Abstract
The development of multicellular organisms is associated with extensive rearrangements of tissues and cell sheets. The driving force for these rearrangements is generated mostly by the actin cytoskeleton. In order to permit the reproducible development of a specific body plan, dynamic reorganization of the actin cytoskeleton must be precisely coordinated in space and time. GTP-exchange factors that activate small GTPases of the Rho family play an important role in this process. Here we review the role of this class of cytoskeletal regulators during important developmental processes such as epithelial morphogenesis, cytokinesis, cell migration, cell polarity, neuronal growth cone extension and phagocytosis in different model systems.
Introduction
Cells in a developing organism are in a continuous state of change. They grow, divide, change their shape, move or die in a magnificently coordinated effort with the objective to reproduce the species-specific body plan of the organism they belong to. The ability of cells to achieve this task relies to a large extent on the actin cytoskeleton. Actin filaments occur dispersed throughout the cell, but are highly concentrated at the cell cortex where they are organized into bundles and networks of filaments. A large number of conserved proteins that cap, sever, cross-link, bundle, nucleate or move actin filaments have been identified.Citation1 The activity of these actin-regulators needs to be precisely coordinated in space and time to ensure normal cell behavior and tissue development.
A broad range of cytoskeletal functions are controlled by small GTPases (Guanosine Triphosphatases) of the Rho family, which act as molecular switches that regulate cell shape, division, migration, polarity, and adhesion.Citation2 The canonical members of this GTPase family, RhoA, Rac1 and Cdc42, have been identified in the early 1990'sCitation2–Citation6 and have more recently also been implicated in the regulation of gene expression, cell cycle progression, microtubule dynamics and cell growth.Citation2 Rho family GTPases relay extrinsic or intrinsic signals to a wide array of effectors many of which are kept in an auto-inhibitory state in the absence of a signal.Citation7,Citation8 Upon activation, the GTPase binds to its cognate effector and triggers a conformational change that activates the effector. Major effector pathways discussed in this review are summarized in .
Three classes of conserved Rho family GTPase regulators have been identified (); (1) Rho-guanine nucleotide exchange factors (RhoGEFs) catalyze the exchange of GDP for GTP, and thereby convert the GTPase into its active state; (2) GTPase activating proteins (RhoGAPs) accelerate the slow intrinsic GTPase activity of Rho family GTPases and convert the GTPase back to it's inactive state; (3) Rho-guanine nucleotide dissociation inhibitors (RhoGDIs) prevent spontaneous activation by sequestering the inactive GDP-bound form of the GTPase in the cytoplasm.
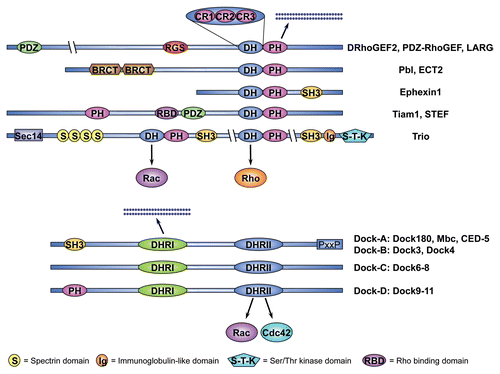
Among these regulators, RhoGEFs play a particularly important role in regulating GTPase signaling. RhoGEFs fall into one of two conserved protein families, the Dbl-GEFs and DHR2/CZH-GEFs, which differ in the conserved domains that mediate membrane attachment and catalyze nucleotide exchange on the cognate GTPase. The mechanism of nucleotide exchange is highly conserved within each family, but differs between families. Members of each group are present in plants and early eukaryotes, revealing an ancient evolutionary origin ().
Animal genomes encode multiple RhoGEFs and many are expressed in spatially and temporally restricted patterns during development. Analysis of the Drosophila and C. elegans genomes has revealed 26 fly and 20 worm genes that fall into the Dbl family, and 4 fly and 3 worm genes that belong to the DHR2/CZH family. The fish and mammalian genomes harbor approximately 70 Dbl-GEFs and 11 DHR2/CZH-GEFs. The human genome encodes 69 Dbl-GEFs and 11 DHR2/CZH-GEFs.Citation9,Citation10
The number of RhoGEFs encoded in the genome is much greater than the number of GTPases they regulate and this disparity has led to the hypothesis that individual RhoGEFs may provide functional specificity by channeling GTPase signaling through one or several of a range of possible effector pathways. Thus, signaling events upstream of Rho family GTPases which involve RhoGEFs and RhoGAPs may specify signaling downstream of Rho family GTPases.Citation11,Citation12 It is possible that RhoGEFs and RhoGAPs cooperate to achieve a distinct level, duration or subcellularly localized activation of Rho family GTPases, which may allow stimulation of specific downstream effector pathways.Citation13,Citation14 Several RhoGEFs are part of multi-protein complexes that include specific GTPase effector proteins, which could provide a mechanism for selective activation of downstream effector pathways.
Here we review recent advancements in characterizing the role of RhoGEFs during animal development. We use six examples of conserved cellular behaviors crucial for animal development such as apical constriction of epithelial cells, cytokinesis, cell migration, establishment of cell polarity, axonal morphogenesis and phagocytosis to illustrate emerging concepts and current directions in the field. In each case, conserved intracellular signaling networks involving RhoGEFs have been identified which impinge on the cytoskeletal machinery that generates the physical force driving the cellular process and, ultimately, the developmental process to which the cellular behavior contributes.
Epithelial Morphogenesis: Drosophila RhoGEF2 Regulates Apical Constriction During Mesoderm Invagination
Epithelial cells that line cavities, tubes and the body surfaceCitation15,Citation16 exhibit polarity that regionalizes their plasma membrane into distinct apical and basolateral domains.Citation15,Citation17 The apical cell membrane is organized into a domain that faces the external or lumenal environment, and a subapical belt of adherens junctions (AJs) that provides a strong mechanical link between adjacent cells. During development, epithelial sheets play important roles in the sculpturing and compartmentalization of the embryo. Sheets of epithelial cells give rise to various three-dimensional structures, including shallow grooves, deep invaginations, plate-like placodes, small pits or hollow tubes by undergoing intricate changes in cellular morphology.Citation18
The mechanisms of epithelial morphogenesis have been extensively investigated during the invagination of mesodermal primordia in the Drosophila embryo, which begins approximately three hours after egg laying.Citation19–Citation21 The prospective mesoderm invaginates from the ventral side of the embryo, forming a transient groove known as the ventral furrow. The ventral furrow encompasses a band of approximately 20 cells along the anterior-posterior axis of the embryo, excluding the terminal areas. The first morphologically distinguishable event in ventral furrow formation is the apical flattening of the ventral-most cells of the presumptive mesoderm. This is followed by rapid apical constriction, which converts the cells from a cuboidal to a wedge-shaped appearance. The change in cell shape bends the epithelium thereby forcing mesodermal cells to move inside the embryo.Citation22
The Rho1-specific Dbl-GEF DRhoGEF2 has been placed at the heart of a signaling pathway that triggers apical constriction of ventral furrow cells. Embryos lacking DRhoGEF2 do not form a ventral furrow.Citation23,Citation24 DRhoGEF2 is the sole fly member of the regulator of G-protein signaling (RGS) domain-containing sub-family, which includes mammalian PDZ-RhoGEF, p115-RhoGEF and LARG,Citation25–Citation28 zebrafish Arhgef11Citation29 and C. elegans CeRhoGEF.Citation30 A large body of evidence supports that this RhoGEF sub-family promotes nucleotide exchange specifically on the GTPase RhoA (Rho1 in Drosophila).Citation25,Citation27,Citation28,Citation31–Citation35
Mesodermal fate is established prior to gastrulation by two genes, twist (twi) and snail (sna), encoding zygotic transcription factors, whose expression is restricted to a band of ventral cells that defines the mesodermal primordium.Citation21,Citation36,Citation37 twi regulates sna,Citation36–Citation38 and also activates two other genes required for efficient gastrulation, folded gastrulation (fog)Citation39–Citation41 and T48,Citation42 which encode an apically secreted ligand and a cortically localized transmembrane protein, respectively. According to a recent model,Citation18,Citation42 Fog and T48 act in parallel to recruit DRhoGEF2 to the apical cell cortex of mesodermal cells (). T48 directly binds to the PDZ domain of DRhoGEF2,Citation42 whereas Fog acts in an autocrine fashion to activate the Gα subunit Concertina (Cta),Citation40,Citation43 presumably by activating an as yet unidentified G-protein coupled receptor. By analogy to mammalian DRhoGEF2 orthologs, Cta is thought to interact with the RGS domain of DRhoGEF2 to promote its apical enrichment and activation. Studies in Drosophila cell culture have suggested that this could be mediated by Cta-induced dissociation of DRhoGEF2 from microtubule tips.Citation44
Once localized to the apical membrane, DRhoGEF2 catalyzes nucleotide exchange on apical Rho1 to induce downstream signaling.Citation23,Citation24 Activated Rho1 appears to signal to two Rho1 effectors, the kinase Rok (Rho kinase)Citation41,Citation45,Citation46 and the formin Diaphanous (Dia)Citation47,Citation48 which may act in parallel to promote assembly and subsequent contraction of an apical actin-myosin network.Citation49 In this process, Rok has a conserved function to increase the phosphorylation state of myosin regulatory light chain (MRLC),Citation45,Citation46 encoded by spaghetti squash (sqh) in Drosophila.Citation50,Citation51 Phosphorylation of Sqh promotes assembly of myosin II into force-generating filaments, which results in increased motor activity.Citation52,Citation53 Phosphorylation also appears to be essential for recruitment of myosin II to the apex of cells prior to gastrulation. Accordingly, apical myosin II accumulation is lost and cells fail to constrict in rok mutantsCitation41 or in embryos treated with the Rok inhibitor Y27632,Citation54 a phenotype mimicked by loss of DRhoGEF2.Citation41,Citation55 In addition, a mutated version of myosin II that lacks the actin-binding domain failed to localize to the apical side of ventral furrow cells, providing strong evidence that myosin II localization in ventral furrow cells is dependent on actin binding and/or contractility.Citation41 Together, these data suggest that apically recruited DRhoGEF2 activates Rok through Rho1 to trigger phosphorylation of Sqh, which, in turn, is a prerequisite for apical enrichment of myosin II. In accordance with this view, overexpression of DRhoGEF2-pathway components in Drosophila Schneider-2 (S2) cells promotes cortical myosin II accumulation and cell contraction that can be inhibited by Rok inactivation.Citation44
The role of Dia in the generation of contractile force as well as its mechanistic link to DRhoGEF2 is less clear. Based on the established role of Diaphanous-related Formins (DRFs) in nucleation and polymerization of unbranched actin filaments, it seems likely that Dia participates in the organization or stabilization of apical actin. However, recent data has revealed a role for Dia in promoting myosin II activity and AJ stability in Drosophila.Citation48,Citation56 Dia may cooperate with the actin-binding non-receptor tyrosine kinase Abelson (Abl),Citation57 which regulates apical constriction of ventral furrow cells, similar to Dia.Citation58 The role of Abl in cell constriction is conserved, as double mutants for the two mouse abl orthologs, abl and arg, exhibit disrupted neural tube closure and defects in actin organization in constricting neuroepithelial cells.Citation59 Interestingly, in Drosophila abl mutants many ventral furrow cells fail to constrict despite the proper localization of DRhoGEF2 and myosin II.Citation58 Thus, Abl may regulate ordered apical actin assembly at the cell cortex in parallel to DRhoGEF2.Citation57,Citation58 By contrast to DRhoGEF2, Abl acts specifically by downregulating the actin anti-capping protein Enabled (Ena)Citation57,Citation60 and embryos mutant for abl exhibit ectopic Ena as well as ectopic actin accumulations in ventral furrow cells.Citation58
It has been difficult to explain how activation of DRhoGEF2 establishes the force-generating mechanism that drives the observed changes in cellular morphology. Recent use of live imaging techniques and detailed measurements of apical cell constriction has shed new light on this important issue. In addition to a contractile actin-myosin network, apical constriction requires assembly of AJs at the apical cell cortex.Citation41,Citation61 According to a simple purse-string model, myosin II-dependent sliding of actin filaments past each other induces contraction of a circumferential microfilament network, which reduces the apical cell perimeter and causes cells constrict. This creates tension in the tissue, which is transmitted from cell to cell through AJs and forces the epithelium to bend. Recent findings suggest that this model for apical constriction may need to be revised. Using live recordings of ventral furrow cells, Martin and colleagues found that cells undergo repeated constriction-stabilization cycles, which occur asynchronously between neighboring cells.Citation62 These pulsed constrictions appear to be driven by actin-myosin contractions at the medial apical cortex and not by contraction of a circumferential actin belt. The authors suggest a “ratchet model” for apical cell constriction in which pulses of subcellular contractions of a cortical actin-myosin network pull discrete AJ sites inwards to narrow the apical area incrementally (). To understand how DRhoGEF2 signaling regulates this form of dynamic contraction at the medial apical cell cortex as well as the subsequent stabilization phase should be an important subject for future investigation.
