Abstract
Small regulatory RNAs (sRNAs) that act by base-pairing were first discovered in so-called accessory DNA elements—plasmids, phages, and transposons—where they control replication, maintenance, and transposition. Since 2001, a huge body of work has been performed to predict and identify sRNAs in a multitude of bacterial genomes. The majority of chromosome-encoded sRNAs have been investigated in E. coli and other Gram-negative bacteria. However, during the past five years an increasing number of sRNAs were found in Gram-positive bacteria. Here, we outline our current knowledge on chromosome-encoded sRNAs from low-GC Gram-positive species that act by base-pairing, i.e., an antisense mechanism. We will focus on sRNAs with known targets and defined regulatory mechanisms with special emphasis on Bacillus subtilis.
Introduction
Bacterial small regulatory RNAs (sRNAs) are the most abundant class of post-transcriptional regulators and had first been discovered in plasmids, phages, and transposons, where they control replication, maintenance, and transposition.Citation1 Whereas until 2001, only a dozen riboregulators were known from bacterial chromosomes, since then, various systematic approaches have been performed to predict and identify chromosome-encoded sRNAs in prokaryotes. The majority of them have been investigated in E. coli and Salmonella, whereas only a few well-studied examples are known from Gram-positive bacteria.
In the past five years, a combination of computational predictions, transcriptome analyses, and RNA sequencing approaches has been applied to identify chromosome-encoded riboregulators in low GC Gram-positive bacteria (see ). Five searches have been performed in B. subtilis. Rasmussen et al. found 84 putative non-coding trans-encoded sRNAs in the B. subtilis genome,Citation2 and Irnov et al. increased the total number to 108.Citation3 A recent RNA-Seq in B. licheniformis identified 461 independently transcribed sRNAs in addition to 855 RNAs transcribed in antisense to known protein and RNA encoding genes.Citation4 Six searches were performed in S. aureus and five in Listeria monocytogenes, three in Streptococcus pyogenes, and four in Streptococcus pneumoniae (see below). Two searches in Enterococcus faecalis identified 29 novel sRNAs, among them an antisense RNA to 6S RNA.Citation5,Citation6 In Clostridium, three searches have been performed (C. difficile,Citation7 C. ljungdahlii,Citation8 and C. acetobutylicumCitation9).
Table 1. Systematic searches for sRNAs in low GC Gram-positive bacteria
sRNAs either regulate translation or RNA stability. The majority of them inhibit translation (), whereas only a few of them activate translation (). Translational inhibition can principally occur in three different ways, (1) by direct blocking of the ribosome-binding site (RBS) (), by induction of structural alterations downstream of the RBS (see or iii) by blocking of a ribosome standby site required for efficient translation (reviewed in ref. Citation10). So far, the latter case has been only found in E. coli.Citation11 In some cases, translational inhibition is accompanied by mRNA degradation (see below, S. aureus sRNAs). Both cis-and trans-encoded sRNAs can inhibit translation () or promote RNA degradation (). Some sRNAs can stabilize their target RNAs (), whereas others process an unstable mRNA into a stable, translationally active RNA (). In 2011, the first sRNA required for the maturation of long RNAs—the CRISPR RNAs—was discoveredCitation12 (). A mechanism of action that can be exclusively used by cis-encoded sRNAs is transcriptional interference (, see below). In this report, we summarize () and discuss all currently known mechanisms employed by chromosome-encoded sRNAs from low-GC Gram-positive bacteria. Thereby, mechanisms discovered for cis-encoded and for trans-encoded sRNAs were assembled. and list all currently known sRNAs with their targets, mechanisms of action, and regulators. In a recent report that also includes plasmid-encoded antisense RNAs that control replication or maintenance of these accessory DNA elements, two additional control mechanisms were considered: transcription and translation attenuation.Citation13 Although both mechanisms are employed by riboswitches, they have not yet been observed for chromosome-encoded bona fide sRNAs.
Figure 1. Mechanisms employed by sRNAs encoded from low-GC Gram-positive bacteria. All currently known mechanisms for sRNAs encoded from chromosomes are summarized. For additional mechanisms employed by plasmid-encoded sRNAs, see reference Citation13. Antisense RNAs are drawn in red, sense RNAs in blue. Black triangles denote promoters. Light blue, ribosome binding sites (RBS). Yellow symbols indicate ribosomes. Green arrows denote RNase III cleavage; black arrows indicate unknown RNase action. The violet symbol represents RNase R. For details, see text. B, C, E, F, and H are based on reference Citation13.
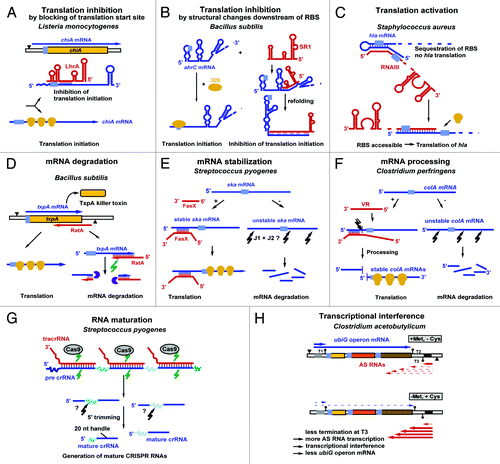
Table 2. Overview of cis-encoded sRNAs from low-GC Gram-positive bacteria
Table 3. Overview of trans-encoded antisense RNAs from low GC Gram-positive bacteria
Chromosome-Encoded sRNAs in Low GC Gram-Positive Bacteria and Their Biological Functions
In the past years, a variety of articles have been published on the identification of sRNAs in Gram-positive bacteria (reviewed in ref. Citation13). On average, ≈100–200 sRNAs have been discovered in a single genome. Despite newly available methods, it is still a challenging task to identify the targets of these novel riboregulators. In the following, we focus on base-pairing sRNAs for which targets have been verified experimentally. For an overview that also includes data on riboswitches and protein-binding sRNAs from Gram-positive bacteria, see reference Citation14.
Chromosome-encoded sRNAs are involved in a wide variety of biological functions. Mostly, they fine-tune metabolic processes and regulate stress adaptation or virulence. Fine-tuning functions are reflected by the lack of severe phenotypes upon deletion or overexpression of such RNAs. Examples for metabolic regulation include arginine catabolism (B. subtilis SR1Citation15) and iron-transport and storage (B. subtilis FsrACitation16) or central metabolism (S. aureus RsaECitation17).
A few trans-encoded sRNAs contain additionally small open reading frames (ORFs) that are translated. Such RNAs were designated dual-function sRNAs. Examples for small ORFs are the 26 codon δ-hemolysin ORF of S. aureus RNAIII,Citation18 the 22 codon psmα-ORF in S. aureus Psm-mec,Citation19 the streptolysin SLS-ORF of Streptococcus Pel RNA,Citation20 and the 39 codon ORF sr1p on B. subtilis SR1 RNA (see belowCitation21). The translation products of the small ORFs can either operate in the same pathway as the base-pairing sRNA (in RNAIII and Pel RNA) or in another pathway: B. subtilis SR1 acts as a base-pairing sRNA in arginine catabolism, whereas SR1P acts in sugar metabolism.Citation21 To date, neither for the 72 codon hyp7 ORF of Clostridium perfringens VRCitation22 nor the 32 codon ORF of Streptococcus pyogenes RivXCitation23 data are available on translation or possible biological function(s).
In different approaches, genome-wide overlapping (antisense) transcription was found (reviewed in ref. Citation24). Antisense transcription in the same bacterial cells yields a collection of short RNA fragments that result from RNase III processing, which appears most prominently in Gram-positive bacteria. Examples are provided below for B. subtilis and S. aureus. The mechanisms through which overlapping transcription can affect sense RNA expression are diverse. Primarily direct base-pairing interactions between sense and antisense transcripts, which result in RNase III cleavage of the complexes, can be imagined. An alternative is transcriptional interference that has, so far, been documented only in one case (see below).
In the following, sRNAs discovered in Bacillus subtilis, Staphylococcus aureus, Listeria monocytogenes, as well as Clostridium and Streptococcus species, and for which targets have been identified, are discussed in detail with regard to their biological function, expression conditions, and mechanism of action.
