biotechnol 2012; 11:4673-5; http://dx.doi.org/10.5897/AJB11.2319
- Asghar S, Ahmad T, Hafiz IA, Yaseen M. In vitro propagation of orchid (Dendrobium nobile) var. Emma white. Afr J Biotechnol 2011; 10:3097-103; http://dx.doi.org/10.5897/AJB10.401
- Chutima R, Lumyong S. Production of indole-3-acetic acid by Thai native orchid-associated fungi. Symbiosis 2012; 56:35-44; http://dx.doi.org/10.1007/s13199-012-0158-2
- Tsavkelova EA, Cherdyntseva TA, Botina SG, Netrusov AI. Bacteria associated with orchid roots and microbial production of auxin. Microbiol Res 2007; 162:69-76; PMID:17140781; http://dx.doi.org/10.1016/j.micres.2006.07.014
- Galdiano RF Jr., Pedrinho EAN, Castellane TCL, Lemos EGDM. Auxin-producing bacteria isolated from the roots of Cattleya walkeriana, an endangered Brazilian orchid, and their role in acclimatization. R Bras Ci Solo 2011; 35:729-37; http://dx.doi.org/10.1590/S0100-06832011000300008
- Shimasaki K, Uemoto S. Micropropagation of a terrestrial Cymbidium species using rhizomes, developed from seeds and pseudobulbs. Plant Cell Tissue Organ Cult 1990; 22:237-44; http://dx.doi.org/10.1007/BF00033642
- Sheelavantmath SS, Murthy HN, Pyati AN, Ashok Kumar HG, Ravishankar BV. In vitro propagation of the endangered orchid Geodorum densiflorum (Lam.) Schltr. through rhizome section culture. Plant Cell Tissue Organ Cult 2000; 60:151-4; http://dx.doi.org/10.1023/A:1006426905052
- Bapat VA, Narayanaswamy S. Rhizogenesis in a tissue culture of the orchid Spathoglottis. Bull Torrey Bot Club 1977; 104:2-4; http://dx.doi.org/10.2307/2484656
- Paek KY, Yeung EC. The effect of 1-naphthalene acetic acid and N6- benzyladenine on the growth of Cymbidium forrestii rhizomes in vitro. Plant Cell Tissue Organ Cult 1991; 24:65-71; http://dx.doi.org/10.1007/BF00039732
- Roy J, Banerjee N. Rhizome and shoot development during in vitro propagation of Geodorum densiflorum (Lam.). Schltr. Sci Hortic (Amsterdam) 2002; 94:181-92; http://dx.doi.org/10.1016/S0304-4238(01)00373-9
- Traw MB, Bergelson J. Interactive effects of jasmonic acid, salicylic acid, and gibberellin on induction of trichomes in Arabidopsis. Plant Physiol 2003; 133:1367-75; PMID:14551332; http://dx.doi.org/10.1104/pp.103.027086
- Ishida T, Kurata T, Okada K, Wada T. A genetic regulatory network in the development of trichomes and root hairs. Annu Rev Plant Biol 2008; 59:365-86; PMID:18257710; http://dx.doi.org/10.1146/annurev.arplant.59.032607.092949
- Chang C, Chen YC, Yen HF. Protocorm or rhizome? The morphology of seed germination in Cymbidium dayanum Reichb. Bot Bull Acad Sin 2005; 46:71-4
- Warcup JH. Rhizanthella gardneri (Orchidaceae), its Rhizoctonia endophyte and close association with Melaleuca uncinata (Myrtaceae) in Western Australia. New Phytol 1985; 99:273-80; http://dx.doi.org/10.1111/j.1469-8137.1985.tb03656.x
- Griesbach RJ. The use of indoleacetylamino acids in the in vitro propagation of Phalaenopsis orchids. Sci Hortic (Amsterdam) 1983; 19:363-6; http://dx.doi.org/10.1016/0304-4238(83)90085-7
- Tokuhara K, Mii M. Micropropagation of Phalaenopsis and Doritaenopsis by culturing shoot tips of flower stalk buds. Plant Cell Rep 1993; 13:7-11; PMID:24196174; http://dx.doi.org/10.1007/BF00232306
- Mohanty P, Paul S, Das CM, Kumaria S, Tandon P. A simple and efficient protocol for the mass propagation of Cymbidium mastersii: an ornamental orchid of Northeast India. AoB Plants 2012; 2012pls023; PMID:22997547; http://dx.doi.org/10.1093/aobpla/pls023
- Chang C, Chang WC. Plant regeneration from callus culture of Cymbidium ensifolium var. misericors. Plant Cell Rep 1998; 17:251-5; http://dx.doi.org/10.1007/s002990050387
- Nasiruddin KM, Begum R, Yasmin S. Protocorm like bodies and plantlet regeneration from Dendrobium formosum leaf callus. As J. Plant Sci 2003; 2:955-7
- Rahman MS, Hasan MF, Das R, Hossain MS, Rahman M. In vitro micropropagation of orchid (Vanda tessellate L.) from shoot tip explant. J Biosci 2009; 17:139-44
- Ogura-Tsujita Y, Okubo H. Promotion of in vitro shoot formation from protocorm-like bodies of a hybrid between tropical and temperate Cymbidium species. J Jpn Soc Hortic Sci 2006; 75:334-6; http://dx.doi.org/10.2503/jjshs.75.334
- Chen TY, Chen JT, Chang WC. Plant regeneration through direct shoot bud formation from leaf cultures of Paphiopedilum orchids. Plant Cell Tissue Organ Cult 2004; 76:11-5; http://dx.doi.org/10.1023/A:1025858211320
- Naing AH, Chung JD, Park IS, Lim KB. Efficient plant regeneration of the endangered medicinal orchid Coelogyne cristata using protocorm-like bodies. Acta Physiol Plant 2011; 33:659-66; http://dx.doi.org/10.1007/s11738-010-0586-7
- Bhadra SK, Hossain MM. In vitro germination and micropropagation of Geodorum densiflorum (Lam.) Schltr., and endangered orchid species. Plant Tissue Cult 13:165-71.