Cytokinesis: Spatial Regulation of Effector Pathways by Pebble/ECT2
Complex cellular behaviors require spatial and temporal coordination of distinct cytoskeletal functions such as actin polymerization, depolymerization, anchoring, crosslinking and movement. These processes, in turn, require the site-specific deployment of multiple effector pathways acting downstream of Rho family GTPases. The role of RhoGEFs in spatial regulation of different effector pathways has been intensely studied in the context of cytokinesis.
Cytokinesis requires the assembly of a membrane-tethered contractile actin-myosin ring, which is involved in physical separation of the two daughter cells that complete mitosis. During cytokinesis, myosin-dependent sliding of actin filaments diminishes the diameter of the contractile ring to produce a circumferential indentation that spans the cells equator and is referred to as the cytokinetic furrow. Continued contraction of the ring leads to separation of the nascent cells as the furrow ingresses between them.Citation63
Contractile ring assembly requires the GTPase RhoA which was initially implicated in cytokinesis because injection of the RhoA-specific inhibitor C3 transferase, stops furrowing in sea urchin and frog embryos.Citation64,Citation65 The first evidence of a RhoGEF regulating cytokinesis emerged from a genetic screen in Drosophila that identified pebble (pbl) as essential for cell division.Citation66 Analysis of the pbl mutant phenotype revealed that embryonic cells lacking pbl failed to assemble a contractile ring resulting in cytokinesis defects.Citation67,Citation68 Subsequently, it was shown that Pbl accumulates at the cell equator during cytokinesis where it acts as a Rho1-specific GEF.Citation69 Drosophila Pbl is the sole fly ortholog of C. elegans and vertebrate ECT2, which are similarly required for contractile ring assembly upstream of RhoA.Citation70–Citation73 In addition to the characteristic tandem DH-PH domain, these RhoGEFs share two N-terminal BRCT (BRCA1 carboxyl terminus) domains (see ), first identified in the familial breast and ovarian cancer susceptibility gene BRCA1. BRCT domains are protein-protein interaction modules and are essential for Pbl recruitment to the cell equator during cytokinesis.Citation74 In the central region, Pbl harbors a PEST sequence, which often serves as a protein degradation signal, and a nuclear localization signal (NLS), which is also present in the human, mouse and worm orthologs.Citation69,Citation71 Similar to other Dbl family GEFs, N-terminally truncated mammalian ECT2 acts as an oncogene in cultured cells.Citation75,Citation76 In flies, overexpression of an analogous truncated form of Pbl, but not wild type Pbl, has a dominant effect.Citation69,Citation77 Together, the data suggest an auto-inhibitory model according to which the GEF activity of Pbl/ECT2 is under negative control by N-terminal domains. Upstream factors may disrupt intramolecular interactions to activate the enzymatic activity of the GEF by binding to such domains, thus triggering local Rho1/RhoA-activation.
Pbl/ECT2 is part of an evolutionary conserved signaling pathway that orchestrates contractile ring dynamics by promoting site-specific activation of Rho1/RhoA at the cell equator (). Following its activation, Rho1/RhoA promotes contractile ring assembly through the recruitment of several protein components.Citation78 GTP-bound Rho1 guides localized production of parallel bundles of actin filaments via its effector DiaCitation79,Citation80, which interacts with the Drosophila Profilin ortholog Chickadee (Chic)Citation81,Citation82 to drive actin polymerization.Citation83 In Drosophila, an alternative Pbl and Rho1-dependent mechanism, which delivers vesicles carrying F-actin that has been assembled elsewhere in the cell to the furrow, has been described.Citation84 The molecular mechanism of this vesicle-mediated delivery of F-actin is currently unclear.
Activation of Rho1/RhoA also results in the recruitment of myosin II, another component of the contractile ring, to the cell equator. Myosin II recruitment depends on the phosphorylation of its MRLC subunit. In vitro studies have implicated at least two effector kinases downstream of Rho1/RhoA in MRLC phosphorylation: Citron kinase (Sticky (Sti) in Drosophila) and ROCK (Rok in Drosophila). However, evidence from Drosophila cell culture suggests that Rok but not Sti is essential for Sqh phosphorylation and localization of myosin II to the equatorial cortex during mitosis.Citation85 Consistent with a conserved role for Rok/ROCK in cytokinesis, human and nematode cells depleted of ROCK activity exhibit defects in actin ring contractility.Citation86–Citation88 In cultured Drosophila cells, Rok depletion results in complete failure to form a furrow. Sti, by contrast, appears to regulate maintenance of the contractile ring during later stages of cytokinesis.Citation89–Citation91 In the mouse, Citron is essential only in a subset of tissues.Citation92
Recent studies in Drosophila have identified another Pbl-regulated Rho1-effector pathway deployed during cytokinesis. In parallel to the activation of Dia, Rok and Sti, Rho1 directs the accumulation of the scaffolding protein Anillin at the future site of cleavage to promote furrow stability. Evidence suggests that Rho1 binds Anillin, which also binds several contractile ring components such as actin, myosin II and septins.Citation93,Citation94 It is believed that Anilin functions to dynamically link the contractile ring to the plasma membrane and to microtubules.Citation95 Thus, several pathways downstream of Pbl/ECT2 cooperate in the establishment, stabilization and contraction of the actin ring during cytokinesis. Precisely how Pbl/ECT2 coordinates the activity of these different effector pathways is currently unknown.
Localized activation of Rho1/RhoA by Pbl/ECT2 at the cell equator appears to define the plane of division in animal cells. However, how is the zone of Pbl/ECT2-activity determined? Studying the distribution of active RhoA in echinoderm and amphibian embryonic cells has demonstrated that the position of RhoA-activation along the equator is dictated by the position of the mitotic spindle.Citation96
The link between microtubules and Rho1/RhoA activation may be mediated by an evolutionary conserved microtubule-associated complex, the centralspindlin complex,Citation97 that captures Pbl/ECT2 at the cell equator.Citation74 Centraspindlin is a two-protein complex composed of a plus-end-directed kinesin-like motor protein, known as ZEN-4 in C. elegans,Citation98,Citation99 Pavarotti (Pav) in DrosophilaCitation100 and MKLP-1/2 (Mitotic Kinesin-Like Protein 1/2) in mammals,Citation101,Citation102 and a Rho family GAP, dubbed CYK-4 in worms,Citation97 RacGAP50C/Tumbleweed (Tum) in fliesCitation74 and MgcRacGAP in vertebrates.Citation103 The centralspindlin complex is required for the earliest steps of animal cytokinesis. Thus, loss of either component of the complex causes a failure in cytokinetic furrow formation or ingression in numerous systems.Citation63 It is believed that the kinesin-like motor protein component ZEN-4/Pav/MKLP-1/2 moves centralspindlin towards the plus-ends of equatorial, astral and midzone microtubules during mitosis. Once properly localized at the cell equator, the centralspindlin complex recruits Pbl/ECT2 to the future cleavage site.Citation74 This triggers local Rho1/RhoA activation, which directs assembly and constriction of the actin ring that powers cytokinesis.
