Abstract
Alternative splicing (AS) allows the production of multiple mRNA variants from a single gene, which contributes to increase the complexity of the proteome. There is evidence that AS is regulated not only by auxiliary splicing factors, but also by components of the core spliceosomal machinery, as well as through epigenetic modifications. However, to what extent these different mechanisms contribute to the regulation of AS in response to endogenous or environmental stimuli is still unclear. Circadian clocks allow organisms to adjust physiological processes to daily changes in environmental conditions. Here we review recent evidence linking circadian clock and AS, and discuss the role of Protein Arginine Methyltransferase 5 (PRMT5) in these processes. We propose that the interactions between daily oscillations in AS and circadian rhythms in the expression of splicing factors and epigenetic regulators offer a great opportunity to dissect the contribution of these mechanisms to the regulation of AS in a physiologically relevant context.
Introduction
The molecular architecture of circadian clocks is similar across eukaryotic organisms. At their core, biological clocks contain a cell autonomous oscillator that is generated, at least in part, by transcription-translation negative-feedback loops with a crucial delay between stimulus and response.Citation1 Transcription is considered the prime mechanism driving daily rhythms in gene expression; in addition, post-translational modifications of clock components modulate their sub-cellular localization, interactions and activities.Citation2 Between these two processes, post-transcriptional regulation is also emerging as another important layer of control.Citation3
Downstream of transcription, messenger RNA suffers many processing and regulatory steps that influence gene expression and allow rapid changes in protein levels and activities.Citation4 Precursor messenger RNA (pre-mRNA) splicing, the excision of intron sequences from mRNAs mediated by the spliceosome, is one of these processes. In addition, AS is a way to generate multiple mRNA products from a single gene, enhancing proteome diversity.Citation5,Citation6 Recent studies indicate that up to 90% of human genes have at least two alternative spliced isoforms. In plants, this percentage seems to be lower, about 40% for Arabidopsis thaliana,Citation7 but still higher than previously anticipated.Citation8 Understanding the biological relevance of alternatively spliced isoforms, as well as deciphering the molecular mechanism regulating this process, is one of the great challenges of this field.
Alternative splicing requires accurate regulation in order to guarantee plasticity, while still displaying high specificity and fidelity.Citation9,Citation10 Traditional studies have focused mainly on the combinatorial effects of auxiliary splicing factors, such as serine/arginine (SR)-rich proteins and heterogeneous nuclear ribonucleoproteins (hnRNP), on the ability of the spliceosome to recognize particular splice sites. However, there is increasing evidence that additional mechanisms help generate the great variety of AS events seen in higher eukaryotes. For example, changes in the activity or concentration of core splicing factors, or regulators of spliceosome assembly, can at least in some cases alter the kinetics of one or more steps in splicing reactions, resulting in changes in splice-site selection and AS for a subset of genes.Citation11,Citation12 In addition, there is evidence that splicing reactions occur mostly co-transcriptionally and, as a corollary of this, the regulation of transcription by RNA polymerase II (pol II), either by factors that interact and regulate its activity or by the nature of the template, would impact on the decisions taken during the process.Citation13,Citation14 Chromatin remodeling complexes and histones post-translational modifications constitute two different ways to produce chromatin changes that regulate transcription.Citation15,Citation16 Responses of AS to several chromatin modifications, which appear to be mediated in some cases through effects on the elongation rate of RNA pol II, and in others, through recruitment of specific splicing factors, have been reported in reference Citation17. It is uncertain, however, to what extent each of the above mechanisms contributes to the generation and/or the modulation of the great extent of AS that is being revealed in plants and animals. Since circadian oscillations depend on dynamic interactions between transcriptional and post-transcriptional regulatory mechanisms, and AS regulation is likely to be influenced by changes in the concentration and/or activity of splicing regulators, core-spliceosome components, and/or proteins involved in the epigenetic regulation of gene expression; the interplay between these processes offers a great opportunity to dissect the contribution of different mechanisms to the regulation of AS.