sRNAs from Bacillus subtilis
Trans-encoded sRNAs
The first trans-encoded sRNA in B. subtilis, FsrA (84 nt) was discovered in 2008Citation16 and resembles E. coli RyhB as it is transcriptionally repressed by Fur and regulates target mRNAs involved in iron metabolism and storage, e.g., sdhCAB (succinate dehydrogenase) and citB (aconitase). Using transcriptomics, additional FsrA targets were identified, among them, gltAB encoding the iron-sulfur-containing enzyme glutamate synthase and lutABC, dctP, resA, and qcrA.Citation25 Consequently, FsrA is a global regulator in B. subtilis. In contrast to E. coli RyhB, which requires the RNA chaperone Hfq, FsrA cooperates with one, two, or three Fur-regulated small basic proteins, FbpA, FbpB, and FbpC, suggested to be RNA chaperones to repress translation of its different targets.Citation16 Under iron-limited growth conditions, both FsrA and FbpB (48 aa) inhibit translation of lutABC encoding iron-sulfur-containing oxidases, which allows iron to be directed to higher-priority target proteins.Citation26 FsrA is predicted to target the region upstream of and including the RBS, while FbpB might facilitate FsrA/lutABC RNA pairing or recruit the RNA degradation machinery.Citation26 Thereby, FsrA is the main regulator, as its modest overexpression can bypass the need for FbpB.Citation26 The authors suggest that FsrA uses a C-rich single-stranded region to interact with the ribosome binding sites of its different target mRNAs.Citation25
In 2009, it has been found that the expression of BsrF (115 nt) is controlled by the global regulator CodY that responds to branched chain amino acids and GTP.Citation27 However, no BsrF targets have been identified so far. In 2011, CsfG, a sporulation-specific, non-coding sRNA highly conserved in endospore formers was found.Citation28 Its target has not yet been determined. The same holds true for a number of non-coding sRNAs that are under sporulation control, which were discovered in 2006Citation29 and further analyzed later on.Citation30 Among them, SurC is transcribed under control of σK and is conserved in the distantly related B. anthracis.Citation29
SR1—The first dual-function sRNA in Bacillus subtilis
The small RNA SR1 was discovered using a bioinformatics approach for sRNAs in intergenic regions of the B. subtilis chromosome combined with northern blotting.Citation15 SR1 comprises 205 nt and is encoded between pdhD and speA. Knockout and overexpression of sr1 were not detrimental for B. subtilis growth. Using 2D gel electrophoresis and northern blotting with a wild-type and an sr1-knockout strain, two arginine catabolic operons—rocABC and rocDEDF—were identified as secondary targets whose expression is upregulated in an sr1-knockout strain. The mRNA encoding AhrC, the transcription activator of these operons, is the primary target of SR1.Citation31 Both RNAs share seven regions of complementarity in the central part of ahrC and the 3′ half of SR1 (). SR1 does not affect the stability or amount of ahrC mRNA, but inhibits its translation by a novel mechanismCitation32: Although it binds ≈100 nt downstream of the ahrC RBS, it induces structural changes 20–40 nt downstream of the ahrC RBS that inhibit translation initiation (). This was shown by secondary structure probing of the ahrC/SR1 complex and toe-printing studies.Citation32 SR1 is only expressed under gluconeogenic conditions and 20–30-fold repressed by CcpN, and, to a minor (3-fold) extent, CcpA, under glycolytic conditions.Citation15 CcpN binds upstream of and overlapping the sr1 promoter,Citation33 whereas CcpA binds 250 upstream of the transcription start site (TSS) at a cre site. CcpN represses sr1 transcription in the presence of ATP and slightly acidic pH (6.5)Citation34 by interacting with the α-subunit of the RNA polymerase, thereby inhibiting promoter escape.Citation35 Transcriptomics and northern blotting suggested a second target for SR1, the gapA operon. In the presence of SR1, gapA mRNA is stabilized, whereas it is barely detectable in the absence of SR1 under gluconeogenic conditions. We demonstrated that the 39 aa peptide encoded by SR1, SR1P, is responsible for this effect: SR1P binds to GapA (glyceraldehyde-3P dehydrogenase A), thereby stabilizing gapA operon mRNA by a hitherto unknown mechanism.Citation21 Consequently, SR1 is a dual-function sRNA: It acts as a base-pairing regulatory RNA on ahrC RNA, and as a peptide-encoding mRNA on the gapA operon.
Figure 2. SR1 and SR4, a trans- and a cis-encoded sRNA from B. subtilis. As in , the antisense RNAs are indicated in red, the sense RNAs in blue, RBS in light blue, ribosomes are in yellow, RNase III in green, and RNase R in violet. (A) SR1, a trans-encoded sRNA, is the first identified dual-function sRNA from B. subtilis. +, activation; -, repression. CcpA and CcpN repress sr1 transcription under glycolytic conditions. TF is a novel transcription factor that activates sr1 transcription at cold-shock. (B) SR4, a cis-encoded sRNA, is the antitoxin of the type I TA system bsrG/SR4. It is the first antitoxin for which two independent functions have been found. For details, see text.
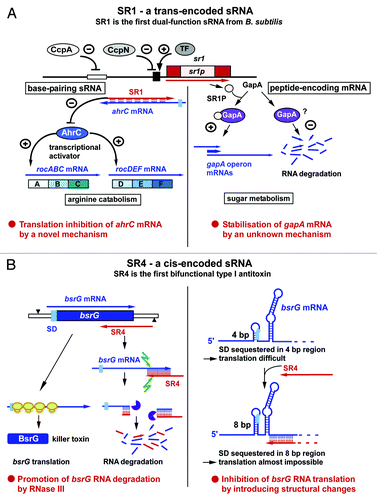
Recently, a computer prediction revealed SR1 homologs in 23 other species belonging to the Bacillales.Citation36 The expression of the SR1 homologs in seven of these species was experimentally verified. Furthermore, the ability of SR1P homologs to bind B. subtilis GapA was investigated in northern blots and co-elution experiments, and the interaction between SR1 homologs and the corresponding ahrC homologs studied in vitro.Citation36 The results demonstrated that both functions of SR1, the base-pairing and the peptide-encoding function, are remarkably conserved over ≈1 billion of years of evolution. represents the two functions of SR1.
To elucidate the interaction surface of SR1P and GapA, a series of peptide mutants were constructed and analyzed in northern blotting and co-elution experiments. To verify the predicted binding regions, both protein-coding genes have to be mutated and expressed simultaneously and independently in B. subtilis. For this purpose, the pMG vector family, a series of modular plasmids suitable for chromosomal integration and gene expression under single copy conditions in B. subtilis, was constructed.Citation37 Preliminary data show that for the stabilizing effect of GapA/SR1P, only part of the gapA operon mRNA must be present, which must contain sequences adjacent to the gapA ORF (Gimpel, unpublished).
Recently, we observed that the amount of SR1 is significantly reduced after an 18 °C cold-shock. This was due to altered transcription initiation and not RNA degradation. Upon investigation of the cold-shock effect, we discovered a novel trigger enzyme (Preis et al., unpublished).
Future work will be aimed at the investigation of the molecular mechanism behind the SR1P–GapA interaction, in particular, the determination of the SR1P binding pocket and properties as KD-value or stoichiometry of the interaction. Moreover, the biological role of SR1P-GapA interaction will be elucidated.
Cis-encoded sRNAs
With the exception of ECF RNA (see below), only cis-encoded sRNAs that act as type I antitoxins have been identified so far in B. subtilis. Currently, five types of toxin-antitoxin systems (TA systems) are known (reviewed in ref. Citation38). In type I TA systems, the antitoxin is a small RNA, and the toxin mRNA encodes a hydrophobic peptide. RNA antitoxins are cis-encoded regulatory RNAs that interact with their target RNAs either at their 5′ or 3′ end by a base-pairing mechanism. In Bacillus subtilis, 14 type I TA systems have been postulated, and three of them verified experimentally: txpA/RatA,Citation39 bsrG/SR4,Citation40 and yonT/as-yonT.Citation41,Citation42 The majority of them are located on prophage elements or phage remnants in the chromosome.
RatA—The first identified RNA antitoxin from B. subtilis
In 2005, txpA/RatA was identified as the first type I TA system on the skin element of the B. subtilis chromosome.Citation39 In the absence of RatA, TxpA causes cell lysis on agar plates. The RatA RNA is 220 nt long and overlaps the 3′ end of txpA mRNA by ≈120 nt.Citation39 The interaction between txpA mRNA and RatA promotes the degradation of txpA mRNA by an RNase III-dependent mechanism.Citation42 Degradation of txpA mRNA by RNase III is essential for viability of B. subtilis.Citation42 The secondary structures of RatA and txpA RNA, as well as their complex, have been determined.Citation42 The ribosome binding site (RBS) of txpA is located in a 5 bp ds region. This sequestration is not altered upon RatA binding.
SR4—An RNA antitoxin with two functions
The bsrG/sr4 module is located on the SPβ prophage of the B. subtilis chromosome. We corroborated experimentally that this module acts as a type I toxin/antitoxin system:Citation40 The bsrG RNA is 294 nt long and codes for a 38 aa toxic peptide with a predicted central transmembrane domain and a charged C terminus (see ref. Citation41). The antisense RNA SR4 is 180 nucleotides long and overlaps the 3′ end of bsrG by 123 nucleotides. The interaction of both RNAs at their 3′ ends promotes degradation of bsrG RNA. RNase III cleaves the bsrG RNA/SR4 duplex at position 185 of bsrG RNA, 8 nt downstream from the stop codon, but is not involved in the degradation of either bsrG RNA or SR4 alone.Citation40 Endonuclease Y and the 3′-5′ exoribonuclease R are responsible for further degradation of both RNAs. PNPase processes SR4 precursors into the mature RNA. As a Δrnc suppressor strain neither lysed on agar plates nor had mutations in the bsrG ORF, RNase III is not essential to the function of the bsrG/SR4 system.Citation40 Later it was found that the Δrnc suppressor strain has a deletion of the skin prophage and a tendency to loose SPβ in order to reduce TxpA and YonT toxicity.Citation41 Hfq is not required for the function of the bsrG/SR4 system,Citation40 since a Δhfq strain does neither show lysis nor altered half-lives of bsrG RNA or SR4.
Recently, the secondary structures of SR4 and bsrG-RNA, as well as the SR4/bsrG RNA complex, were experimentally determined. The results demonstrated that SR4 induces structural alterations around the RBS of bsrGCitation43 (): A 4 bp region that sequesters the bsrG RBS is extended to 8 bp in the presence of SR4. It was shown experimentally that this extended double-stranded region inhibits translation. Consequently, SR4 is the first type I antitoxin with two clearly separable functions: it promotes degradation of bsrG mRNA and impedes ribosome access to the bsrG SD, thereby preventing translation. Complex formation assays with wild-type bsrG RNA/SR4 yielded an apparent binding constant kapp of 6.5 × 105 M−1 s−1,Citation43 which is in the same order of magnitude as those of other cis-encoded sense/antisense RNA pairs.Citation44 The binding pathway of bsrG mRNA and SR4 was elucidated: Binding starts with a single loop–loop contact between loop 3 of bsrG RNA and loop 4 of the SR4 terminator stem-loop. Intermolecular base-pairing progresses via the single-stranded region between L4 and L3 toward loop 3 of SR4, and, finally to loop 2, which pairs with the bsrG terminator-stem-loop. However, the latter interaction was not required for efficient binding.Citation43 Loop 3 of bsrG RNA contains a 5′ YUNR motif, which apparently forms a U-turn that provides a scaffold for the efficient initial interaction between both RNAs.Citation43 The sr4 promoter is about 6- to 10-fold stronger than the bsrG promoter,Citation40 which should result in an excess of the antitoxin over the toxin, as was shown for txpA/RatA.Citation42
Other cis-encoded sRNAs in B. subtilis
A recent review summarizes the current knowledge about other cis-encoded sRNAs that act as type I antitoxins in B. subtilis.Citation41 Among them is yonT80, which is regulated by as-yonT, and was recently confirmed to be a toxin in E. coli, and indirectly in B. subtilis.Citation42 Furthermore, BsrE, regulated by as-BsrE (renamed SR5) acts as a toxin in B. subtilis (Maiwald, Jahn, and Brantl, unpublished).