Several lines of evidence support the centralspindlin-Pbl/ECT2 model for furrow positioning. Three-dimensional reconstructions of Pbl, RacGAP50C/Tum and Pav localization during cytokinesis in Drosophila embryonic epithelial cells have revealed a double ring arrangement in which a cortical microtubule-associated ring of centralspindlin complexes is juxtaposed to a ring of Pbl.Citation74 Ectopically localized RacGAP50C/Tum induces ectopic furrowing,Citation104 centralspindlin decorates microtubules at the site of cleavage prior to the onset of cytokinesis,Citation105 and Pbl localization depends on RacGAP50C/Tum and Pav.Citation106 These data are further supported by evidence of a direct interaction between Pbl and RacGAP50C/Tum, as well as RacGAP50C/Tum and Pav.Citation74,Citation107–Citation110 Somers and SaintCitation74 have shown that RacGAP50C/Tum binds to the extended N-terminal BRCT domain of Pbl through an N-terminal coiled-coil domain. This might activate the GEF-activity of Pbl by relieving the auto-inhibitory constraint that the N-terminus imposes on the Dbl domain.
In summary, it appears that an evolutionary conserved core mechanism regulates site-specific recruitment of Pbl/ECT2, which results in activation of a set of conserved Rho1/RhoA-effector pathways acting in concert to orchestrate cytokinesis.
Migration of Mesodermal Cells: Pebble Integrates Different Upstream Signals and Regulates Different GTPases in a Context-Dependent Fashion
Drosophila Pbl also illustrates the concept that individual RhoGEFs can be deployed in more than one process, as it has been found that Pbl controls the lateral migration of mesoderm cells during gastrulation in addition to its role in cytokinesis. Interestingly, in the mesoderm Pbl is regulated by different upstream signals and exhibits altered GTPase specificity.
Cell migration requires cells to generate a polarized pushing force that is coordinated in space and time. Recent in vivo data regarding the molecular mechanism of cell migration have emerged from studies using the lateral spreading of mesodermal cells in the early Drosophila embryo as a model. Mesoderm progenitors originate on the ventral side of the early fly embryo. During gastrulation, mesodermal cells invaginate, undergo epithelial-mesenchymal transition and then migrate laterally over the inner surface of the ectoderm.Citation111 Migrating mesodermal cells adhere to the overlaying ectoderm and extend multiple cellular protrusions in the direction of migration. The signal to migrate is provided by two Fibroblast Growth Factor 8 (FGF8)-like ligands, Thisbe (Ths) and Pyramus (Pyr),Citation112,Citation113 which activate the FGF receptor Heartless (Htl) in a partially redundant fashion.Citation114–Citation118 Interestingly, pbl mutants have a mesoderm migration defect that is reminiscent of the htl or ths, pyr double mutant phenotypes.Citation119,Citation120 Whereas internalization of the mesoderm occurs normally in pbl mutants, subsequent lateral migration of mesodermal cells fails due to the inability of mesoderm cells to attach to the overlaying ectoderm and to support protrusive activity. Interestingly, C. elegans ECT2, has also been implicated in cell migration during developmentCitation71 and it will be interesting to see if the role of Pbl/ECT2 proteins in cell migration represents a universally conserved mechanism.
Functional dissection of Pbl has shown that distinct protein domains mediate the roles in cytokinesis and mesodermal cell migration as expression of truncated versions of Pbl partially rescues mesodermal cell migration but not the cytokinesis defects.Citation77,Citation119 Both the cell migration and cytokinesis functions of Pbl require GEF activity. Intriguingly, van Impel and colleagues could show that Pbl may act downstream of Htl to activate Rac1 and its paralogsCitation77 (collectively termed Rac GTPases) instead of Rho1 and that the C-terminal tail of Pbl is important for substrate specificity. C-terminally truncated Pbl interacts with Rac GTPases but not Rho1, which is consistent with the idea that the C-terminus may impose a negative constraint on the GEF activity of Pbl towards Rac GTPases. It is possible that FGF-signaling modifies the C-terminus of Pbl and thus converts Pbl from a Rho1-specific to a Rac GTPase-specific GEF ().
Anterior-Posterior Polarity Determination: The Role of C. elegans ECT2
Similar to its fly ortholog Pbl, C. elegans ECT2 is not only required for cytokinesis but plays an essential role early in development during anterior-posterior (head-tail) polarity determination in the zygote. In C. elegans, anterior-posterior polarity is established at the one-cell stage by the site of sperm entry, which triggers asymmetric distribution of polarity determinants in the embryo. The sperm-derived centrosome settles near the sperm entry site where it makes contact with the oocyte cortex and nucleates microtubules. This zone becomes the future posterior pole of the embryo. Once localized, the centrosome transfers the initial asymmetry to the actin cytoskeleton resulting in a gradient of contractile actin-myosin directed towards the anterior pole that pulls the polarity determinants PAR-3, PAR-6 and atypical protein kinase C (aPKC, PKC-3 in C. elegans) towards the prospective anterior pole of the embryo. Contraction of the actin-myosin network is essential for anterior-posterior axis establishment and for anterior PAR-3, PAR-6 and PKC-3 localization in the zygote.Citation121,Citation122
Prior to fertilization, the cortical actin-myosin network of the oocyte is unpolarized and proteins associated with the cortex, such as the PAR-complexes, are uniformly distributed.Citation123 Following fertilization, this network begins to contract uniformly. Contractions are triggered by ubiquitous cortical ECT2-dependent activation of RhoA (also known as Rho-1) and myosin II.Citation124–Citation126 Consistent with this, ECT2 co-localizes with myosin II and depletion of ECT2 or RhoA from the zygote causes defects in contractility and polarity, in addition to failure of cytokinesis.
In addition to the paternal centrosome, the sperm contributes a pool of the Rho family GAP CYK-4 to the oocyte.Citation126 Using an antibody against CYK-4, Jenkins and co-workers showed that the sperm-donated CYK-4 localizes around the centrosomes and to the overlaying cortex (i.e., at the future posterior end) where it regulates early contractile events that are important for the segregation of polarity determinants. Thus, initial symmetry is broken as sperm-derived CYK-4 mediates a local down-regulation of RhoA activity at the cortex overlaying the centrosomes.Citation126 At the same time, ECT2 becomes eliminated from this region of the embryo,Citation124 reinforcing the asymmetry in RhoA activation along the anterior-posterior axis. The localized downregulation of RhoA activity results in a local loss of contractility and in partial collapse of the actin-myosin network at the future posterior pole. Consistent with this, the earliest detectable sign of polarity establishment is the cessation of contractions in a small region of the cortex overlying the centrosomes.Citation121 The local collapse of the actin-myosin network is believed to promote a progressive spreading of the non-contractile domain throughout the future anterior half of the embryo in which the actin-myosin network continues to contract under the influence of ECT2 and RhoA. In this way, two domains are set up in the one-cell embryo; one domain containing a contractile actin-myosin network with associated anterior determinants; the other devoid of such a network and anterior determinants. Instead, the non-contractile cortical domain accumulates factors that confer posterior identity, such as PAR-1 and PAR-2.Citation123 In conclusion, local accumulation of the Rho family GAP CYK-4 and exclusion of the RhoGEF ECT2, results in spatial differences in actin-myosin contractility in the early embryo, which direct the asymmetric localization of polarity determining factors along the anterior-posterior axis.
Multiple RhoGEFs Regulate Axonal Growth Cone Morphogenesis
Complex cellular behaviors require the deployment of multiple effector pathways acting downstream of different Rho family GTPases. The complexity of these GTPase-signaling networks that control cellular morphology has been extensively studied in the context of neurogenesis. The development of neuronal progenitors to mature neurons relies on the combined and localized activation of several effectors downstream of RhoA, Rac1 and Cdc42 at different stages of cellular maturation, and on extensive crosstalk between these GTPases.Citation127,Citation128
Mature neurons are characterized by one long axon that sends signals, and multiple shorter dendrites that receive signals. Shape, polarity and size of neurons are determined during development and depend on the dynamic properties of an underlying dendritic and axonal cytoskeleton. During neuronal development, the axonal cytoskeleton undergoes reorganization in response to a variety of extracellular cues that can serve as either attractive or repulsive signals by virtue of their ability to induce extension or retraction of axonal growth cones.Citation129 Extracellular cues are received by receptors at the cell surface, and transmitted by a signaling network to Rho family GTPases, which regulate the cytoskeletal machinery throughout the maturation process from neuronal progenitor to fully functional neuron. During growth cone morphogenesis, local activation of Cdc42 and Rac1 promote the extension of filopodia and lamellipodia, respectively, which is important for cone outgrowth, while activated RhoA generates contractile forces that may drive forward translocation of the growth cone body. Growth cone retraction is believed to result from RhoA-mediated contractility concurrent with decreases in Cdc42 and Rac1 activity. Recent progress has helped to understand how RhoGEFs coordinate the GTPase signaling network that directs growth cone specification and morphogenesis in vivo.
The signaling network that underlies growth cone morphology has been extensively studied in cultured mammalian primary hippocampal neurons.Citation127 In this context, the exchange factors Ephexin1 and PDZ-RhoGEF have been found to trigger growth cone collapse through RhoA in response to the activation of Ephrin and PlexinB1 receptors on the growth cone surface of primary cells ().Citation130,Citation131 Ephexin1 knockout mice exhibit no apparent defects in axon pathfinding during embryonic development,Citation132 however, adult ephexin1-/- mice have defects in structural maturation and neurotransmission at neuromuscular junctionsCitation133 and shRNA-mediated knockdown of the single chick ortholog resulted in motor axon defects in the embryo.Citation132 Expression of Ephexin1 in fibroblasts elicits morphological changes consistent with the activation of RhoA, Rac1, and Cdc42 suggesting that Ephexin1 has a broad GTPase specificity.Citation130 Interestingly, following Ephrin receptor activation, Ephexin1 becomes phosphorylated on Tyr87 and this enhances the exchange activity of Ephexin1 towards RhoA but not Rac1 and Cdc42,Citation132 which is reminiscent of the switch of GTPase activity that has been observed for Pbl (see above).