Alternative Splicing of Circadian Network Components
A role for AS in the regulation of circadian rhythms was first suggested by the identification of alternative spliced isoforms of the clock gene period (per) in Drosophila, a key component of the transcriptional-feedback loop operating at the core of the circadian clock in this organism. Two per mRNA isoforms differ in the removal or retention of intron 8 (dmpi8) in the 3′UTR. This AS event has no effect on the coding sequence, but appears to modulate PER protein accumulation.Citation18 Interestingly, AS of dmpi8 is regulated by the circadian clock, temperature and photoperiod, and its regulation contributes to seasonal adjustments in locomotor behavior.Citation18–Citation20 Although the molecular mechanism through which the clock controls per splicing is unknown, suboptimal splice sites appear to mediate the thermal sensitivity of this AS event ().Citation21 Interestingly, temperature also regulates AS of core-clock genes in other species. In Neurospora crassa, the core clock gene frequency can give rise to either a long (l-FRQ) or a short (s-FRQ) isoform, whose ratio is regulated through thermosensitive AS of an intron containing the initiation codon for l-FRQ. Temperature regulation of the s-FRQ/l-FRQ ratio appears to be important for fine-tuning of the circadian period, allowing robust circadian oscillations in this species under a wide range of temperatures ().Citation22 Furthermore, evolutionary comparisons within the Sordariaceae family of perithecial fungi (which includes N. crassa) reveal significant conservation of this AS event, supporting its relevance for the proper regulation of clock function.Citation22
In Arabidopsis, the central oscillatory mechanism involves the Myb transcription factors CCA1 and LHY, and the PSEUDO RESPONSE REGULATOR 1 (PRR1), also known as TIMING OF CAB EXPRESSION 1 (TOC1), and its homo-logs PRR7 and PRR9.Citation23,Citation24 A recent study, in which the Arabidopsis transcriptome was characterized through high throughput sequencing, has revealed the existence of a temperature-sensitive AS event involving intron 4 retention in CCA1 mRNA ().Citation7 Interestingly, this AS event is present not only in Arabidopsis thaliana, but also in Oryza sativa, Brachypodium distachyon and Populus trichocarpa, mono- and dicotyledonous species that diverged from a common ancestor 120–170 million years ago.Citation25,Citation26 It is also interesting to note that the regulation of this splicing event is conserved despite the low sequence similarity in the region involved.
Regulation of AS of circadian components in Arabidopsis is not restricted to core-clock genes. Glycine Rich Binding Protein 7 (GRP7) encodes an hnRNP-like protein whose expression is regulated by the circadian clock and temperature in A. thaliana.Citation27 This clock-regulated RNA-binding protein constitutes a clock output gene that influences circadian oscillations of its own transcripts and of its homolog AtGRP8 by negative feedback at the post-transcriptional level.Citation28 In addition to this particular example, a recent analysis of the Arabidopsis transcriptome using whole genome tiling arrays revealed the existence of circadian oscillation in the level of several hundred introns, many of which are associated with genes whose expression is not clock-regulated.Citation29 Thus, these most likely constitute clock-regulated intron retention events that might contribute to the mediation of clock regulation of protein levels.
All the above evidences indicate that regulation of AS may represent a common way of fine-tuning biological rhythms to changes in environmental conditions across eukaryotes. Even more, according to this line of reasoning, AS regulation would be another tool to modulate circadian clocks (). Although the molecular mechanisms linking these two cellular processes are still poorly understood, we have recently reported a role for Protein Arginine Methyl Transferase 5 (PRMT5) in the regulation of circadian rhythms and AS in A. thaliana, as well as in D. melanogaster. In the following section, we will discuss the evidence pointing towards PRMT5 as a molecule responsible, at least in part, for connecting those processes.Citation30
PRMT5, an Important Piece of the Elusive Circadian Clock and Alternative Splicing Connection
PRMT5 is part of a small family of conserved enzymes (methyl arginine transferases, PRMTs) able to transfer methyl groups to different kinds of proteins. These methyl transferases are classified according to the characteristic location where they attach methyl groups to the arginine residue; type I PRMTs transfer two methyl groups to the same nitrogen atom, resulting in asymetrically dimethylated arginines, while type II originates symmetrically dimethylated arginines by adding two methyl groups, one on each opposite terminal nitrogen atom.Citation31 In mammals, PRMT5 is known to be the only bona fide type II PRMT.Citation31 In Arabidopsis thaliana, several PRMT homologs have been identified and their biochemical function characterized. These include type I PRMTs, such as PRMT1a and PRMT1b (both homologs of human PRMT1),Citation32 PRMT4a and PRMT4b (both homologs of mammalian CARM1/PRMT4),Citation33 PRMT10,Citation34 PRMT11,Citation35 and a type II PRMT, PRMT5.Citation36,Citation37