The only other cis-encoded sRNA known to date is the long (750 nt) ECF RNA, which is expressed under control of extracytoplasmic sigma factors σX and σM and was found to regulate an autolysin encoded by the yabE gene.Citation45
A transcriptome analysis indicated a widespread antisense transcription in bacterial chromosomes: Using micorarrays, for 2.9% of all B. subtilis genes, antisense transcripts were detected,Citation2 and a dRNA Seq approach found in total 29 cis-encoded antisense RNAs.Citation3 To date, the biological role of these antisense RNAs is unknown.
In Staphylococcus, Streptococcus, Listeria, and Clostridium species, mostly sRNAs implicated in the regulation of pathogenesis and virulence, but also a few metabolic riboregulators, have been discovered and investigated in more detail.
sRNAs from Staphylococcus aureus
Meanwhile, six different approaches have identified about 100 trans- and 100 cis-encoded sRNAs in the human pathogen S. aureus (). The majority of the hitherto characterized sRNAs are involved in pathogenesis. The most prominent and best-characterized of them, which was already discovered in 1995, is the unusually long RNAIII (514 nt).Citation18 Transcription of RNAIII is induced by AgrA, the response regulator of the quorum-sensing agr (accessory gene regulation) system, and RNAIII is the effector of this system. Its secondary structure was mapped in vitro and in vivo and revealed 14 hairpin structures, out of which the conserved hairpin 13 was involved in repression of protein A expression.Citation46 RNAIII was the first antisense RNA for which an activating function has been foundCitation18 (). However, it does not only activate translation of the α-hemolysin mRNA, but also inhibits translation of a variety of targets:Citation47 spa (main surface adhesin protein), SA1000 (novel fibrinogen-binding protein), sa2261 (ABC transporter), rot (pleiotropic transcriptional factor RotCitation48), lytM (peptidoglycan hydrolase), and coagulase mRNA.Citation49 To exert its inhibitory effect, RNAIII employs a combination of translation inhibition by a base-pairing interaction of its two redundant 3′ hairpin loops with the target mRNA and recruitment of RNase III for target degradation.Citation50 As shown for spa, RNA degradation was required for permanent translational arrest. Specificity for RNAIII on all translationally inhibited targets is obtained by either propagating the first loop–loop contact at the RBS into the stem regions (sa1000 and sa2353 mRNAs) or by addition of a second loop–loop interaction (rot and coa mRNAs). The RNAIII/coa mRNA duplex comprises an imperfect duplex masking the SD sequence and a loop–loop interaction in the coa ORF. The imperfect duplex is sufficient to prevent translation initiation. RNase III cleaves the two regions of the coa mRNA bound to RNAIII that may contribute to the degradation of the repressed mRNA.Citation49 Interestingly, RNAIII represents the first identified dual-function sRNA as it is on the one hand a regulatory sRNA that acts by base-pairing and on the other hand a protein-encoding mRNA with an ORF for the 26 aa δ-hemolysin.Citation18
The second dual-function sRNA in S. aureus, Psm-mec (157 nt), was discovered only in 2013.Citation19 The psm-mec gene is located on the mobile genetic element SCCmec that confers—via mecA—methicillin resistance to MRSA strains. Psm-mec encodes the 22 aa-secreted cytolytic toxin PSMα (α phenol-soluble modulin). Additionally, it inhibits translation of agrA mRNA by base-pairing of its 5′ nt 21–50 with the agrA coding sequence (most important are nt 199 to nt 267). Furthermore, it causes a ≈2-fold RNase III-dependent decrease in agrA half-life, which was—in contrast to the inhibitory effect of RNAIII on rot mRNA—independent of the translation inhibition effect. Psm-mec RNA itself is stable (half-life 20 min) and not affected by agrA mRNA. AgrA activates transcription of RNAIII and of the psmα 3 operon. Twenty-five percent of 325 analyzed clinical isolates (HA strains) have a promoter mutation that causes attenuated psm-mec transcription, and 9% have no psm-mec, which results in high virulence. By contrast, community-acquired MRSA strains (CA strains) have no Psm-mec. Kaito et al. proposed that Psm-mec sRNA attenuates virulence in HA comparison to CA strains.Citation19
Recently, a second AgrA-regulated sRNA, ArtA, was discovered that controls α-toxin expression by targeting the 5′ UTR of sarT mRNA.Citation51
In 2005, a search in the clinical agr negative S. aureus strain N315 resulted in seven experimentally confirmed small pathogenicity islands RNAs = Spr (SprA to SprG), among which SprA, E, F, and G were present in multiple copies, partly also on the core genome.Citation52 For SprA (202 nt), in vitro data suggested an interaction with three putative mRNA targets, among them a 3.5 kb ABC transporter mRNA.Citation52 However, in 2012 it was found instead that SprA1 encodes a 30 aa peptide toxin, which inserts into the cell membrane and kills S. aureus cells, and is regulated by a cis-encoded antisense RNA (SprA1AS) that facilitates degradation of SprA1 mRNA. Therefore, SprA1/SprA1AS constitutes a type I toxin-antitoxin system.Citation53 SprA1AS combines—similar to RNAI from E. faecalis plasmid pAD1 (reviewed in ref. Citation13)—features of a cis- and a trans-encoded sRNA.Citation54 Surprisingly, the SprA1 peptide was previously also found to act as cytolysin on human erythrocytes.Citation54 In 2010, SprD (142 nt), another sRNA of the first search, was shown to inhibit translation initiation of the abundant secreted immune-evasion protein Sbi by an interaction between its central region and the 5′ 41 nt of sbi mRNA, including RBS and start codon.Citation55 In contrast to RNAIII and Psm-mec, SprD did not affect the sbi mRNA levels. In an animal septicaemia model, SprD impaired both adaptive and innate host immune responses.Citation54
The first staphylococcal sRNA involved in metabolic regulation, RsaE, was discovered in 2009Citation17 among 11 novel Hfq-independent sRNAs (RsaA–RsaK). RsaE (96 nt) is highly conserved in four differential S. aureus species and was also found in Macrococcus and Bacillus.Citation17 It co-regulates several metabolic pathways involved in amino acid and peptide transport, cofactor synthesis, carbohydrate and folate metabolism, and TCA cycle by inhibiting translation of two cistrons of an oligopeptide transporter operon, opp-3ACitation56 and opp-3B,Citation17 and sucC/sucD-encoding succinyl-CoA synthetase subunits α and β, and by targeting sA0873.Citation56 Expression of rsaE is AgrA-dependent and very low in pre-stationary phase. Similar to B. subtilis FsrA and S. aureus RNAIII, RsaE seems to recognize its target mRNAs at the RBS via a conserved C-rich loop (UCCC motif). In another screen for sRNAs, RsaOX (129 nt) and RsaOW were proposed to target the transposase genes sa0062 and is1181, respectively, possibly by promoting RNA degradation.Citation56
Recent tiling arrays revealed long antisense RNAs that are rapidly degraded by RNase III.Citation57 It is suggested that such long sRNAs might play an important role in staphylococcal gene regulation, in particular, of genes involved in pathogenesis and virulence. One example is SSR42 (891 nt) that affects erythrocyte lysis and pathogenesis in a murine infection model.Citation58 SSR42 is stabilized in stationary phase, upregulates genes involved in capsule biosynthesis, and downregulates ≈80 genes, among them, virulence factors. However, its role seems to be indirect as no SSR42 binding to the corresponding mRNAs was found.Citation58 A summary of all kinds of regulatory RNAs hitherto found and investigated in Staphylococcus areus and their biological functions has been published recently.Citation59
sRNAs from Clostridium
In 2013, RNA-seq approaches in three Clostridium species discovered between 36 and 182 sRNAs (). So far, for only a few of them, targets are known. Already > 20 y ago, a cis-encoded antisense RNA was found in the biotechnologically important Clostridium acetobutylicum, which is involved in control of nitrogen metabolism by interacting with the 5′ UTR of the glutamin synthetase gene glnA.Citation60,Citation61 However, no further reports were published on this issue. In the same species, four antisense RNAs were discovered in 2008 that are encoded downstream of the ubiG operon and act in concert with an S-box riboswitch to regulate sulfur metabolism,Citation62 . These long antisense RNAs (between 200–1000 nt) represent the so-far-unique example for transcriptional interference as mechanism of action of base-pairing sRNAs. In the human pathogen Clostridium difficile, a genome-wide association study identified 94 trans-encoded sRNAs and 91 cis-encoded sRNAs, and confirmed 35 of them experimentally.Citation7
In the food-born pathogen Clostridium perfringens, two sRNAs have been characterized in more detail: The sRNA VR is part of the VirR/S regulon that controls toxin production and induces collagenase (K-toxin) and b2-toxin synthesis,Citation63 . VR has been shown to regulate five genes by direct binding to their mRNAs: pfoA, vrr, virT, ccp, and virU. Additionally, VR positively affects synthesis of CPE1447 and CPE1446, which form a protein heterodimer that controls toxin gene expression.Citation64 The function of the small ORF encoded on VR is still unclear. In 2013, another sRNA, VirX, which had been shown beforeCitation65 to regulate pfoA, plc, and colA mRNAs independent of the VirR/VirS system has been found to repress genes encoding positive sporulation regulators like Spo0A and sigma factors E, F, and K.Citation66 Inactivation of virX led to higher levels of sporulation and enterotoxin production. Data on sRNAs in Clostridium species available until 2011 have been summarized.Citation67
sRNAs from Listeria
Although five different approaches have discovered sRNAs in Listeria monocytogenes and L. innocua (), only a few targets have been identified so far. One screen for Hfq-binding sRNAs in L. monocytogenes identified LhrA, LhrB, and LhrC.Citation68 LhrA is stabilized by Hfq and targets at least three mRNAs directly, chiA mRNA encoding two chitinases and two genes of unknown function (lmo0850 and lmo0302).Citation69,Citation70 In a global screen, 300 genes were found to be affected by LhrA. In the presence of Hfq, LhrA inhibits translation of chiA mRNA (). LhrA is, so far, the only example from Gram-positive bacteria for which an effect of the RNA chaperone Hfq on target binding was foundCitation69 (see below). However, the majority of the sRNAs from Listeria do not seem to need Hfq for stabilization or interaction with their targets (reviewed in ref. Citation69). In the case of lmo0850, LhrA both inhibits translation and promotes RNA degradation. For a few recently discovered sRNAs, targets were predicted, but their mechanism of action on them is still elusive71: RliB is proposed to target lmo2104/5 involved in iron transport, RliE the competence factors comEA/FA, and RliL a phosphotransferase system (lmo1035). The absence of 15 of the 29 sRNAs recently found in L. monocytogenesCitation72 in the non-pathogenic L. innocua underlines the importance of riboregulators for pathogenesis and virulence. Rli38 from L. monocytogenes is 25-fold higher expressed in blood and in the presence of H2O2, i.e., under oxygen stress.