Other exchange factors that have been analyzed include the closely related Rac GTPase-specific GEFs Tiam1 (T-lymphoma invasion and metastasis 1)Citation134,Citation135 and STEF (Sif and Tiam1-like exchange factor, also called Tiam2)Citation136, which are orthologs of Drosophila Still life.Citation137,Citation138 Tiam1 and STEF are expressed at high levels in the developing murine brainCitation139,Citation140 and both proteins have been implicated in axonal development.Citation141–Citation143 Overexpression of Tiam1 in primary cells induces the extension of multiple axon-like neurites whereas treatment with an antisense oligonucleotide resulted in a dramatic decrease in the number of cells displaying an axon-like neurite.Citation142 However, tiam1 knockout mice show no clear embryonic phenotype, even though oncogenesis is reduced.Citation144 This suggests that other GEFs capable of activating Rac1 in neural cells, most notably STEF, but also Dock4,Citation145 Dock7,Citation146 β-PixCitation147 and Vav sub-family GEFsCitation148,Citation149 might act redundantly. Recent studies have provided interesting insights into the upstream signals that regulate Tiam1 and STEF during axonal specification. In brain lysates, these GEFs form a complex with the polarity proteins Par3, Par6 and aPKC, which act downstream of Cdc42. Specifically, Tiam1 and STEF bind Par3. Cell culture experiments suggest that Cdc42 may act first to select which neurite should become the future axon, and then signals to Tiam1/STEF through the Par3/Par6/aPKC complex to activate Rac1, which promotes extension of the pre-selected axon through actin remodeling ().Citation150
Another well-studied Rac1 activator in the nervous system is Trio, which was originally identified as a binding partner for the human receptor-like tyrosin phosphatase LARCitation151 that conveys extracellular cues to the actin cytoskeleton during development. Trio belongs to a sub-family of Dbl-GEFs that includes the mammalian GEFs DuetCitation152 and KalirinCitation153 and C. elegans UNC-73 (see ).Citation154 Trio-related proteins are unique in that they possess two Dbl domains arranged in tandem of which the N-terminal GEF domain (GEF1) has been shown to induce nucleotide exchange on Rac1 or the Rac1-like GTPase MIG-2Citation155 while the C-terminal (GEF2) domain is specific for Rho1/RhoA.Citation156 In addition, mammalian Trio-related proteins contain a C-terminal serine-threonine kinase domain.
The in vivo roles of Trio and Kalirin have been analyzed using knockout mice. Trio is essential in the embryo and mutant animals exhibit abnormal skeletal muscle and aberrant organization in several brain regions, including the hippocampus and olfactory bulb, late in development.Citation157 Kalirin knockout mice are viable until adulthood but show behavioral phenotypes associated with reduced cortical Rac GTPase signaling.Citation158,Citation159 The lack of more severe phenotypes may reflect functional redundancy between several RhoGEFs.
Further evidence for a role of Trio in neuronal development comes from analyses in C. elegans and Drosophila. C. elegans unc-73 mutants exhibit strong axon outgrowth, axon guidance and cell migration defects.Citation154,Citation160 A detailed genetic analysis has uncovered that unc-73 is a complex locus encoding at least eight differentially expressed mRNAs. One of these transcripts encodes a protein with tandem Dbl domains, one encodes a protein with only the GEF1 domain and the remaining six encode proteins containing only the GEF2 domain.Citation161 Phenotypic comparisons of animals with impaired GEF1 or GEF2 domains have shown that only the GEF1 domain is essential for axon guidance, supporting that UNC-73 acts on Rac GTPases to promote axonal growth. The GEF2 domain, however, is required to modulate synaptic neurotransmission upstream of RhoA.Citation161,Citation162 Different isoforms have also been identified for the mammalian Trio and Kalirin orthologs many of which are neuronal specific, suggesting that the regulatory mechanism may be conserved from worms to mammals.Citation163,Citation164
In Drosophila, photoreceptor cells and mushroom body neurons deficient for trio display axon guidance and growth defects.Citation138,Citation165,Citation166 Removing trio from Drosophila embryos results in CNS and PNS defects, including axon stalling and guidance errors.Citation13,Citation165,Citation167,Citation168 The neural defects of trio mutant flies are reminiscent of defects observed after simultaneous removal of all three Drosophila Rac-like genes, and epistasis and biochemical experiments using the Trio GEF1 domain confirm that Trio acts through Rac1, Rac2 and the Rac1-like GTPase Mtl.Citation13,Citation166 Moreover, Trio has been functionally linked to the Rac GTPase-effector Pak, and genetic evidence indicates that the Trio/Rac1/Pak module regulates cytoskeletal dynamics in the axonal growth cone ().Citation13,Citation138,Citation166
The signals that regulate Trio activity in the axonal growth cone are not well understood. Studies in mouse, fly and worm have revealed physical and functional interactions between Trio sub-family members and attractive Netrin receptors, known as Deleted in Colorectal Cancer (DCC) in mammals, UNC-40 in C. elegans and Frazzled (Fra) in Drosophila. This suggests a conserved role for Trio proteins downstream of attractive Netrin receptors to regulate Rac GTPase-mediated axon outgrowth.Citation167,Citation169–Citation171 In Drosophila, further dissection of the pathway suggested that Trio may cooperate with the non-receptor tyrosin kinase Abl and its target, the actin-binding protein Ena, downstream of Fra.Citation167,Citation169 Binding studies have revealed that Abl physically interacts with Fra, and since Trio also binds Fra, this may allow Abl to phosphorylate Trio, which could modulate its exchange factor activity.Citation169 In conclusion, a network of factors, including Trio and Rac GTPases, may act at the interface of cell surface receptors and cytoskeletal regulators in the axonal growth cone. Notably, this network may also include other RhoGEFs such as the Drosophila Ras/RacGEF Sos that regulates Rac1-dependent cytoskeletal reorganizations downstream of the repulsive Slit receptor Roundabout (Robo)Citation172 and the Rho1-specific activator GEF64C that counteracts Robo dependent repulsion.Citation173 It is important to note that Trio proteins might engage with different upstream factors and downstream regulators in a context-dependent fashion. Thus, at a later stage of neuronal maturation, Trio has been linked to the Drosophila LAR receptor and to the Rho1-effector Dia to regulate presynaptic growth ().Citation174
Studies on C. elegans UNC-73 function have provided additional information on the role of this GEF in growth cone development.Citation175 As in Drosophila, the GEF1 domain of UNC-73 has been positioned downstream of the Netrin receptor UNC-40,Citation170 and upstream of Rac1 and Pak during axonal outgrowth.Citation176 Intriguingly, UNC-73 can also act upstream of the netrin receptor and the Slit receptor SAX-3 (Robo) to direct axonal growth cone migration.Citation177,Citation178 This is surprising since it is generally assumed that Rho family GTPases operate downstream of guidance receptors to regulate cytoskeletal reorganization. Genetic evidence suggests a mechanism whereby UNC-73 acts together with the Rac1-related GTPase MIG-2 and the kinesin-related protein VAB-8 to specify the subcellular distribution of UNC-40 and SAX-3 receptors at the cell surface (). Consistent with this, yeast two-hybrid analysis has confirmed physical interactions of UNC-73 with VAB-8 and SAX-3, in addition to UNC-40.Citation177 A recent study has identified CRML-1 (the C. elegans homologue of the mammalian actin-uncapping protein CARMIL), which binds to UNC-73 and negatively regulates its ability to recruit SAX-3 to the cell membrane.Citation179 Thus, VAB-8 may signal through UNC-73 to positively regulate receptor levels at the cell surface, whereas CRML-1 may signal through UNC-73 to counteract this effect. A role of Rho family GTPases in the intracellular trafficking of receptor complexes has also been describedCitation180 and it will be interesting to see if Trio proteins play a similar role in other systems.
DHR2/CZH Family GTP Exchange Factors: Roles in Cell Migration and Phagocytosis
During Drosophila oogenesis a cluster of 6–10 specialized somatic cells, termed border cells, located at the anterior end of the egg chamber delaminate from the monolayered follicular epithelium and move posteriorly as a coherent group of cells until they, after travelling a distance of approximately 100 µm in six hours, reach the oocyte.
The mechanism of border cell migration and the signaling pathways involved have been a subject of intense investigation, since the behavior of border cells is reminiscent of invasive cells.Citation181,Citation182 Time-lapse recordings of migrating border cell clusters have revealed two distinct phases.Citation183 In an early phase, one or two highly polarized leading edge cells exhibit extensive and dynamic actin-rich protrusionsCitation184 that can extend several cell diameters in length.Citation185 Cell behavior during this phase is reminiscent of solitary migrating cells in culture. However, at half way through the egg chamber, cell behavior changes and the border cells now round up, extend shorter protrusions and enter into a “shuffling” mode. This second phase is characterized by position changes within the cluster as the cells collectively move posteriorly.Citation183
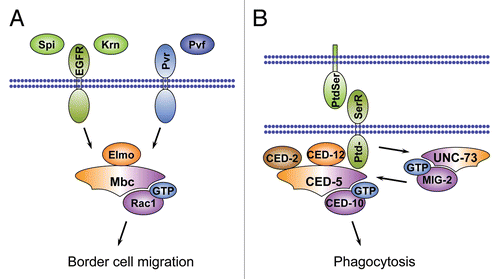
Mutant analyses have revealed that both phases require signaling downstream of the receptor tyrosine kinases (RTKs), EGFR and PVR.Citation186,Citation187 Moreover, several RTK ligands have been identified that act redundantly as chemo-attractants during this processCitation186–Citation189 and the atypical Rac GTPase-specific GEF Myoblast city (Mbc) has been positioned downstream of the RTKsCitation187 (). Mbc is a member of the DHR2/CZH family of RhoGEFs, which includes the mammalian genes Dock1 (also known as Dock180Citation190) to Dock11, C. elegans CED-5Citation191 and Drosophila Sponge (Spg, the fly Dock4 orthologCitation192,Citation193).Citation10,Citation194 These proteins share a conserved catalytic Docker domain (DHR2), which mediates GDP-GTP exchange on Rac GTPases or Cdc42 (see ).Citation9,Citation194 The Drosophila mbc gene was originally identified through its requirement for myoblast fusionCitation195 and has subsequently been implicated in many other Rac1-dependent processes.Citation184,Citation196–Citation198 Cell clusters simultaneously mutant for mbc and spg exhibit an early arrest in border cell migration.Citation184 The same is true for cell clusters that lack the conserved adaptor protein and Mbc/Spg binding partner Elmo,Citation184,Citation199 or for cells expressing a dominant negative form of Rac1.Citation200
Studies of mosaic border cell clusters (i.e., clusters in which some cells are wild-type and others are mutant for a particular gene) have provided additional insights into the molecular mechanisms of border cell migration. In order to examine the precise requirement of RTK-Mbc/Elmo-Rac1 signaling, Bianco and colleagues created mosaic clusters containing individual border cells devoid of Elmo function in an otherwise wild type environment.Citation183 Strikingly, such elmo mutant cells exhibited defects in the early but not in the late phase of migration. Thus, during the early-phase, elmo mutant cells are located at the rear of the migrating cluster from where they appeared to piggyback on their wild type neighbors. Elmo is not required during the later phase of migration, which is controlled by the Ras/Raf/MAPK pathway and phospholipase Cγ.Citation183
Mbc-related proteins have been shown to act together with the SH2 and SH3 domain-containing adapter protein CrkCitation201 in several contexts and the Mbc ortholog Dock180 was originally identified in a screen for novel binding partners of vertebrate Crk.Citation190 It is believed that binding to Crk proteins may concentrate Dock180 homologs at specific sites at the cell membrane. The Dock180-Crk interaction is conserved in fliesCitation202 and both genes have been shown to regulate closure of the adult thorax during metamorphosis.Citation198 However, a role for Drosophila Crk in border cell migration has not been reported and evidence from myoblasts suggests that Crk may not be essential for Mbc function in all cell types.Citation203 During myoblast fusion, binding of the conserved DHR1 domain to the membrane lipid-derived second messenger phosphatidyl-inositol 3,4,5-triphosphate (PIP3) appears to be important for Mbc function.Citation203 PIP3 is known to accumulate at the front of chemotactic cells where it polarizes the cell along the axis of migration.Citation204 The Mbc-PIP3 interaction is conserved between flies and mammals,Citation205 and in migrating cells in culture the Dock180-PIP3 interaction has been shown to mediate the localization of this GEF and its binding partner Elmo to the leading edge.Citation206 It will be interesting to see if Mbc-PIP3 interactions play a role during border cell migration.