Although the biochemical and molecular activities of many of these proteins have been known for several years, their biological functions have just started to be elucidated through the characterization of mutants in model organisms. Indeed, a forward genetic approach allowed us to determine that PRMT5 is involved in the proper functioning of the circadian clock in Arabidopsis,Citation30 and a similar finding was obtained by Hong et al. through an independent genetic screen.Citation38 Both studies have shown that prmt5 mutants display a long period phenotype for multiple circadian rhythms, and have also revealed that PRMT5 expression follows itself a circadian pattern. PRMT5 is known to act as an epigenetic regulator of gene expression and, interestingly, PRR9 is the gene showing the greatest enhancement in expression of prmt5 mutants compared to wild type plants, according to micro-array data. The PRR9 gene is involved in the morning loop of the core oscillator, but its overexpression has been shown to produce a short period phenotype, instead of the long period characteristic of prmt5 mutants.Citation39 This apparent contradiction, together with the fact that PRMT5 methylates spliceosomal proteins in addition to histones, led us to speculate that PRMT5 might control the clock through the regulation of AS. Indeed, intron retention and alterntative donor site (5′ss) events give rise to different PRR9 mRNA isoforms that present premature stop codons and prmt5 mutants have higher proportion of these non-productive isoforms than wild type plants, and actually reduced levels of the functional isoform. Furthermore, genetic evidence supports the role of PRR9, as well as its homolog PRR7, as the genes responsible for the circadian alteration observed in prmt5 mutants. Taken together, the above results indicate that PRMT5 affects circadian rhythms in Arabidopsis, at least in part, through its effect on AS of the core-clock gene PRR9.
In addition to the effects on PRR9, whole genome tiling arrays have revealed that prmt5 mutants present alterations in a few hundred splicing events, out of approximately sixty thousand evaluated. Moreover, a high resolution RT-PCR panel of well characterized AS events has shown a high percentage of AS alterations that indicate defective selection of weak 5′splice sites in prmt5 mutant plants.Citation30 Interestingly, detailed analysis of some of these events has revealed a circadian pattern of AS mediated by PRMT5, indicating that PRMT5 contributes to linking these two processes.Citation30
A role for PRMT5 in regulating a subset of pre-mRNA splicing events is also supported by recent results from two independent research groups,Citation40,Citation41 which used ultra high-throughput RNA sequencing (RNAseq) to compare the transcriptome of wild type and prmt5 mutant plants in Arabidopsis. These studies have revealed roles for PRMT5 in modulating splicing of genes involved in flowering time regulation and abiotic stress responses, two physiological processes also altered in prmt5 mutants.
Finally, similarly to the effects observed in A. thaliana, mutant flies for the prmt5 gene show wide-spread defects in pre-mRNA splicing, which are particularly enriched in intron retention events associated with weak 5′splice sites. Interestingly, these flies are arrhythmic at the behavioral level, and this phenotype is associated with alterations in AS of the core-clock gene per and several clock-output genes. However, strong rhythmic expression of core-clock genes is still observed in prmt5 mutant flies, suggesting that the arrhythmic phenotype is caused by alterations in the pathway connecting the clock to the regulatory mechanisms controlling locomotor behavior. Taken together, these results point to a conserved role for PRMT5 in the regulation of AS, but indicate a different connection between this methyl transferase and the central oscillator in flies compared to plants, suggesting a more elusive bond in the former.Citation30