In RNaseq approaches, unusually long antisense RNAs (las RNA) complementary to more than one ORF or operon were found in Listeria species. The authors designated such a genomic locus excludon.Citation72,Citation73 Thereby, the 5′ or 3′ non-coding part of a lasRNA negatively affects the expression of one or several gene(s) on the complementary strand, whereas the remaining (major) part functions as mRNA for the downstream or upstream genes. The first reported excludon in L. monocytogenes controls flagellum biosynthesis at the motility gene repressor locus. MogR is the transcriptional repressor of flagellum and motility genes in Listeria species, and mogR is transcribed from a promoter 45 nt upstream of the AUG. In opposite direction, the flagellum operon with lmo0675 (unknown), lmo0676 (fli), lmo0677 (fliQ), and lmo0678 (fliR) is transcribed. FliP and FliQ form the flagellum export apparatus. Additionally, transcription initiated at a third promoter upstream of pmogR results in an excludon transcript, Anti0677, whose 5′ region is antisense to lmo0675-lmo0677, and whose 3′ part contains the mogR ORF. Anti0677 is under control of stress-activated σB located within the lmo0677 ORF. Other recently summarized examplesCitation73 comprise two putative permease-efflux pump excludons and a putative carbon source utilization excludon. However, the detailed mechanism of action of the proposed lasRNAs has not yet been elucidated.
In 2009, two trans-acting S-adenosylmethionine riboswitches (SreA and SreB) that can function as trans-encoded sRNAs were discovered in L. monocytogenes.Citation74 SreA upregulates argD and represses translation of prfA encoding the virulence master regulator by base-pairing upstream of the RBS. SreB (179 nt) regulates prfA and lmo2230 (arsenate reductase homolog).Citation74 By targeting PrfA, SreA and SreB link virulence to nutrient availability.
sRNAs from Streptococcus
Streptococci include species that cause severe human diseases. In the group A (GAS) Streptococcus pyogenes, 75 sRNAs were identified (), and three of them were implicated in virulence control: Pel, FasX and RivX. The 450 nt long bifunctional sRNA Pel regulates M- and M-related proteins and codes for streptolysin S (SLS).Citation20 It exerts pleiotropic effects on virulence. Pel expression is repressed both by a multitude of transcription factors, in fact CcpA, CovRS/CsRS, LuxS, Mga, Nra, and RopB/Rgg, and, additionally, by FasX RNA, but activated at amino acid starvation by CodY, Irr, and SLS itself.
FasX (≈200 nt) is encoded in the fasBCAX operon and transcriptionally activated by response regulator FasA. FasX stabilizes the ska mRNA encoding the secreted plasminogen activator streptokinaseCitation75 (). It also controls sagS mRNA encoding streptolysin S (see above) and, by unknown mechanisms, transcription of fbp54 mRNA and mrp mRNA encoding two fibronectin-binding proteins. In S. dysgalactiae ssp. equisimilis (group C streptococci GCS), FasX also affects ska and streptolysin.Citation76
The third recently found sRNA, RivX, is processed from a longer mRNA-encoding transcription regulator RivR and has, thus, three alternative 5′ ends resulting in 189, 237, 289 nt-long species. Together with transcription factor RivR, RivX upregulates the global virulence gene regulator mgA by enhancing its translation.Citation23 Mga itself controls emm, C5a peptidase, and cysteine protease speB. As mentioned above, RivX contains a 23 codon ORF of unknown function.
In 2011, tracrRNA (89 nt), a trans-encoded sRNA involved in maturation of CRISPR RNA was discovered.Citation12 CRISPR protects its host against prophage-derived DNA. In type II CRISPR systems, tracrRNA induces—together with Cas9 and RNase III—cleavage of pre-crRNA to yield mature crRNA (), which, upon phage infection, can target phage DNA. By probing selected loci, functional tracrRNA homologs were also found in Streptococcus mutans, S. thermophiles, and Listeria innocua, and even the Gram-negative Neisseria meningitidis.Citation12
Details about expression regulation and the involvement of streptococcal sRNAs in global networks controlling virulence and pathogenesis have been summarized recently.Citation77
sRNAs from Streptococcus pneumoniae
In Streptococcus pneumoniae, far more than one hundred sRNAs have been identified mainly by high-throughput approaches (). One sRNA, srn206, has been implicated in competence controlCitation78 and the target was suggested to be comD, the gene encoding the histidine kinase of the two-component regulatory system ComDE, which is essential for initiation of competence development.Citation79 Several sRNAs were shown to be involved in the control of various aspects of virulence and a number of differentially expressed proteins were detected in sRNA mutants.Citation80 However, no direct regulatory link was established between sRNAs and putative targets. In addition, two-component regulatory systems appeared to be involved in sRNA expression control, but the underlying mechanism was not determined.Citation80 Despite the identification of numerous sRNAs in whole genome approaches, no clearly defined targets or regulatory mechanisms were determined so far.
More information is available for the first sRNAs described in S. pneumoniae, which have been detected in an analysis to define the regulon of the two-component regulatory system CiaRH.Citation81 The five strongest promoters in the CiaRH regulon were found to drive expression of sRNAs between 87–151 nt in size. These non-coding sRNAs, designated csRNAs (cia-dependent small RNA), show a high degree of similarity to each other. They are predicted to adopt a secondary structure with two stem-loops at the 5′- and 3′-ends, respectively. Sequences complementary to the SD sequence and the start codon AUG are present in the unpaired region between the stem-loops. The csRNAs appear to affect pneumococcal physiology pleiotropically. Stationary phase autolysis was affected by csRNA4 and csRNA5,Citation81 and a csRNA5 mutant was defective in lung infection.Citation80 Furthermore, csRNA1 was shown to act negatively on competence development.Citation82 The csRNAs were originally detected in S. pneumoniae R6,Citation81 but are found in all S. pneumoniae genome sequences available to date. Curiously, Hungarian S. pneumoniae serotype 19A isolates carry and express longer versions of csRNA5, which apparently arose by internal sequence duplication (R. Brückner, unpublished observations). Expression of CiaR-controlled csRNAs was also confirmed in Streptococcus mitis, Streptococcus oralis, Streptococcus sanguinis,Citation83 and Streptococcus pyogenes.Citation84 The presence of multiple csRNAs genes could be predicted in all streptococcal genomesCitation83 suggesting that they serve an important function in this group of organisms.
In a recent study, csRNA target predictions in S. pneumoniae R6 were evaluated by analyzing translational fusions of candidate genes.Citation85 Six targets could be identified, which were all downregulated by the csRNAs. At least for the three genes tested, each of the csRNAs could act upon the targets reflecting the high degree of csRNA similarity. Regulation by the csRNAs was additive, no single csRNA was as effective as all csRNAs together. Four of the regulated genes, spr0081, spr0371, spr0551, spr1097, encode transport proteins of various protein families, but their physiological roles in S. pneumoniae are currently unknown. A putative transcriptional regulator spr0159 and comC, the gene encoding the precursor of the competence stimulating peptide CSPCitation86 were the remaining csRNA targets. Especially the identification of the latter was intriguing, since CiaRH was known to act negatively on competence development.Citation87,Citation88 Mutation of comC between the SD sequence and the start codon partially disrupting complementarity to the csRNAs greatly diminished csRNA-mediated repression of a translational fusion. Replacing wild-type comC by the mutated version in the genome of S. pneumoniae R6 relieved competence from CiaRH-dependent control.Citation85 Therefore, the csRNAs block CSP precursor production thereby interfering with pheromone signaling that initiates competence.
Interestingly, CiaRH controls production of the serine protease HtrA, which is also able to act negatively on competence by degradation of CSP.Citation89 Which negative CiaRH-dependent control mechanism prevails, csRNA- or HtrA-mediated, depends strongly on the growth conditions.Citation85
In addition to competence control, csRNAs are involved in another CiaRH-dependent phenotype. Mutations in the histidine kinase gene ciaH leading to a hyperactive CiaRH systemCitation87,Citation88 have been shown to increase β-lactam resistance. Without csRNAs, these CiaRH hyperactive strains are no longer resistant, but the target(s) involved in this phenotype has not yet been identified. Thus, the csRNAs certainly control at least one more target in S. pneumoniae.