The role of the Mbc-Elmo complex in cell motility upstream of Rac1 is likely conserved in other systems. In C. elegans, the Mbc ortholog CED-5 is essential for proper migration of two specific gonadal cells, the distal tip cells, during larval development.Citation191 CED-5 acts in a pathway together with CED-2, CED-12 and CED-10 (orthologs of Drosophila Crk, Elmo and Rac1, respectively).Citation207–Citation210 The worm CED-2, CED-5 and CED-12 proteins interact with each other to form a ternary complex, and a similar complex has been documented in mammalian systems. Consistent with a conserved role for this complex in cytoskeletal reorganization, co-transfection of Elmo1, Dock180 and Crk in cultured fibroblasts induces extensive membrane ruffling that can be suppressed by co-transfection of dominant negative Rac1N17.Citation208
The CED-2/CED-12/CED-5-CED-10 signaling casette regulating migration of distal tip cells is also deployed during engulfment of apoptotic cells by macrophages (hence the acronym CED, for Cell Death Abnormal) and reveals mechanistic links between cell migration and engulfment (). Both processes involve actin remodeling and polarized extension of cell surfaces regulated by Rac1. The recognition of phosphatidylserine (PtdSer) on the surface of apoptotic cells triggers signaling during cell corpse clearance.Citation211 Early in the apoptotic process, PtdSer is translocated from the inner to the outer leaflet of the lipid bilayer of the dying cell. Exposure to PtdSer stimulates the PtdSer receptor on the phagocyte cell, which has been reported to physically interact with CED-5 (Mbc/Dock180) and CED-12 through its intracellular domain.Citation212 Alternatively, the Rac-like GTPase MIG-2 and its GEF UNC-73 can channel signaling from surface receptors to trigger CED-2/CED-12/CED-5-CED-10 pathway activation during cell engulfment.Citation213 The role of the CED-2/CED-12/CED-5-CED-10-pathway in cell engulfment and the function of PtdSer as a recognition signal for phagocytosisCitation214–Citation216 is conserved between worms and mammals.Citation217,Citation218 However, whether PtdSer-receptors bind the mammalian CED-5 and CED-12 homologs DOCK180 and Elmo is still an open question. Together, these studies highlight the CED-2/Crk-CED-5/DOCK180-CED-12/Elmo tripartite complex as a conserved signaling module that links extracellular cues to localized Rac1 activation, which, in turn, induces cytoskeletal rearrangements at the cell membrane to drive cell motility and phagocytosis of apoptotic cells. The function of this RhoGEF-mediated signaling cassette upstream of Rac1 to regulate both cell migration and phagocytosis illustrates the deployment of the same intracellular signaling cassette to control distinct processes during development.
Conclusion and Future Directions
This review has presented several examples of RhoGEF function during development, highlighting how RhoGEFs coordinate different GTPase effector pathways and how individual RhoGEFs control different cell behaviors at different stages during the development of an organism. However, the mechanisms that connect RhoGEFs to upstream organizers of tissue morphogenesis and lead to RhoGEF activation and recruitment to specific subcellular sites are still not well understood and should be a particular focus of future efforts.
The examples of RhoGEF function presented in this review focus on genes that are particularly accessible to genetic analysis in model organisms since their mutation leads to interpretable phenotypes. However, these genes represent only a small fraction of RhoGEFs encoded in the genome and many RhoGEFs remain uncharacterized. There is increasing evidence that many RhoGEFs may act redundantly during tissue morphogenesis and future analysis should focus on the use of innovative approaches such as RNA interference to attempt to inactivate several RhoGEFs simultaneously during specific developmental processes.
Figures and Tables
Figure 1 Regulation of GTPase activity by RhoGEFs, RhoGAPs and RhoGDIs. Upon activation by upstream factors many RhoGEFs undergo a conformational change that enables them to bind a specific GTPase and promote nucleotide exchange. The GTP-bound GTPase interacts with effector proteins to activate cytoskeletal reorganization through a variety of effector pathways (see ). RhoGAPs activate the intrinsic GTPase activity of the GTPase and promote conversion to the inactive GDP-bound form. RhoGDIs stabilize the GDP-bound form of the GTPase and promote sequestration in the cytosol.
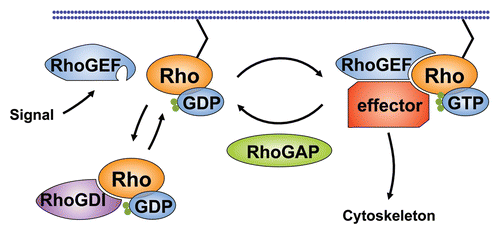
Figure 2 Signaling pathways regulating contractile force during mesoderm invagination in Drosophila. (A) Activation of an unidentified receptor by the secreted signal Fog is transmitted to the heterotrimeric G-protein α-subunit Cta at the cytosolic side of the cell membrane. Cta binds to the RGS domain of DRhoGEF2, promoting membrane recruitment and interaction of DRhoGEF2 with Rho1. Membrane localization of DRhoGEF2 is reinforced by interaction of the DRhoGEF2 PDZ domain with the transmembrane protein T48. GTP-bound Rho1 activates actin polymerization through the Formin Dia and the actin binding protein Chic. Rho1 also activates the serine-threonine kinase Rok that stimulates the formation of contractile actin-myosin fibers partly by inhibiting MLCP and partly by directly phosphorylating Sqh. (B) Pulsed contractions of an apical network of actin-myosin filaments drive apical constriction of epithelial cells on the ventral side of the embryo. The contractile force is transmitted from cell to cell by adherens junctions and causes the tissue to bend. Pulsed contractions require activity of the transcription factor Sna. Stabilization of the contracted state between contractions requires activity of the transcription factor Twi. Ventral twi-expressing nuclei are shown in blue. Apical actin networks and the apical side of cells are shown in red. Spot adherens junctions are shown in purple.
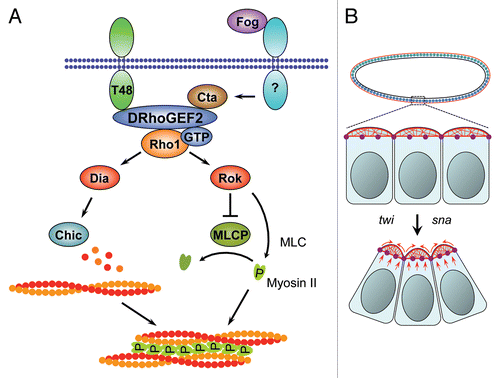
Figure 3 Role of Pbl during contractile ring formation in cytokinesis. (1) Pbl is delivered to the cell equator by the kinesin-like motor protein Pav and Tum/RacGAP50C through plus-end-directed microtubule-mediated transport. The Drosophila Anillin ortholog Scraps is one of the first factors recruited to the cytokinetic furrow and is required for proper actin ring organization. (2) Pbl-mediated GTP exchange on Rho1 promotes formation of an actin ring by the Formin Dia. (3) Pbl-dependent delivery of actin-associated vesicles to the equator on microtubules has been observed. (4) Rok-mediated formation of actin-myosin filaments drives ring contraction. In some cell types later stages of cytokinesis require the function of the kinase Sticky.
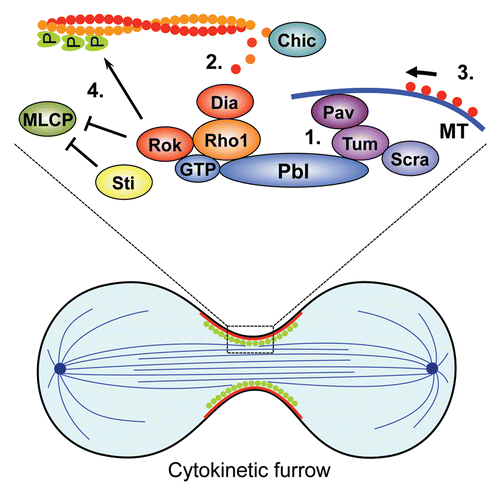
Figure 4 Context-dependent GTPase specificity of the RhoGEF Pbl. During cytokinesis Pbl interacts with the GTPase Rho1 to organize actin ring formation and contraction. In the Drosophila gastrula Pbl interacts with Rac GTPases to control lateral migration of mesodermal cells in response to a signal from the FGF-receptor Htl that is activated by the FGF ligands Pyr and Ths.