A priori, four different mechanisms could explain the involvement of PRMT5 in AS. First, histone methylation mediated by PRMT5 could impact on chromatin structure through ATP-dependent remodelingCitation42 and it would be completely reasonable to expect this modification to have a role in pre-mRNA processing.Citation17 Second, PRMT5, a known transcriptional regulator,Citation36,Citation37,Citation43 could modify the availability of different splicing factors and indirectly regulate this process. Third, PRMT5 methylation of auxiliary splicing factors could act as an additional step of AS regulation. Fourth, Sm proteins, which are core components of spliceosomal snRNPs,Citation44 are also proven targets of this methyltransferase.Citation40,Citation41 Although, in principle, alterations in the activity of a core spliceosomal component would be expected to affect splicing at a global level, there is accumulated evidence that in some cases certain splicing events are preferentially affected.Citation11,Citation45 For instance, knock-down of the general splicing factor SmB/B' affects the splicing of less than 2% of constitutive exons, and the splicing of 18% of the alternative exons evaluated in human cells.Citation11 These findings are remarkably similar to those observed in Arabidopsis prmt5 mutants, where the lack of methylation of Sm proteins is associated with defects in splicing of less than 1% of the total number of introns evaluated, but results in changes in 15% of the AS events analyzed with a high resolution RT-PCR panel.Citation30 Previous studies in yeast have shown that the C-terminal region of Sm proteins, which is methylated by PRMT5 in higher eukaryotes, stabilizes interactions between U1snRNA and the 5′SS of pre-mRNA.Citation46 Thus, we propose that PRMT5 effects on AS result, to a great extent, from failure to stabilize weak interactions between the U1 snRNA and 5′SS that deviate from the consensus sequence. Notably, this effect could be comparable to the role of temperature in regulating the AS of D. melanogaster per gene (dmpi8), which affects recognition of suboptimal 5′SS and 3′SS.Citation21 In principle, all the above mechanisms are not mutually exclusive and, therefore, it will be important to evaluate the contribution of each of them independently.
Concluding Remarks and Perspectives
A growing body of evidence is revealing important links between circadian and AS regulatory networks. Altogether, our results plus previous evidence demonstrate that PRMT5 is a key modulator of AS and that this characteristic, coupled with circadian regulation of PRMT5 expression, contributes to connecting these important processes. In the near future, genetic approaches should be used to test the role of additional splicing regulators in adjusting the pace of the clock, focusing on those whose levels are under circadian control. At the same time, high-throughput sequencing technologies should allow us to expand the knowledge of AS events and the corresponding biological processes that are regulated by the clock through this mechanism. Correlation analysis of clock-regulated AS events and daily changes in the expression of auxiliary splicing factors and/or core spliceosome components should help identify both cis and trans regulatory elements underlying AS oscillations. This information should also contribute to the development of models capable of predicting changes in AS in response to additional environmental or endogenous stimuli. Finally, there is ample evidence that epigenetic mechanisms contribute to the modulation of circadian properties. It is tempting to speculate that some of the proteins that affect chromatin structure modulate the circadian tick-tock through effects on AS.
Figures and Tables
Figure 1 Alternative splicing, a new layer of control of circadian clock gene expression in different eukaryotes. The figure shows that alternative splicing regulates the expression of different components of the central oscillator in Drosophila, Neurospora and Arabidopsis. Exons are shown as boxes, introns as lines, 5′ss are 5′ splicing sites or donors, and arrowheads at the end of exons represent that the gene continues. Black arrows indicate activation and black lines with a bar at the end indicate inhibition. Left upper part, Drosophila melanogaster circadian oscillator's scheme. The cartoon shows the central genes involved in the circadian clock central loops in this organism. Per, period; Tim, timeless; dClk, Drosophila Clock; Cyc, cycle. Left bottom part, per alternative splicing isoforms. Middle upper part, Neurospora crassa circadian oscillator's scheme. l-Frq and s-Frq, long and short isoforms of Frequency respectively, which arise from the two different translation initiation sites (flag AUG); WC, white collar. PU and PD are alternative promoters of frequency. Middle bottom part, frq alternative splicing isoforms depending on the promoter used. Right upper part, Arabidopsis thaliana circadian oscillator's scheme. The cartoon shows the central genes involved in the circadian clock in this organism. TOC1, TIMING OF CAB EXPRESSION 1; LHY, LATE ELONGATED HYPOCOTYL; CCA1, CIRCADIAN CLOCK ASSOCIATED 1, PRR7 and PRR9, PSEUDO RESPONSE REGULATOR 7 and 9, respectively. Right bottom part, PRR9 and CCA1 alternative splicing isoforms.