Role of RNA Chaperones in sRNA-Mediated Gene Regulation in Low GC-Gram-Positive Bacteria
An important characteristic of many trans-encoded antisense RNAs from E. coli is their ability to bind the RNA chaperone Hfq (reviewed in ref. Citation90). Hfq is present in 50% of all sequenced bacterial species, and a few species like Bacillus anthracis encode even two Hfq proteins. Hfq is a homohexamer that is very similar to the eukaryotic Sm proteins involved in splicing.Citation90 It binds to AU-rich sequences in single-stranded regions flanked by one or two stem-loops. Among others, Hfq is involved in mRNA stability, polyadenylation, and translation.Citation90 In Gram-negative bacteria, the majority of trans-encoded sRNAs need Hfq either for their stability or for sRNA/target interaction (reviewed in ref. Citation10). In 2010, it was shown that sRNAs can displace each other on Hfq on a short time scale by RNA concentration-driven cycling.Citation91
Currently, the only example for Hfq-dependent antisense regulation in Gram-positive bacteria is LhrA from L. monocytogenes (see above).Citation68,Citation69 For two trans-encoded sRNAs in Gram-positive bacteria, B. subtilis SR1Citation32 and S. aureus RNAIII,Citation92 Hfq does not impact sRNA/target interaction. In the latter case, this was even tested in three virulent genetic backgrounds.Citation92 However, as hfq expression levels differ between strains, the role of Hfq in S. aureus is discussed controversially (e.g. ref. Citation93). By contrast, for the trans-encoded sRNA FsrA from B. subtilis, a role of other small putative RNA binding proteins that might act as RNA chaperones—in particular FbpB—has been proposed.Citation16,Citation26 However, it has not yet been demonstrated experimentally that FbpA, B, or C indeed bind RNA. Interestingly, Streptococcus species do not encode Hfq. A study on the interchangeability of Hfq-like proteins between Gram-negative and Gram-positive bacteria demonstrated that neither S. aureus nor Borrelia Hfq expressed chromosomally in S. enterica Typhimurium from the location of endogenous Hfq could functionally substitute Salmonella Hfq in sRNA-mediated regulation and protection from degradation.Citation94 Future research will show whether the LhrA case is an exception and whether other, yet-unidentified RNA binding proteins function as additional RNA chaperones in Gram-positive bacteria. In addition to the Fbp proteins, the SMc01113 protein could be a possible candidate, as it alters sRNA/target mRNA accumulation in Sinorhizobius meliloti,Citation95 but is highly conserved and present in almost all bacteria, also those that lack Hfq.
Future Perspectives
In the near future, the multitude of newly discovered sRNAs in low-GC Gram-positive bacteria will be investigated in detail to identify their targets, to analyze their biological role, and to elucidate their mechanisms of action. It can be expected that novel mechanisms or such known so far only from plasmid-encoded antisense RNAs or from sRNAs in Gram-negative bacteria, will be found for these sRNAs. Likewise, one sRNA might act in cis on one target and in trans on one or several others, thereby employing different modes of action.
As only in one case, LhrA from Listeria monocytogenes, a role for Hfq has been established, it might well be that other chaperones will be detected that play equivalent roles in Gram-positive bacteria. First possible candidates are the small basic proteins FbpA, FbpB, and FbpC, which were linked to the function of B. subtilis FsrA (see above).
Like in E. coli or Salmonella, target mRNAs will be found that are regulated by different sRNAs. In this context, global regulatory networks will be uncovered that implicate both sRNAs and transcriptional repressors and activators.
Furthermore, the small number of dual-function sRNAs will increase considerably, and new unprecedented functions for small peptides encoded by sRNAs will be detected.
The excludon concept recently established for long antisense RNAs (lasRNAs) from Listeria might be confirmed for novel lasRNAs from other low-GC-Gram-positive bacteria. The modes of action used by the lasRNAs may not only comprise classical antisense RNA concepts or RNA interference, but so far unanticipated mechanisms. Additionally, sRNAs might be found that act directly on the genome like e.g., the siRNAs from Schizosaccharomyces pombe that are involved in chromatin silencing (reviewed in ref. Citation96).
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
This study was supported by grants BR1552/6-1 to 6-3 and by grants BR1552/7-1 and 7-2 of the priority program SPP1258 from the Deutsche Forschungsgemeinschaft to Brantl S and BR974/5-1 of SPP1258 to Brückner R.
References
- Brantl S. Antisense RNAs in plasmids: control of replication and maintenance. Plasmid 2002; 48:165 - 73; http://dx.doi.org/10.1016/S0147-619X(02)00108-7; PMID: 12460531
- Rasmussen S, Nielsen HB, Jarmer H. The transcriptionally active regions in the genome of Bacillus subtilis. Mol Microbiol 2009; 73:1043 - 57; http://dx.doi.org/10.1111/j.1365-2958.2009.06830.x; PMID: 19682248
- Irnov I, Sharma CM, Vogel J, Winkler WC. Identification of regulatory RNAs in Bacillus subtilis.. Nucleic Acids Res 2010; 38:6637 - 51; http://dx.doi.org/10.1093/nar/gkq454; PMID: 20525796
- Wiegand S, Dietrich S, Hertel R, Bongaerts J, Evers S, Volland S, Daniel R, Liesegang H. RNA-Seq of Bacillus licheniformis: active regulatory RNA features expressed within a productive fermentation. BMC Genomics 2013; 14:667; http://dx.doi.org/10.1186/1471-2164-14-667; PMID: 24079885
- Fouquier d’Hérouel A, Wessner F, Halpern D, Ly-Vu J, Kennedy SP, Serror P, Aurell E, Repoila F. A simple and efficient method to search for selected primary transcripts: non-coding and antisense RNAs in the human pathogen Enterococcus faecalis.. Nucleic Acids Res 2011; 39:e46; http://dx.doi.org/10.1093/nar/gkr012; PMID: 21266481
- Shioya K, Michaux C, Kuenne C, Hain T, Verneuil N, Budin-Verneuil A, Hartsch T, Hartke A, Giard J-C. Genome-wide identification of small RNAs in the opportunistic pathogen Enterococcus faecalis V583. PLoS One 2011; 6:e23948; http://dx.doi.org/10.1371/journal.pone.0023948; PMID: 21912655
- Soutourina OA, Monot M, Boudry P, Saujet L, Pichon C, Sismeiro O, Semenova E, Severinov K, Le Bouguenec C, Coppée JY, et al. Genome-wide identification of regulatory RNAs in the human pathogen Clostridium difficile.. PLoS Genet 2013; 9:e1003493; http://dx.doi.org/10.1371/journal.pgen.1003493; PMID: 23675309
- Tan Y, Liu J, Chen X, Zheng H, Li F. RNA-seq-based comparative transcriptome analysis of the syngas-utilizing bacterium Clostridium ljungdahlii DSM 13528 grown autotrophically and heterotrophically. Mol Biosyst 2013; 9:2775 - 84; http://dx.doi.org/10.1039/c3mb70232d; PMID: 24056499
- Venkataramanan KP, Jones SW, McCormick KP, Kunjeti SG, Ralston MT, Meyers BC, Papoutsakis ET. The Clostridium small RNome that responds to stress: the paradigm and importance of toxic metabolite stress in C. acetobutylicum.. BMC Genomics 2013; 14:849; http://dx.doi.org/10.1186/1471-2164-14-849; PMID: 24299206
- Brantl S. Bacterial chromosome-encoded small regulatory RNAs. Future Microbiol 2009; 4:85 - 103; http://dx.doi.org/10.2217/17460913.4.1.85; PMID: 19207102
- Darfeuille F, Unoson C, Vogel J, Wagner EG. An antisense RNA inhibits translation by competing with standby ribosomes. Mol Cell 2007; 26:381 - 92; http://dx.doi.org/10.1016/j.molcel.2007.04.003; PMID: 17499044
- Deltcheva E, Chylinski K, Sharma CM, Gonzales K, Chao Y, Pirzada ZA, Eckert MR, Vogel J, Charpentier E. CRISPR RNA maturation by trans-encoded small RNA and host factor RNase III. Nature 2011; 471:602 - 7; http://dx.doi.org/10.1038/nature09886; PMID: 21455174
- Brantl S. Acting antisense: plasmid- and chromosome-encoded sRNAs from Gram-positive bacteria. Future Microbiol 2012; 7:853 - 71; http://dx.doi.org/10.2217/fmb.12.59; PMID: 22827307
- Romby P, Charpentier E. An overview of RNAs with regulatory functions in gram-positive bacteria. Cell Mol Life Sci 2010; 67:217 - 37; http://dx.doi.org/10.1007/s00018-009-0162-8; PMID: 19859665