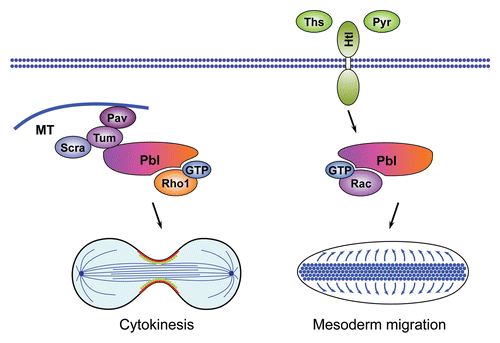
Figure 5 The role of Ephexin1 and Tiam1 in axon growth cone morphogenesis. (A) Ephrin-receptor-mediated phosphorylation of Ephexin1 increases the GEF activity of Ephexin1 towards RhoA and leads to axon growth cone collapse through the Rho-kinase pathway. (B) Cdc42 acts upstream of the Par3/Par6/aPKC complex to promote axon growth cone extension through Tiam1-mediated activation of Rac1.
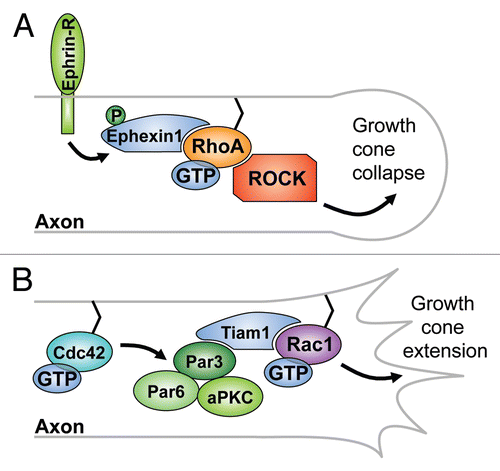
Figure 6 The role of Trio-related RhoGEFs during axon growth cone morphogenesis. (A) Trio has two GEF domains and can regulate axon outgrowth through its Rac-specific GEF1 domain downstream of the Drosophila Netrin receptor Frazzled or, alternatively, controls presynaptic growth by modulating actin reorganization through its Rho1-specific GEF2 domain and the Formin Dia. (B) In addition to its role downstream of Netrin receptors, the C. elegans Trio ortholog UNC-73 also acts upstream to regulate cell surface distribution of the Netrin receptor UNC-40 and the Robo ortholog SAX-3 through the Rac-like GTPase MIG-2. Activity of UNC-73 in this process is counteracted by the actin uncapping protein CRML-1, an ortholog of mammalian CARMIL.
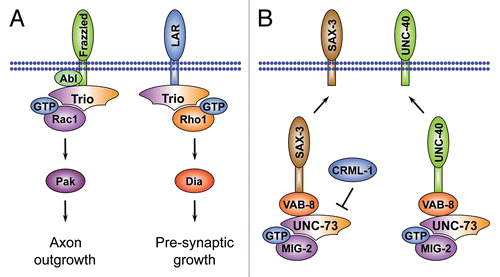
Figure 7 Role of DHR2/CZH family RhoGEFs in Drosophila border cell migration and phagocytosis in C. elegans. (A) The EGF-receptor ligands Spitz (Spi) and Keren (Krn) and the Pvr-receptor ligand Pvf act redundantly to activate the DHR2/CZH family GEF Mbc that signals through Rac1 to control border cell migration in the Drosophila ovaries. The early phase of border cell migration requires interaction of Mbc with its binding partner Elmo. (B) The Mbc/Elmo/Rac1 signaling cassette is conserved in C. elegans, where the orthologs CED-5, CED-12 and CED-10 regulate phagocytosis of apoptotic cells in addition to their function in cell migration. CED-5 is concentrated at specific subcellular sites by the adaptor protein CED-2. During phagocytosis CED-5 is activated the PtdSer receptor that binds to PtdSer on neighboring cells or, alternatively, by the RhoGEF UNC-73 and the GTPase MIG-2.
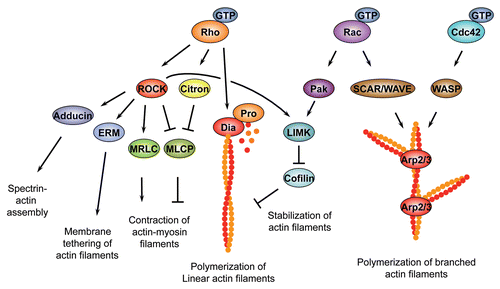
Box 1 Rho family GTPase effector pathways.Regulation of actin polymerization. Rho family GTPases control the rate and site of actin polymerization by regulating the head-to-tail assembly of monomeric, globular G-actin subunits into long, polar filamentous polymers, known as F-actin. The assembly of actin filaments is a multi-step process, with a slow and rate limiting nucleation phase followed by a rapid elongation phase.Citation219 Rho family GTPases control F-actin assembly via two main effectors, the Diaphanous-related Formins (DRFs) which promote the formation of linear filaments and the multi-subunit actin related protein complex Arp2/3, which drives the formation of branched actin filaments. Both factors increase the rate of actin polymerization by enhancing the rate-limiting nucleation step. Arp2/3 binds to the sides of pre-existing filaments to initiate growth of a new filament at a distinctive 70-degree angle from the original filament.Citation219 Both Cdc42 and Rac GTPases can control Arp2/3 activation; however, while Cdc42 activates Arp2/3 via members of the WASP (Wiskott-Aldrich Syndrome protein) family, Rac-dependent activation relies on the structurally related SCAR/WAVE (WASP family Verprolin-homologous protein) family.Citation219, Citation220 In addition to several other functions, Rac GTPases and Cdc42 activate Arp2/3 to promote formation of broad actin-based protrusions termed lamellipodia that extend from the leading edge of migratory cells and developing neurons.Citation221 In contrast to Rac GTPases and Cdc42, Rho1/RhoA stimulates actin polymerization mainly through DRFsCitation83, Citation222, which promote the formation of linear filaments. Many DRF members, such as the Drosophila founding member diaCitation47, Citation79 or the murine mDia1Citation82 are specific for Rho1/RhoA. However, individual DRFs such as mDia2 and mDia3 can be the target of multiple Rho family GTPases.Citation223–Citation225 Following nucleation, DRFs remain stably associated with the growing end of actin filamentsCitation226 and can add new actin monomers to the growing filament or can antagonize the inhibitory activities of filament capping proteins.Citation83 In this way, DRFs promote formation of various actin-rich structures, such as the contractile ring that separates daughter cells during cytokinesisCitation47, Citation79, Citation81, or filopodia that protrude from the membrane front of migrating cells.Citation227, Citation228Regulation of actin filament dynamics. In addition to regulating actin filament nucleation, elongation and branching, Rho family GTPases control the spatial and temporal arrangement of actin filaments, regulate the formation of filament bundles, promote tethering of the actin network to the plasma membrane and trigger myosin II-dependent sliding of actin filaments past each other to create contractile force. In all of these processes, RhoA-specific effectors of the Rho-kinase (ROCK/ROK) family of serine/threonine kinases play an important role.Citation229, Citation230 Various conserved ROCK substrates such as the myosin II Regulatory Light-Chain (MRLC)Citation231, the myosin II Binding Subunit (MBS) of myosin II Light-Chain Phosphatase (MLCP)Citation232, Citation233, LIM-kinase (LIMK)Citation234, AdducinCitation235 and the ERM proteins Ezrin, Radixin and MoesinCitation236 have been identified. RhoA-mediated activation of ROCK promotes the assembly of contractile actin-myosin filaments in non-muscle cells.Citation52, Citation53 Non-muscle myosin II is a hexameric protein consisting of two heavy chains, two light chains, and two regulatory light chains. It is believed that phosphorylation of MRLC on two conserved residues triggers assembly of myosin II into force-generating filaments and promotes the interaction of myosin II with F-actin. ROCK can increase the phosphorylation state of MRLC either directly, or indirectly by phoshorylating the MBS of MLCP, which inactivates this enzyme. In addition to regulating actin-myosin interactions through MRLC, ROCK controls actin-filament dynamics by phosphorylating and thereby activating LIMK. In response to activation by ROCK, LIMK inactivates the actin severing factor Cofilin via phosphorylation, which leads to a reduced turnover and increased stability of actin filaments.Citation237, Citation238 In addition to its role as a ROCK substrate, LIMK also acts downstream of the Rac1 or Cdc42 effector p21-associated kinase (Pak) to inhibit actin de-polymerization.Citation239 Two other targets of ROCK, Adducin and the ERM proteins, promote Spectrin-actin network assemblyCitation240 and tether actin filaments to the plasma membraneCitation241, respectively.
Box 2 Guanine nucleotide exchange factors of the Rho family.The Dbl family. The first RhoGEF gene to be identified was the Dbl (Diffuse B-cell Lymphoma) oncogene.Citation242 In subsequent studies Dbl was shown to induce nucleotide exchange on Cdc42Citation243 by means of a catalytic domain that encompasses approximately 180 amino acids.Citation244 This domain, now known as the DH domain (for Dbl homology), is essential for GEF activity and is conserved in all RhoGEFs of the Dbl family, which includes 69 members in humans.Citation9 In the course of the exchange reaction, the DH domain drives the displacement of GDP from the inactive GTPase. The subsequent step, the addition of GTP to the GTPase, is promoted by the high intracellular ratio of GTP over GDP. With the exception of three conserved regions (CR1, CR2, CR3), each 10–30 amino acid long, DH domains share limited sequence similarity with each other. Structure-function analyses have revealed that CR1, CR2, and CR3 form helical structures that, in the case of CR1 and CR3, are exposed to the surface of the RhoGEF and participate in formation of the Rho family GTPase binding pocket.Citation9, Citation245 Dbl-family GEFs typically possess a pleckstrin homology (PH) domain carboxy (C)-terminally adjacent to the catalytic DH domain. This DH-PH module is the minimal structural unit that can promote nucleotide exchange in vivo. Evidence suggests that PH domains can regulate GEF activity by direct modulation of DH domain function as well as by targeting of the RhoGEF to its proper intracellular location, likely via binding to membrane phospholipids.Citation246 However, the PH domains of several RhoGEFs have been shown to be dispensable for membrane localization, implicating other domains or motifs in proper protein targeting.Citation9, Citation247 Analyses of RhoGEF specificity have revealed that several RhoGEFs act exclusively on a single GTPase while others may have a broader spectrum of target GTPases. Trio-related GEFs share two DH-PH motifs of which the amino (N)-terminal DH domain induces nucleotide exchange on Rac GTPases whereas the C-terminal DH domain is specific for Rho1/RhoA. In addition to the DH-PH module Dbl-family GEFs contain a variety of conserved protein motifs that receive signals, mediate protein-protein interaction, regulate subcellular localization or modulate GEF activity. The DHR2/CZH family. A second family of RhoGEFs that is not related to the Dbl family GEFs has been described more recently.Citation10 Members of this so-called CZH (for CED-5/Dock180/Myoblast City (CDM)-Zizimin Homology) family share two highly conserved domains, designated CZH1 and CZH2 or Dock-homology region-1 and -2 (DHR1 and DHR2), of which the CZH2/DHR2 domain has been implicated in regulating nucleotide exchange on Rho family GTPases.Citation248 The DHR2/CZH family has been subdivided into the Dock-A to Dock-D groups of which Dock-A encompasses the prototypical members of this family CED-5/Dock180/Myoblast City, which have been shown to function upstream of Rac1 in several contexts.Citation194 In addition to the family typical DHR1 and DHR2 domains Dock-A GEFs posses an N-terminal SH3 domain and a C-terminal PxxP motif that is also found in Dock-B GEFs and mediates interaction with other factors such as the adaptor protein Crk. Members of the Dock-D group have an N-terminal Pleckstrin homology (PH) domain
Acknowledgements
Work in our laboratory is supported by grants from the Swedish Research Council and the Swedish Cancer Society to U.H.