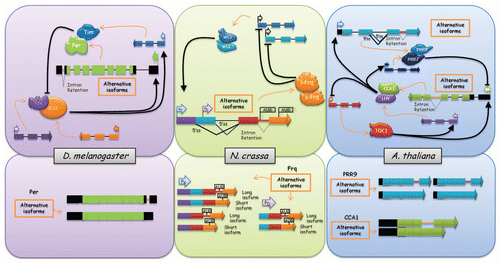
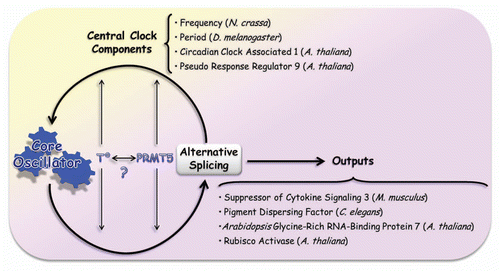
Figure 2 Alternative splicing and its relationship with circadian clocks. The curved arrows indicate that the central core of the circadian clock is known to modulate alternative splicing, allowing a certain isoform to peak in a particular momentCitation29,Citation30 and that, in turn, alternative splicing plays a regulatory role over the central oscillator by modulating pre-mRNA splicing of essential pieces of this mechanism. As explained in the text, these interactions are regulated by temperature and PRMT5 protein (straight and thin arrows). It is still unknown whether there is a connection between those factors. There are several examples of alternative spliced genes which are involved in the modulation of circadian outputs. Among them, we mentioned the case of Suppressor of Cytokine Signaling 3 (SOCS3) in M. musculus,Citation47 Pigment Dispersing Factor (PDF) in C. elegans,Citation48 and Glycine-Rich RNA-Binding Protein 7,Citation49 and Rubisco ActivaseCitation30,Citation50 in A. thaliana. Gene targets of alternative splicing and components of the central core are cited: Frequency (N. crassa),Citation22 Period (D. melanogaster),Citation19,Citation20 Circadian Clock Associated 1,Citation7 and Pseudo Response Regulator 9 Citation30 (A. thaliana).
Acknowledgments
This work has been supported by grants from the Howard Hughes Medical Institute (International Scholar Award) and from Agencia Nacional de Promoción Científica y Tecnológica (ANPCyT) to M.Y. and A.R.K., and grants from Fundación Antorchas, CONICET and Universidad de Buenos Aires to M.Y.
References
- Gallego M, Virshup D. Post-translational modifications regulate the ticking of the circadian clock. Nature Rev Mol Cell Biol 2007; 8:139 - 148
- Mehra A, Baker CL, Loros JJ, Dunlap JC. Post-translational modifications in circadian rhythms. Trends in Biochemical Sciences 2009; 34:483 - 490
- Staiger D, Koster T. Spotlight on post-transcriptional control in the circadian system. Cell Mol Life Sci 2011; 68:71 - 83
- Kojima S, Shingle DL, Green CB. Post-transcriptional control of circadian rhythms. J Cell Sci 2011; 124:311 - 320
- Kim E, Magen A, Ast G. Different levels of alternative splicing among eukaryotes. Nucleic Acids Res 2007; 35:125 - 131
- Graveley BR. Alternative splicing: increasing diversity in the proteomic world. Trends Genet 2001; 17:100 - 107
- Filichkin SA, Priest HD, Givan SA, Shen R, Bryant DW, Fox SE, et al. Genome-wide mapping of alternative splicing in Arabidopsis thaliana. Genome Research 2010; 20:45 - 58
- Campbell MA, Haas BJ, Hamilton JP, Mount SM, Buell CR. Comprehensive analysis of alternative splicing in rice and comparative analyses with Arabidopsis. BMC Genomics 2006; 7:327
- de la Mata M, Lafaille C, Kornblihtt AR. First come, first served revisited: factors affecting the same alternative splicing event have different effects on the relative rates of intron removal. RNA 2010; 16:904 - 912
- Kornblihtt AR, de la Mata M, Fededa JP, Munoz MJ, Nogues G. Multiple links between transcription and splicing. RNA 2004; 10:1489 - 1498
- Saltzman AL, Pan Q, Blencowe BJ. Regulation of alternative splicing by the core spliceosomal machinery. Genes Dev 2011; 25:373 - 384
- Chen M, Manley JL. Mechanisms of alternative splicing regulation: insights from molecular and genomics approaches. Nat Rev Mol Cell Biol 2009; 10:741 - 754