- Licht A, Preis S, Brantl S. Implication of CcpN in the regulation of a novel untranslated RNA (SR1) in Bacillus subtilis.. Mol Microbiol 2005; 58:189 - 206; http://dx.doi.org/10.1111/j.1365-2958.2005.04810.x; PMID: 16164558
- Gaballa A, Antelmann H, Aguilar C, Khakh S-K, Song K-B, Smaldone GT, Helmann JD. The Bacillus subtilis iron-sparing response is mediated by a Fur-regulated small RNA and three small, basic proteins. Proc Natl Acad Sci U S A 2008; 105:11927 - 32; http://dx.doi.org/10.1073/pnas.0711752105; PMID: 18697947
- Geissmann T, Chevalier C, Cros M-J, Boisset S, Fechter P, Noirot C, Schrenzel J, François P, Vandenesch F, Gaspin C, et al. A search for small noncoding RNAs in Staphylococcus aureus reveals a conserved sequence motif for regulation. Nucleic Acids Res 2009; 37:7239 - 57; http://dx.doi.org/10.1093/nar/gkp668; PMID: 19786493
- Morfeldt E, Taylor D, von Gabain A, Arvidson S. Activation of alpha-toxin translation in Staphylococcus aureus by the trans-encoded antisense RNA, RNAIII. EMBO J 1995; 14:4569 - 77; PMID: 7556100
- Kaito C, Saito Y, Ikuo M, Omae Y, Mao H, Nagano G, Fujiyuki T, Numata S, Han X, Obata K, et al. Mobile genetic element SCCmec-encoded psm-mec RNA suppresses translation of agrA and attenuates MRSA virulence. PLoS Pathog 2013; 9:e1003269; http://dx.doi.org/10.1371/journal.ppat.1003269; PMID: 23592990
- Mangold M, Siller M, Roppenser B, Vlaminckx BJ, Penfound TA, Klein R, Novak R, Novick RP, Charpentier E. Synthesis of group A streptococcal virulence factors is controlled by a regulatory RNA molecule. Mol Microbiol 2004; 53:1515 - 27; http://dx.doi.org/10.1111/j.1365-2958.2004.04222.x; PMID: 15387826
- Gimpel M, Heidrich N, Mäder U, Krügel H, Brantl S. A dual-function sRNA from B. subtilis: SR1 acts as a peptide encoding mRNA on the gapA operon. Mol Microbiol 2010; 76:990 - 1009; http://dx.doi.org/10.1111/j.1365-2958.2010.07158.x; PMID: 20444087
- Shimizu T, Yaguchi H, Ohtani K, Banu S, Hayashi H. Clostridial VirR/VirS regulon involves a regulatory RNA molecule for expression of toxins. Mol Microbiol 2002; 43:257 - 65; http://dx.doi.org/10.1046/j.1365-2958.2002.02743.x; PMID: 11849553
- Roberts SA, Scott JR. RivR and the small RNA RivX: the missing links between the CovR regulatory cascade and the Mga regulon. Mol Microbiol 2007; 66:1506 - 22; PMID: 18005100
- Lasa I, Toledo-Arana A, Gingeras TR. An effort to make sense of antisense transcription in bacteria. RNA Biol 2012; 9:1039 - 44; http://dx.doi.org/10.4161/rna.21167; PMID: 22858676
- Smaldone GT, Revelles O, Gaballa A, Sauer U, Antelmann H, Helmann JD. A global investigation of the Bacillus subtilis iron-sparing response identifies major changes in metabolism. J Bacteriol 2012; 194:2594 - 605; http://dx.doi.org/10.1128/JB.05990-11; PMID: 22389480
- Smaldone GT, Antelmann H, Gaballa A, Helmann JD. The FsrA sRNA and FbpB protein mediate the iron-dependent induction of the Bacillus subtilis lutABC iron-sulfur-containing oxidases. J Bacteriol 2012; 194:2586 - 93; http://dx.doi.org/10.1128/JB.05567-11; PMID: 22427629
- Preis H, Eckart RA, Gudipati RK, Heidrich N, Brantl S. CodY activates transcription of a small RNA in Bacillus subtilis.. J Bacteriol 2009; 191:5446 - 57; http://dx.doi.org/10.1128/JB.00602-09; PMID: 19542274
- Marchais A, Duperrier S, Durand S, Gautheret D, Stragier P. CsfG, a sporulation-specific, small non-coding RNA highly conserved in endospore formers. RNA Biol 2011; 8:358 - 64; http://dx.doi.org/10.4161/rna.8.3.14998; PMID: 21532344
- Silvaggi JM, Perkins JB, Losick R. Genes for small, noncoding RNAs under sporulation control in Bacillus subtilis.. J Bacteriol 2006; 188:532 - 41; http://dx.doi.org/10.1128/JB.188.2.532-541.2006; PMID: 16385044
- Schmalisch M, Maiques E, Nikolov L, Camp AH, Chevreux B, Muffler A, Rodriguez S, Perkins J, Losick R. Small genes under sporulation control in the Bacillus subtilis genome. J Bacteriol 2010; 192:5402 - 12; http://dx.doi.org/10.1128/JB.00534-10; PMID: 20709900
- Heidrich N, Chinali A, Gerth U, Brantl S. The small untranslated RNA SR1 from the Bacillus subtilis genome is involved in the regulation of arginine catabolism. Mol Microbiol 2006; 62:520 - 36; http://dx.doi.org/10.1111/j.1365-2958.2006.05384.x; PMID: 17020585
- Heidrich N, Moll I, Brantl S. In vitro analysis of the interaction between the small RNA SR1 and its primary target ahrC mRNA. Nucleic Acids Res 2007; 35:4331 - 46; http://dx.doi.org/10.1093/nar/gkm439; PMID: 17576690
- Licht A, Brantl S. Transcriptional repressor CcpN from Bacillus subtilis compensates asymmetric contact distribution by cooperative binding. J Mol Biol 2006; 364:434 - 48; http://dx.doi.org/10.1016/j.jmb.2006.09.021; PMID: 17011578
- Licht A, Golbik R, Brantl S. Identification of ligands affecting the activity of the transcriptional repressor CcpN from Bacillus subtilis.. J Mol Biol 2008; 380:17 - 30; http://dx.doi.org/10.1016/j.jmb.2008.05.002; PMID: 18511073
- Licht A, Brantl S. The transcriptional repressor CcpN from Bacillus subtilis uses different repression mechanisms at different promoters. J Biol Chem 2009; 284:30032 - 8; http://dx.doi.org/10.1074/jbc.M109.033076; PMID: 19726675
- Gimpel M, Preis H, Barth E, Gramzow L, Brantl S. SR1--a small RNA with two remarkably conserved functions. Nucleic Acids Res 2012; 40:11659 - 72; http://dx.doi.org/10.1093/nar/gks895; PMID: 23034808
- Gimpel M, Brantl S. Construction of a modular plasmid family for chromosomal integration in Bacillus subtilis.. J Microbiol Methods 2012; 91:312 - 7; http://dx.doi.org/10.1016/j.mimet.2012.09.003; PMID: 22982324
- Brantl S. Bacterial type I toxin-antitoxin systems. RNA Biol 2012; 9:1488 - 90; http://dx.doi.org/10.4161/rna.23045; PMID: 23324552
- Silvaggi JM, Perkins JB, Losick R. Small untranslated RNA antitoxin in Bacillus subtilis. J Bacteriol 2005; 187:6641 - 50; http://dx.doi.org/10.1128/JB.187.19.6641-6650.2005; PMID: 16166525
- Jahn N, Preis H, Wiedemann C, Brantl S. BsrG/SR4 from Bacillus subtilis--the first temperature-dependent type I toxin-antitoxin system. Mol Microbiol 2012; 83:579 - 98; http://dx.doi.org/10.1111/j.1365-2958.2011.07952.x; PMID: 22229825
- Durand S, Jahn N, Condon C, Brantl S. Type I toxin-antitoxin systems in Bacillus subtilis.. RNA Biol 2012; 9:1491 - 7; http://dx.doi.org/10.4161/rna.22358; PMID: 23059907
- Durand S, Gilet L, Condon C. The essential function of B. subtilis RNase III is to silence foreign toxin genes. PLoS Genet 2012; 8:e1003181; http://dx.doi.org/10.1371/journal.pgen.1003181; PMID: 23300471
- Jahn N, Brantl S. One antitoxin--two functions: SR4 controls toxin mRNA decay and translation. Nucleic Acids Res 2013; 41:9870 - 80; http://dx.doi.org/10.1093/nar/gkt735; PMID: 23969414
- Brantl S. Regulatory mechanisms employed by cis-encoded antisense RNAs. Curr Opin Microbiol 2007; 10:102 - 9; http://dx.doi.org/10.1016/j.mib.2007.03.012; PMID: 17387036
- Eiamphungporn W, Helmann JD. Extracytoplasmic function sigma factors regulate expression of the Bacillus subtilis yabE gene via a cis-acting antisense RNA. J Bacteriol 2009; 191:1101 - 5; http://dx.doi.org/10.1128/JB.01530-08; PMID: 19047346
- Benito Y, Kolb FA, Romby P, Lina G, Etienne J, Vandenesch F. Probing the structure of RNAIII, the Staphylococcus aureus agr regulatory RNA, and identification of the RNA domain involved in repression of protein A expression. RNA 2000; 6:668 - 79; http://dx.doi.org/10.1017/S1355838200992550; PMID: 10836788
- Boisset S, Geissmann T, Huntzinger E, Fechter P, Bendridi N, Possedko M, Chevalier C, Helfer AC, Benito Y, Jacquier A, et al. Staphylococcus aureus RNAIII coordinately represses the synthesis of virulence factors and the transcription regulator Rot by an antisense mechanism. Genes Dev 2007; 21:1353 - 66; http://dx.doi.org/10.1101/gad.423507; PMID: 17545468
- Geisinger E, Adhikari RP, Jin R, Ross HF, Novick RP. Inhibition of rot translation by RNAIII, a key feature of agr function. Mol Microbiol 2006; 61:1038 - 48; http://dx.doi.org/10.1111/j.1365-2958.2006.05292.x; PMID: 16879652