References
- dos Remedios CG, Chhabra D, Kekic M, Dedova IV, Tsubakihara M, Berry DA, et al. Actin binding proteins: regulation of cytoskeletal microfilaments. Physiol Rev 2003; 83:433 - 473
- Jaffe AB, Hall A. Rho GTPases: biochemistry and biology. Annu Rev Cell Dev Biol 2005; 21:247 - 269
- Ridley AJ, Hall A. The small GTP-binding protein rho regulates the assembly of focal adhesions and actin stress fibers in response to growth factors. Cell 1992; 70:389 - 399
- Ridley AJ, Paterson HF, Johnston CL, Diekmann D, Hall A. The small GTP-binding protein rac regulates growth factor-induced membrane ruffling. Cell 1992; 70:401 - 410
- Etienne-Manneville S, Hall A. Rho GTPases in cell biology. Nature 2002; 420:629 - 635
- Van Aelst L, Symons M. Role of Rho family GTPases in epithelial morphogenesis. Genes Dev 2002; 16:1032 - 1054
- Bishop AL, Hall A. Rho GTPases and their effector proteins. Biochem J 2000; 348:Pt 2 241 - 255
- Bustelo XR, Sauzeau V, Berenjeno IM. GTP-binding proteins of the Rho/Rac family: regulation, effectors and functions in vivo. Bioessays 2007; 29:356 - 370
- Rossman KL, Der CJ, Sondek J. GEF means go: turning on RHO GTPases with guanine nucleotide-exchange factors. Nat Rev Mol Cell Biol 2005; 6:167 - 180
- Meller N, Merlot S, Guda C. CZH proteins: a new family of Rho-GEFs. J Cell Sci 2005; 118:4937 - 4946
- Settleman J. Rac 'n Rho: the music that shapes a developing embryo. Dev Cell 2001; 1:321 - 331
- Buchsbaum RJ. Rho activation at a glance. J Cell Sci 2007; 120:1149 - 1152
- Hakeda-Suzuki S, Ng J, Tzu J, Dietzl G, Sun Y, Harms M, et al. Rac function and regulation during Drosophila development. Nature 2002; 416:438 - 442
- Pertz OC, Wang Y, Yang F, Wang W, Gay LJ, Gristenko MA, et al. Spatial mapping of the neurite and soma proteomes reveals a functional Cdc42/Rac regulatory network. Proc Natl Acad Sci USA 2008; 105:1931 - 1936
- Tepass U, Tanentzapf G, Ward R, Fehon R. Epithelial cell polarity and cell junctions in Drosophila. Annu Rev Genet 2001; 35:747 - 784
- Schöck F, Perrimon N. Molecular mechanisms of epithelial morphogenesis. Annu Rev Cell Dev Biol 2002; 18:463 - 493
- Nelson WJ. Adaptation of core mechanisms to generate cell polarity. Nature 2003; 422:766 - 774
- Lecuit T, Lenne PF. Cell surface mechanics and the control of cell shape, tissue patterns and morphogenesis. Nat Rev Mol Cell Biol 2007; 8:633 - 644
- Kam Z, Minden JS, Agard DA, Sedat JW, Leptin M. Drosophila gastrulation: analysis of cell shape changes in living embryos by three-dimensional fluorescence microscopy. Development 1991; 112:365 - 370
- Sweeton D, Parks S, Costa M, Wieschaus E. Gastrulation in Drosophila: the formation of the ventral furrow and posterior midgut invaginations. Development 1991; 112:775 - 789
- Leptin M. Drosophila gastrulation: from pattern formation to morphogenesis. Annu Rev Cell Dev Biol 1995; 11:189 - 212
- Leptin M. Gastrulation movements: the logic and the nuts and bolts. Dev Cell 2005; 8:305 - 320
- Barrett K, Leptin M, Settleman J. The Rho GTPase and a putative RhoGEF mediate a signaling pathway for the cell shape changes in Drosophila gastrulation. Cell 1997; 91:905 - 915
- Häcker U, Perrimon N. DRhoGEF2 encodes a member of the Dbl family of oncogenes and controls cell shape changes during gastrulation in Drosophila. Genes Dev 1998; 12:274 - 284
- Hart MJ, Jiang X, Kozasa T, Roscoe W, Singer WD, Gilman AG, et al. Direct stimulation of the guanine nucleotide exchange activity of p115 RhoGEF by Galpha13. Science (New York, NY) 1998; 280:2112 - 2114
- Kozasa T, Jiang X, Hart MJ, Sternweis PM, Singer WD, Gilman AG, et al. p115 RhoGEF, a GTPase activating protein for Galpha12 and Galpha13. Science (New York, NY) 1998; 280:2109 - 2111
- Fukuhara S, Murga C, Zohar M, Igishi T, Gutkind JS. A novel PDZ domain containing guanine nucleotide exchange factor links heterotrimeric G proteins to Rho. J Biol Chem 1999; 274:5868 - 5879
- Fukuhara S, Chikumi H, Gutkind JS. Leukemia-associated Rho guanine nucleotide exchange factor (LARG) links heterotrimeric G proteins of the G(12) family to Rho. FEBS Lett 2000; 485:183 - 188
- Panizzi JR, Jessen JR, Drummond IA, Solnica-Krezel L. New functions for a vertebrate Rho guanine nucleotide exchange factor in ciliated epithelia. Development 2007; 134:921 - 931
- Yau DM, Yokoyama N, Goshima Y, Siddiqui ZK, Siddiqui SS, Kozasa T. Identification and molecular characterization of the G alpha12-Rho guanine nucleotide exchange factor pathway in Caenorhabditis elegans. Proc Natl Acad Sci USA 2003; 100:14748 - 14753
- Reuther GW, Lambert QT, Booden MA, Wennerberg K, Becknell B, Marcucci G, et al. Leukemia-associated Rho guanine nucleotide exchange factor, a Dbl family protein found mutated in leukemia, causes transformation by activation of RhoA. J Biol Chem 2001; 276:27145 - 27151
- Suzuki N, Nakamura S, Mano H, Kozasa T. Galpha 12 activates Rho GTPase through tyrosine-phosphorylated leukemia-associated RhoGEF. Proc Natl Acad Sci USA 2003; 100:733 - 738
- Derewenda U, Oleksy A, Stevenson AS, Korczynska J, Dauter Z, Somlyo AP, et al. The crystal structure of RhoA in complex with the DH/PH fragment of PDZRhoGEF, an activator of the Ca(2+) sensitization pathway in smooth muscle. Structure 2004; 12:1955 - 1965
- Kristelly R, Gao G, Tesmer JJ. Structural determinants of RhoA binding and nucleotide exchange in leukemia-associated Rho guanine-nucleotide exchange factor. J Biol Chem 2004; 279:47352 - 47362
- Oleksy A, Opalinski L, Derewenda U, Derewenda ZS, Otlewski J. The molecular basis of RhoA specificity in the guanine nucleotide exchange factor PDZ-RhoGEF. J Biol Chem 2006; 281:32891 - 32897
- Kosman D, Ip YT, Levine M, Arora K. Establishment of the mesoderm-neuroectoderm boundary in the Drosophila embryo. Science (New York, NY) 1991; 254:118 - 122
- Leptin M. twist and snail as positive and negative regulators during Drosophila mesoderm development. Genes Dev 1991; 5:1568 - 1576
- Ip YT, Park RE, Kosman D, Yazdanbakhsh K, Levine M. dorsal-twist interactions establish snail expression in the presumptive mesoderm of the Drosophila embryo. Genes Dev 1992; 6:1518 - 1530
- Costa M, Wilson ET, Wieschaus E. A putative cell signal encoded by the folded gastrulation gene coordinates cell shape changes during Drosophila gastrulation. Cell 1994; 76:1075 - 1089
- Morize P, Christiansen AE, Costa M, Parks S, Wieschaus E. Hyperactivation of the folded gastrulation pathway induces specific cell shape changes. Development 1998; 125:589 - 597
- Dawes-Hoang RE, Parmar KM, Christiansen AE, Phelps CB, Brand AH, Wieschaus EF. folded gastrulation, cell shape change and the control of myosin localization. Development 2005; 132:4165 - 4178
- Kölsch V, Seher T, Fernandez-Ballester GJ, Serrano L, Leptin M. Control of Drosophila gastrulation by apical localization of adherens junctions and RhoGEF2. Science (New York, NY) 2007; 315:384 - 386
- Parks S, Wieschaus E. The Drosophila gastrulation gene concertina encodes a G alpha-like protein. Cell 1991; 64:447 - 458
- Rogers SL, Wiedemann U, Häcker U, Turck C, Vale RD. Drosophila RhoGEF2 associates with microtubule plus ends in an EB1-dependent manner. Curr Biol 2004; 14:1827 - 1833
- Mizuno T, Amano M, Kaibuchi K, Nishida Y. Identification and characterization of Drosophila homolog of Rho-kinase. Gene 1999; 238:437 - 444
- Winter CG, Wang B, Ballew A, Royou A, Karess R, Axelrod JD, et al. Drosophila Rho-associated kinase (Drok) links Frizzled-mediated planar cell polarity signaling to the actin cytoskeleton. Cell 2001; 105:81 - 91
- Afshar K, Stuart B, Wasserman SA. Functional analysis of the Drosophila diaphanous FH protein in early embryonic development. Development 2000; 127:1887 - 1897
- Homem CC, Peifer M. Diaphanous regulates myosin and adherens junctions to control cell contractility and protrusive behavior during morphogenesis. Development 2008; 135:1005 - 1018