- Alexander R, Beggs JD. Cross-talk in transcription, splicing and chromatin: who makes the first call?. Biochem Soc Trans 2010; 38:1251 - 1256
- Narlikar GJ, Fan HY, Kingston RE. Cooperation between complexes that regulate chromatin structure and transcription. Cell 2002; 108:475 - 487
- Kouzarides T. Chromatin Modifications and Their Function. Cell 2007; 128:693 - 705
- Berger SL. The complex language of chromatin regulation during transcription. Nature 2007; 447:407 - 412
- Luco RF, Allo M, Schor IE, Kornblihtt AR, Misteli T. Epigenetics in Alternative Pre-mRNA Splicing. Cell 2011; 144:16 - 26
- Majercak J, Sidote D, Hardin PE, Edery I. How a Circadian Clock Adapts to Seasonal Decreases in Temperature and Day Length. Neuron 1999; 24:219 - 230
- Majercak J, Chen W, Edery I. Splicing of the period gene 3′-terminal intron is regulated by light, circadian clock factors and Phospholipase C. Mol Cell Biol 2004; 24:3359 - 3372
- Collins BH, Rosato E, Kyriacou CP. Seasonal behavior in Drosophila melanogaster requires the photoreceptors, the circadian clock and phospholipase C. Proc Natl Acad Sci USA 2004; 101:1945 - 1950
- Low KH, Lim C, Ko HW, Edery I. Natural Variation in the Splice Site Strength of a Clock Gene and Species-Specific Thermal Adaptation. Neuron 2008; 60:1054 - 1067
- Colot HV, Loros JJ, Dunlap JC. Temperature-modulated Alternative Splicing and Promoter Use in the Circadian Clock Gene frequency. Mol Biol Cell 2005; 16:5563 - 5571
- Alabadí D, Oyama T, Yanovsky MJ, Harmon FG, Mas P, Kay SA. Reciprocal regulation between TOC1 and LHY/CCA1 within the Arabidopsis circadian clock. Science 2001; 293:880 - 883
- Farré EM, Kay SA. PRR7 protein levels are regulated by light and the circadian clock in Arabidopsis. Plant J 2007; 52:548 - 560
- Lynch M, Conery JS. The evolutionary fate and consequences of duplicate genes. Science 2000; 290:1151 - 1155
- Tuskan GA, Difazio S, Jansson S, Bohlmann J, Grigoriev I, Hellsten U, et al. The genome of black cottonwood, Populus trichocarpa (Torr. & Gray). Science 2006; 313:1596 - 1604
- Schoning JC, Streitner C, Meyer IM, Gao Y, Staiger D. Reciprocal regulation of glycine-rich RNA-binding proteins via an interlocked feedback loop coupling alternative splicing to nonsense-mediated decay in Arabidopsis. Nucl Acids Res 2008; 36:6977 - 6987
- Schoning JC, Streitner C, Page DR, Hennig S, Uchida K, Wolf E, et al. Auto-regulation of the circadian slave oscillator component AtGRP7 and regulation of its targets is impaired by a single RNA recognition motif point mutation. Plant J 2007; 52:1119 - 1130
- Hazen S, Naef F, Quisel T, Gendron J, Chen H, Ecker J, et al. Exploring the transcriptional landscape of plant circadian rhythms using genome tiling arrays. Genome Biol 2009; 10:17
- Sanchez SE, Petrillo E, Beckwith EJ, Zhang X, Rugnone ML, Hernando CE, et al. A methyl transferase links the circadian clock to the regulation of alternative splicing. Nature 2010; 468:112 - 116
- Bedford MT, Clarke SG. Protein Arginine Methylation in Mammals: Who, What and Why. Molecular Cell 2009; 33:1 - 13
- Yan D, Zhang Y, Niu L, Yuan Y, Cao X. Identification and characterization of two closely related histone H4 arginine 3 methyltransferases in Arabidopsis thaliana. Biochem J 2007; 408:113 - 121
- Niu L, Zhang Y, Pei Y, Liu C, Cao X. Redundant Requirement for a Pair of PROTEIN ARGININE METHYLTRANSFERASE4 Homologs for the Proper Regulation of Arabidopsis Flowering Time. Plant Physiol 2008; 148:490 - 503