- Chevalier C, Boisset S, Romilly C, Masquida B, Fechter P, Geissmann T, Vandenesch F, Romby P. Staphylococcus aureus RNAIII binds to two distant regions of coa mRNA to arrest translation and promote mRNA degradation. PLoS Pathog 2010; 6:e1000809; http://dx.doi.org/10.1371/journal.ppat.1000809; PMID: 20300607
- Huntzinger E, Boisset S, Saveanu C, Benito Y, Geissmann T, Namane A, Lina G, Etienne J, Ehresmann B, Ehresmann C, et al. Staphylococcus aureus RNAIII and the endoribonuclease III coordinately regulate spa gene expression. EMBO J 2005; 24:824 - 35; http://dx.doi.org/10.1038/sj.emboj.7600572; PMID: 15678100
- Xue T, Zhang X, Sun H, Sun B. ArtR, a novel sRNA of Staphylococcus aureus, regulates α-toxin expression by targeting the 5′ UTR of sarT mRNA. Med Microbiol Immunol 2013; Forthcoming http://dx.doi.org/10.1007/s00430-013-0307-0; PMID: 23955428
- Pichon C, Felden B. Small RNA genes expressed from Staphylococcus aureus genomic and pathogenicity islands with specific expression among pathogenic strains. Proc Natl Acad Sci U S A 2005; 102:14249 - 54; http://dx.doi.org/10.1073/pnas.0503838102; PMID: 16183745
- Sayed N, Nonin-Lecomte S, Réty S, Felden B. Functional and structural insights of a Staphylococcus aureus apoptotic-like membrane peptide from a toxin-antitoxin module. J Biol Chem 2012; 287:43454 - 63; http://dx.doi.org/10.1074/jbc.M112.402693; PMID: 23129767
- Sayed N, Jousselin A, Felden B. A cis-antisense RNA acts in trans in Staphylococcus aureus to control translation of a human cytolytic peptide. Nat Struct Mol Biol 2012; 19:105 - 12; http://dx.doi.org/10.1038/nsmb.2193; PMID: 22198463
- Chabelskaya S, Gaillot O, Felden B. A Staphylococcus aureus small RNA is required for bacterial virulence and regulates the expression of an immune-evasion molecule. PLoS Pathog 2010; 6:e1000927; http://dx.doi.org/10.1371/journal.ppat.1000927; PMID: 20532214
- Bohn C, Rigoulay C, Chabelskaya S, Sharma CM, Marchais A, Skorski P, Borezée-Durant E, Barbet R, Jacquet E, Jacq A, et al. Experimental discovery of small RNAs in Staphylococcus aureus reveals a riboregulator of central metabolism. Nucleic Acids Res 2010; 38:6620 - 36; http://dx.doi.org/10.1093/nar/gkq462; PMID: 20511587
- Lasa I, Toledo-Arana A, Dobin A, Villanueva M, de los Mozos IR, Vergara-Irigaray M, Segura V, Fagegaltier D, Penadés JR, Valle J, et al. Genome-wide antisense transcription drives mRNA processing in bacteria. Proc Natl Acad Sci U S A 2011; 108:20172 - 7; http://dx.doi.org/10.1073/pnas.1113521108; PMID: 22123973
- Morrison JM, Miller EW, Benson MA, Alonzo F 3rd, Yoong P, Torres VJ, Hinrichs SH, Dunman PM. Characterization of SSR42, a novel virulence factor regulatory RNA that contributes to the pathogenesis of a Staphylococcus aureus USA300 representative. J Bacteriol 2012; 194:2924 - 38; http://dx.doi.org/10.1128/JB.06708-11; PMID: 22493015
- Tomasini A, François P, Howden BP, Fechter P, Romby P, Caldelari I. The importance of regulatory RNAs in Staphylococcus aureus.. Infect Genet Evol 2013; Forthcoming http://dx.doi.org/10.1016/j.meegid.2013.11.016; PMID: 24291227
- Janssen PJ, Jones DT, Woods DR. Studies on Clostridium acetobutylicum glnA promoters and antisense RNA. Mol Microbiol 1990; 4:1575 - 83; http://dx.doi.org/10.1111/j.1365-2958.1990.tb02069.x; PMID: 1981087
- Fierro-Monti IP, Reid SJ, Woods DR. Differential expression of a Clostridium acetobutylicum antisense RNA: implications for regulation of glutamine synthetase. J Bacteriol 1992; 174:7642 - 7; PMID: 1360004
- André G, Even S, Putzer H, Burguière P, Croux C, Danchin A, Martin-Verstraete I, Soutourina O. S-box and T-box riboswitches and antisense RNA control a sulfur metabolic operon of Clostridium acetobutylicum.. Nucleic Acids Res 2008; 36:5955 - 69; http://dx.doi.org/10.1093/nar/gkn601; PMID: 18812398
- Okumura K, Ohtani K, Hayashi H, Shimizu T. Characterization of genes regulated directly by the VirR/VirS system in Clostridium perfringens.. J Bacteriol 2008; 190:7719 - 27; http://dx.doi.org/10.1128/JB.01573-07; PMID: 18790863
- Obana N, Nakamura K. A novel toxin regulator, the CPE1446-CPE1447 protein heteromeric complex, controls toxin genes in Clostridium perfringens.. J Bacteriol 2011; 193:4417 - 24; http://dx.doi.org/10.1128/JB.00262-11; PMID: 21725013
- Ohtani K, Bhowmik SK, Hayashi H, Shimizu T. Identification of a novel locus that regulates expression of toxin genes in Clostridium perfringens.. FEMS Microbiol Lett 2002; 209:113 - 8; http://dx.doi.org/10.1111/j.1574-6968.2002.tb11118.x; PMID: 12007663
- Ohtani K, Hirakawa H, Paredes-Sabja D, Tashiro K, Kuhara S, Sarker MR, Shimizu T. Unique regulatory mechanism of sporulation and enterotoxin production in Clostridium perfringens. J Bacteriol 2013; 195:2931 - 6; http://dx.doi.org/10.1128/JB.02152-12; PMID: 23585540
- Chen Y, Indurthi DC, Jones SW, Papoutsakis ET. Small RNAs in the genus Clostridium. MBio 2011; 2:e00340 - 10; http://dx.doi.org/10.1128/mBio.00340-10; PMID: 21264064
- Christiansen JK, Nielsen JS, Ebersbach T, Valentin-Hansen P, Søgaard-Andersen L, Kallipolitis BH. Identification of small Hfq-binding RNAs in Listeria monocytogenes.. RNA 2006; 12:1383 - 96; http://dx.doi.org/10.1261/rna.49706; PMID: 16682563
- Nielsen JS, Lei LK, Ebersbach T, Olsen AS, Klitgaard JK, Valentin-Hansen P, Kallipolitis BH. Defining a role for Hfq in Gram-positive bacteria: evidence for Hfq-dependent antisense regulation in Listeria monocytogenes.. Nucleic Acids Res 2010; 38:907 - 19; http://dx.doi.org/10.1093/nar/gkp1081; PMID: 19942685
- Nielsen JS, Larsen MH, Lillebæk EM, Bergholz TM, Christiansen MH, Boor KJ, Wiedmann M, Kallipolitis BH. A small RNA controls expression of the chitinase ChiA in Listeria monocytogenes.. PLoS One 2011; 6:e19019; http://dx.doi.org/10.1371/journal.pone.0019019; PMID: 21533114
- Toledo-Arana A, Dussurget O, Nikitas G, Sesto N, Guet-Revillet H, Balestrino D, Loh E, Gripenland J, Tiensuu T, Vaitkevicius K, et al. The Listeria transcriptional landscape from saprophytism to virulence. Nature 2009; 459:950 - 6; http://dx.doi.org/10.1038/nature08080; PMID: 19448609
- Wurtzel O, Sesto N, Mellin JR, Karunker I, Edelheit S, Bécavin C, Archambaud C, Cossart P, Sorek R. Comparative transcriptomics of pathogenic and non-pathogenic Listeria species. Mol Syst Biol 2012; 8:583; http://dx.doi.org/10.1038/msb.2012.11; PMID: 22617957
- Sesto N, Wurtzel O, Archambaud C, Sorek R, Cossart P. The excludon: a new concept in bacterial antisense RNA-mediated gene regulation. Nat Rev Microbiol 2013; 11:75 - 82; http://dx.doi.org/10.1038/nrmicro2934; PMID: 23268228
- Loh E, Dussurget O, Gripenland J, Vaitkevicius K, Tiensuu T, Mandin P, Repoila F, Buchrieser C, Cossart P, Johansson J. A trans-acting riboswitch controls expression of the virulence regulator PrfA in Listeria monocytogenes.. Cell 2009; 139:770 - 9; http://dx.doi.org/10.1016/j.cell.2009.08.046; PMID: 19914169
- Ramirez-Peña E, Treviño J, Liu Z, Perez N, Sumby P. The group A Streptococcus small regulatory RNA FasX enhances streptokinase activity by increasing the stability of the ska mRNA transcript. Mol Microbiol 2010; 78:1332 - 47; http://dx.doi.org/10.1111/j.1365-2958.2010.07427.x; PMID: 21143309
- Steiner K, Malke H. Dual control of streptokinase and streptolysin S production by the covRS and fasCAX two-component regulators in Streptococcus dysgalactiae subsp. equisimilis. Infect Immun 2002; 70:3627 - 36; http://dx.doi.org/10.1128/IAI.70.7.3627-3636.2002; PMID: 12065504
- Le Rhun A, Charpentier E. Small RNAs in streptococci. RNA Biol 2012; 9:414 - 26; http://dx.doi.org/10.4161/rna.20104; PMID: 22546939
- Acebo P, Martin-Galiano AJ, Navarro S, Zaballos A, Amblar M. Identification of 88 regulatory small RNAs in the TIGR4 strain of the human pathogen Streptococcus pneumoniae.. RNA 2012; 18:530 - 46; http://dx.doi.org/10.1261/rna.027359.111; PMID: 22274957
- Håvarstein LS, Gaustad P, Nes IF, Morrison DA. Identification of the streptococcal competence-pheromone receptor. Mol Microbiol 1996; 21:863 - 9; http://dx.doi.org/10.1046/j.1365-2958.1996.521416.x; PMID: 8878047
- Mann B, van Opijnen T, Wang J, Obert C, Wang YD, Carter R, McGoldrick DJ, Ridout G, Camilli A, Tuomanen EI, et al. Control of virulence by small RNAs in Streptococcus pneumoniae.. PLoS Pathog 2012; 8:e1002788; http://dx.doi.org/10.1371/journal.ppat.1002788; PMID: 22807675
- Halfmann A, Kovács M, Hakenbeck R, Brückner R. Identification of the genes directly controlled by the response regulator CiaR in Streptococcus pneumoniae: five out of 15 promoters drive expression of small non-coding RNAs. Mol Microbiol 2007; 66:110 - 26; http://dx.doi.org/10.1111/j.1365-2958.2007.05900.x; PMID: 17725562
- Tsui H-CT, Mukherjee D, Ray VA, Sham LT, Feig AL, Winkler ME. Identification and characterization of noncoding small RNAs in Streptococcus pneumoniae serotype 2 strain D39. J Bacteriol 2010; 192:264 - 79; http://dx.doi.org/10.1128/JB.01204-09; PMID: 19854910
- Marx P, Nuhn M, Kovács M, Hakenbeck R, Brückner R. Identification of genes for small non-coding RNAs that belong to the regulon of the two-component regulatory system CiaRH in Streptococcus.. BMC Genomics 2010; 11:661; http://dx.doi.org/10.1186/1471-2164-11-661; PMID: 21106082
- Perez N, Treviño J, Liu Z, Ho SC, Babitzke P, Sumby P. A genome-wide analysis of small regulatory RNAs in the human pathogen group A Streptococcus.. PLoS One 2009; 4:e7668; http://dx.doi.org/10.1371/journal.pone.0007668; PMID: 19888332
- Schnorpfeil A, Kranz M, Kovács M, Kirsch C, Gartmann J, Brunner I, Bittmann S, Brückner R. Target evaluation of the non-coding csRNAs reveals a link of the two-component regulatory system CiaRH to competence control in Streptococcus pneumoniae R6. Mol Microbiol 2013; 89:334 - 49; http://dx.doi.org/10.1111/mmi.12277; PMID: 23710838
- Håvarstein LS, Coomaraswamy G, Morrison DA. An unmodified heptadecapeptide pheromone induces competence for genetic transformation in Streptococcus pneumoniae.. Proc Natl Acad Sci U S A 1995; 92:11140 - 4; http://dx.doi.org/10.1073/pnas.92.24.11140; PMID: 7479953
- Guenzi E, Gasc AM, Sicard MA, Hakenbeck R. A two-component signal-transducing system is involved in competence and penicillin susceptibility in laboratory mutants of Streptococcus pneumoniae.. Mol Microbiol 1994; 12:505 - 15; http://dx.doi.org/10.1111/j.1365-2958.1994.tb01038.x; PMID: 8065267
- Müller M, Marx P, Hakenbeck R, Brückner R. Effect of new alleles of the histidine kinase gene ciaH on the activity of the response regulator CiaR in Streptococcus pneumoniae R6. Microbiology 2011; 157:3104 - 12; http://dx.doi.org/10.1099/mic.0.053157-0; PMID: 21903754
- Cassone M, Gagne AL, Spruce LA, Seeholzer SH, Sebert ME. The HtrA protease from Streptococcus pneumoniae digests both denatured proteins and the competence-stimulating peptide. J Biol Chem 2012; 287:38449 - 59; http://dx.doi.org/10.1074/jbc.M112.391482; PMID: 23012372
- Vogel J, Luisi BF. Hfq and its constellation of RNA. Nat Rev Microbiol 2011; 9:578 - 89; http://dx.doi.org/10.1038/nrmicro2615; PMID: 21760622
- Fender A, Elf J, Hampel K, Zimmermann B, Wagner EGH. RNAs actively cycle on the Sm-like protein Hfq. Genes Dev 2010; 24:2621 - 6; http://dx.doi.org/10.1101/gad.591310; PMID: 21123649
- Bohn C, Rigoulay C, Bouloc P. No detectable effect of RNA-binding protein Hfq absence in Staphylococcus aureus.. BMC Microbiol 2007; 7:10; http://dx.doi.org/10.1186/1471-2180-7-10; PMID: 17291347
- Liu Y, Wu N, Dong J, Gao Y, Zhang X, Mu C, Shao N, Yang G. Hfq is a global regulator that controls the pathogenicity of Staphylococcus aureus.. PLoS One 2010; 5:e13069; http://dx.doi.org/10.1371/journal.pone.0013069; PMID: 20927372
- Rochat T, Bouloc P, Yang Q, Bossi L, Figueroa-Bossi N. Lack of interchangeability of Hfq-like proteins. Biochimie 2012; 94:1554 - 9; http://dx.doi.org/10.1016/j.biochi.2012.01.016; PMID: 22326874
- Pandey SP, Minesinger BK, Kumar J, Walker GC. A highly conserved protein of unknown function in Sinorhizobium meliloti affects sRNA regulation similar to Hfq. Nucleic Acids Res 2011; 39:4691 - 708; http://dx.doi.org/10.1093/nar/gkr060; PMID: 21325267
- Grewal SI. RNAi-dependent formation of heterochromatin and its diverse functions. Curr Opin Genet Dev 2010; 20:134 - 41; http://dx.doi.org/10.1016/j.gde.2010.02.003; PMID: 20207534
- Lee JM, Zhang S, Saha S, Santa Anna S, Jiang C, Perkins J. RNA expression analysis using an antisense Bacillus subtilis genome array. J Bacteriol 2001; 183:7371 - 80; http://dx.doi.org/10.1128/JB.183.24.7371-7380.2001; PMID: 11717296
- Saito S, Kakeshita H, Nakamura K. Novel small RNA-encoding genes in the intergenic regions of Bacillus subtilis.. Gene 2009; 428:2 - 8; http://dx.doi.org/10.1016/j.gene.2008.09.024; PMID: 18948176
- Nicolas P, Mäder U, Dervyn E, Rochat T, Leduc A, Pigeonneau N, Bidnenko E, Marchadier E, Hoebeke M, Aymerich S, et al. Condition-dependent transcriptome reveals high-level regulatory architecture in Bacillus subtilis. Science 2012; 335:1103 - 6; http://dx.doi.org/10.1126/science.1206848; PMID: 22383849
- Marchais A, Naville M, Bohn C, Bouloc P, Gautheret D. Single-pass classification of all noncoding sequences in a bacterial genome using phylogenetic profiles. Genome Res 2009; 19:1084 - 92; http://dx.doi.org/10.1101/gr.089714.108; PMID: 19237465
- Beaume M, Hernandez D, Farinelli L, Deluen C, Linder P, Gaspin C, Romby P, Schrenzel J, François P. Cartography of methicillin-resistant S. aureus transcripts: detection, orientation and temporal expression during growth phase and stress conditions. PLoS One 2010; 5:e10725; http://dx.doi.org/10.1371/journal.pone.0010725; PMID: 20505759
- Abu-Qatouseh LF, Chinni SV, Seggewiss J, Proctor RA, Brosius J, Rozhdestvensky TS, Peters G, von Eiff C, Becker K. Identification of differentially expressed small non-protein-coding RNAs in Staphylococcus aureus displaying both the normal and the small-colony variant phenotype. J Mol Med (Berl) 2010; 88:565 - 75; http://dx.doi.org/10.1007/s00109-010-0597-2; PMID: 20151104
- Howden BP, Beaume M, Harrison PF, Hernandez D, Schrenzel J, Seemann T, Francois P, Stinear TP. Analysis of the small RNA transcriptional response in multidrug-resistant Staphylococcus aureus after antimicrobial exposure. Antimicrob Agents Chemother 2013; 57:3864 - 74; http://dx.doi.org/10.1128/AAC.00263-13; PMID: 23733475
- Mandin P, Repoila F, Vergassola M, Geissmann T, Cossart P. Identification of new noncoding RNAs in Listeria monocytogenes and prediction of mRNA targets. Nucleic Acids Res 2007; 35:962 - 74; http://dx.doi.org/10.1093/nar/gkl1096; PMID: 17259222
- Oliver HF, Orsi RH, Ponnala L, Keich U, Wang W, Sun Q, Cartinhour SW, Filiatrault MJ, Wiedmann M, Boor KJ. Deep RNA sequencing of L. monocytogenes reveals overlapping and extensive stationary phase and sigma B-dependent transcriptomes, including multiple highly transcribed noncoding RNAs. BMC Genomics 2009; 10:641; http://dx.doi.org/10.1186/1471-2164-10-641; PMID: 20042087
- Mraheil MA, Billion A, Mohamed W, Mukherjee K, Kuenne C, Pischimarov J, Krawitz C, Retey J, Hartsch T, Chakraborty T, et al. The intracellular sRNA transcriptome of Listeria monocytogenes during growth in macrophages. Nucleic Acids Res 2011; 39:4235 - 48; http://dx.doi.org/10.1093/nar/gkr033; PMID: 21278422
- Mellin JR, Cossart P. The non-coding RNA world of the bacterial pathogen Listeria monocytogenes.. RNA Biol 2012; 9:372 - 8; http://dx.doi.org/10.4161/rna.19235; PMID: 22336762
- Patenge N, Billion A, Raasch P, Normann J, Wisniewska-Kucper A, Retey J, Boisguérin V, Hartsch T, Hain T, Kreikemeyer B. Identification of novel growth phase- and media-dependent small non-coding RNAs in Streptococcus pyogenes M49 using intergenic tiling arrays. BMC Genomics 2012; 13:550; http://dx.doi.org/10.1186/1471-2164-13-550; PMID: 23062031
- Tesorero RA, Yu N, Wright JO, Svencionis JP, Cheng Q, Kim JH, Cho KH. Novel regulatory small RNAs in Streptococcus pyogenes.. PLoS One 2013; 8:e64021; http://dx.doi.org/10.1371/journal.pone.0064021; PMID: 23762235
- Kumar R, Shah P, Swiatlo E, Burgess SC, Lawrence ML, Nanduri B. Identification of novel non-coding small RNAs from Streptococcus pneumoniae TIGR4 using high-resolution genome tiling arrays. BMC Genomics 2010; 11:350; http://dx.doi.org/10.1186/1471-2164-11-350; PMID: 20525227