- Niu L, Lu F, Pei Y, Liu C, Cao X. Regulation of flowering time by the protein arginine methyltransferase AtPRMT10. EMBO Rep 2007; 8:1190 - 1195
- Scebba F, Bastiani MD, Bernacchia G, Andreucci A, Galli A, Pitto L. PRMT11: a new Arabidopsis MBD7 protein partner with arginine methyltransferase activity. Plant J 2007; 52:210 - 222
- Wang X, Zhang Y, Ma Q, Zhang Z, Zue Y, Bao S, et al. SKB1-mediated symmetric dimethylation of his-tone H4R3 controls flowering time in Arabidopsis. EMBO J 2007; 26:1934 - 1941
- Pei Y, Niu L, Lu F, Liu C, Zhai J, Kong X, et al. Mutations in the type II protein arginine methyltransferase AtPRMT5 result in pleiotropic developmental defects in Arabidopsis. Plant Physiol 2007; 44:1913 - 1923
- Hong S, Song HR, Lutz K, Kerstetter RA, Michael TP, McClung CR. Type II protein arginine methyltransferase 5 (PRMT5) is required for circadian period determination in Arabidopsis thaliana. Proc Natl Acad Sci USA 2010; 107:21211 - 21216
- Matsushika A, Imamura A, Yamashino T, Mizuno T. Aberrant expression of the light-inducible and circadian-regulated APRR9 gene belonging to the circadian-associated APRR1/TOC1 quintet results in the phenotype of early flowering in Arabidopsis thaliana. Plant Cell Physiol 2002; 43:833 - 843
- Zhang Z, Zhang S, Zhang Y, Wang X, Li D, Li Q, et al. Arabidopsis Floral Initiator SKB1 Confers High Salt Tolerance by Regulating Transcription and Pre-mRNA Splicing through Altering Histone H4R3 and Small Nuclear Ribonucleoprotein LSM4 Methylation. Plant Cell 2011; 23:396 - 411
- Deng X, Gu L, Liu C, Lu T, Lu F, Lu Z, et al. Arginine methylation mediated by the Arabidopsis homolog of PRMT5 is essential for proper premRNA splicing. Proc Natl Acad Sci USA 2010; 107:19114 - 19119
- Dacwag CS, Ohkawa Y, Pal S, Sif S, Imbalzano AN. The Protein Arginine Methyltransferase Prmt5 Is Required for Myogenesis because It Facilitates ATP-Dependent Chromatin Remodeling. Mol Cell Biol 2007; 27:384 - 394
- Schmitz R, Sung S, Amasino R. Histone arginine methylation is required for vernalization-induced epigenetic silencing of FLC in winter-annual Arabidopsis thaliana. Proc Natl Acad Sci USA 2008; 105:411 - 416
- Chari A, Fischer U. Cellular strategies for the assembly of molecular machines. Trends Biochem Sci 2010; 35:676 - 683
- Boon KL, Grainger RJ, Ehsani P, Barrass JD, Auchynnikava T, Inglehearn CF, et al. prp8 mutations that cause human retinitis pigmentosa lead to a U5 snRNP maturation defect in yeast. Nat Struct Mol Biol 2007; 14:1077 - 1083
- Zhang D, Rosbash M. Identification of eight proteins that cross-link to pre-mRNA in the yeast commitment complex. Genes Dev 1999; 13:581 - 592
- Ptitsyn A, Gimble J. Analysis of circadian pattern reveals tissue-specific alternative transcription in leptin signaling pathway. BMC Bioinformatics 2007; 8:15
- Janssen T, Husson SJ, Lindemans M, Mertens I, Rademakers S, Donck KV, et al. Functional Characterization of Three G Protein-coupled Receptors for Pigment Dispersing Factors in Caenorhabditis elegans. J Biolog Chem 2008; 283:15241 - 15249
- Staiger D, Zecca L, Kirk DAW, Apel K, Eckstein L. The circadian clock regulated RNA-binding protein AtGRP7 autoregulates its expression by influencing alternative splicing of its own pre-mRNA. Plant J 2003; 33:361 - 371
- Zhang N, Kallis RP, Ewy RG, Portis AR. Light modulation of Rubisco in Arabidopsis requires a capacity for redox regulation of the larger Rubisco activase isoform. Proc Natl Acad Sci USA 2002; 99:3330 - 3334