- Sahai E, Marshall CJ. ROCK and Dia have opposing effects on adherens junctions downstream of Rho. Nat Cell Biol 2002; 4:408 - 415
- Karess RE, Chang XJ, Edwards KA, Kulkarni S, Aguilera I, Kiehart DP. The regulatory light chain of nonmuscle myosin is encoded by spaghetti-squash, a gene required for cytokinesis in Drosophila. Cell 1991; 65:1177 - 1189
- Jordan P, Karess R. Myosin light chain-activating phosphorylation sites are required for oogenesis in Drosophila. J Cell Biol 1997; 139:1805 - 1819
- Tan JL, Ravid S, Spudich JA. Control of nonmuscle myosins by phosphorylation. Annu Rev Biochem 1992; 61:721 - 759
- Somlyo AP, Somlyo AV. Signal transduction by G-proteins, rho-kinase and protein phosphatase to smooth muscle and non-muscle myosin II. J Physiol 2000; 522:Pt 2 177 - 185
- Pilot F, Lecuit T. Compartmentalized morphogenesis in epithelia: from cell to tissue shape. Dev Dyn 2005; 232:685 - 694
- Nikolaidou KK, Barrett K. A Rho GTPase Signaling Pathway Is Used Reiteratively in Epithelial Folding and Potentially Selects the Outcome of Rho Activation. Curr Biol 2004; 14:1822 - 1826
- Mulinari S, Barmchi MP, Häcker U. DRhoGEF2 and Diaphanous Regulate Contractile Force during Segmental Groove Morphogenesis in the Drosophila Embryo. Mol Biol Cell 2008; 19:1883 - 1892
- Grevengoed EE, Fox DT, Gates J, Peifer M. Balancing different types of actin polymerization at distinct sites: roles for Abelson kinase and Enabled. J Cell Biol 2003; 163:1267 - 1279
- Fox DT, Peifer M. Abelson kinase (Abl) and RhoGEF2 regulate actin organization during cell constriction in Drosophila. Development 2007; 134:567 - 578
- Koleske AJ, Gifford AM, Scott ML, Nee M, Bronson RT, Miczek KA, et al. Essential roles for the Abl and Arg tyrosine kinases in neurulation. Neuron 1998; 21:1259 - 1272
- Gates J, Mahaffey JP, Rogers SL, Emerson M, Rogers EM, Sottile SL, et al. Enabled plays key roles in embryonic epithelial morphogenesis in Drosophila. Development 2007; 134:2027 - 2039
- Cox RT, Kirkpatrick C, Peifer M. Armadillo is required for adherens junction assembly, cell polarity, and morphogenesis during Drosophila embryogenesis. J Cell Biol 1996; 134:133 - 148
- Martin AC, Kaschube M, Wieschaus EF. Pulsed contractions of an actin-myosin network drive apical constriction. Nature 2009; 457:495 - 499
- Glotzer M. The molecular requirements for cytokinesis. Science (New York, NY) 2005; 307:1735 - 1739
- Kishi K, Sasaki T, Kuroda S, Itoh T, Takai Y. Regulation of cytoplasmic division of Xenopus embryo by rho p21 and its inhibitory GDP/GTP exchange protein (rho GDI). J Cell Biol 1993; 120:1187 - 1195
- Mabuchi I, Hamaguchi Y, Fujimoto H, Morii N, Mishima M, Narumiya S. A rho-like protein is involved in the organisation of the contractile ring in dividing sand dollar eggs. Zygote 1993; 1:325 - 331
- Jürgens G, Wieschaus E, Nusslein-Volhard C, Kluding H. Mutations affecting the pattern of the larval cuticle in Drosophila melanogaster. II. Zygotic loci on the third chromosome. Roux Arch Dev Biol 1984; 193:283 - 295
- Hime G, Saint R. Zygotic expression of the pebble locus is required for cytokinesis during the postblastoderm mitoses of Drosophila. Development 1992; 114:165 - 171
- Lehner CF. The pebble gene is required for cytokinesis in Drosophila. J Cell Sci 1992; 103:Pt 4 1021 - 1030
- Prokopenko SN, Brumby A, O'Keefe L, Prior L, He Y, Saint R, et al. A putative exchange factor for Rho1 GTPase is required for initiation of cytokinesis in Drosophila. Genes Dev 1999; 13:2301 - 2314
- Dechant R, Glotzer M. Centrosome separation and central spindle assembly act in redundant pathways that regulate microtubule density and trigger cleavage furrow formation. Dev Cell 2003; 4:333 - 344
- Morita K, Hirono K, Han M. The Caenorhabditis elegans ect-2 RhoGEF gene regulates cytokinesis and migration of epidermal P cells. EMBO Rep 2005; 6:1163 - 1168
- Tatsumoto T, Xie X, Blumenthal R, Okamoto I, Miki T. Human ECT2 is an exchange factor for Rho GTPases, phosphorylated in G2/M phases, and involved in cytokinesis. J Cell Biol 1999; 147:921 - 928
- Kimura K, Tsuji T, Takada Y, Miki T, Narumiya S. Accumulation of GTP-bound RhoA during cytokinesis and a critical role of ECT2 in this accumulation. J Biol Chem 2000; 275:17233 - 17236
- Somers WG, Saint R. A RhoGEF and Rho family GTPase-activating protein complex links the contractile ring to cortical microtubules at the onset of cytokinesis. Dev Cell 2003; 4:29 - 39
- Fields AP, Justilien V. The guanine nucleotide exchange factor (GEF) Ect2 is an oncogene in human cancer. Adv Enzyme Regul 2010; 50:190 - 200
- Miki T, Smith CL, Long JE, Eva A, Fleming TP. Oncogene ect2 is related to regulators of small GTP-binding proteins. Nature 1993; 362:462 - 465
- van Impel A, Schumacher S, Draga M, Herz HM, Grosshans J, Muller HA. Regulation of the Rac GTPase pathway by the multifunctional Rho GEF Pebble is essential for mesoderm migration in the Drosophila gastrula. Development 2009; 136:813 - 822
- Piekny A, Werner M, Glotzer M. Cytokinesis: welcome to the Rho zone. Trends Cell Biol 2005; 15:651 - 658
- Castrillon DH, Wasserman SA. Diaphanous is required for cytokinesis in Drosophila and shares domains of similarity with the products of the limb deformity gene. Development 1994; 120:3367 - 3377
- Severson AF, Baillie DL, Bowerman B. A Formin Homology protein and a profilin are required for cytokinesis and Arp2/3-independent assembly of cortical microfilaments in C. elegans. Curr Biol 2002; 12:2066 - 2075
- Chang F, Drubin D, Nurse P. cdc12p, a protein required for cytokinesis in fission yeast, is a component of the cell division ring and interacts with profilin. J Cell Biol 1997; 137:169 - 182
- Watanabe N, Madaule P, Reid T, Ishizaki T, Watanabe G, Kakizuka A, et al. p140mDia, a mammalian homolog of Drosophila diaphanous, is a target protein for Rho small GTPase and is a ligand for profilin. EMBO J 1997; 16:3044 - 3056
- Faix J, Grosse R. Staying in shape with formins. Dev Cell 2006; 10:693 - 706
- Albertson R, Cao J, Hsieh TS, Sullivan W. Vesicles and actin are targeted to the cleavage furrow via furrow microtubules and the central spindle. J Cell Biol 2008; 181:777 - 790
- Dean SO, Spudich JA. Rho kinase's role in myosin recruitment to the equatorial cortex of mitotic Drosophila S2 cells is for myosin regulatory light chain phosphorylation. PLoS ONE 2006; 1:e131
- Kosako H, Yoshida T, Matsumura F, Ishizaki T, Narumiya S, Inagaki M. Rho-kinase/ROCK is involved in cytokinesis through the phosphorylation of myosin light chain and not ezrin/radixin/moesin proteins at the cleavage furrow. Oncogene 2000; 19:6059 - 6064
- Piekny AJ, Mains PE. Rho-binding kinase (LET-502) and myosin phosphatase (MEL-11) regulate cytokinesis in the early Caenorhabditis elegans embryo. J Cell Sci 2002; 115:2271 - 2282
- Dean SO, Rogers SL, Stuurman N, Vale RD, Spudich JA. Distinct pathways control recruitment and maintenance of myosin II at the cleavage furrow during cytokinesis. Proc Natl Acad Sci USA 2005; 102:13473 - 13478
- Naim V, Imarisio S, Di Cunto F, Gatti M, Bonaccorsi S. Drosophila citron kinase is required for the final steps of cytokinesis. Mol Biol Cell 2004; 15:5053 - 5063
- Echard A, Hickson GR, Foley E, O'Farrell PH. Terminal cytokinesis events uncovered after an RNAi screen. Curr Biol 2004; 14:1685 - 1693
- D'Avino PP, Savoian MS, Glover DM. Mutations in sticky lead to defective organization of the contractile ring during cytokinesis and are enhanced by Rho and suppressed by Rac. J Cell Biol 2004; 166:61 - 71
- Di Cunto F, Imarisio S, Hirsch E, Broccoli V, Bulfone A, Migheli A, et al. Defective neurogenesis in citron kinase knockout mice by altered cytokinesis and massive apoptosis. Neuron 2000; 28:115 - 127
- D'Avino PP, Takeda T, Capalbo L, Zhang W, Lilley KS, Laue ED, et al. Interaction between Anillin and RacGAP50C connects the actomyosin contractile ring with spindle microtubules at the cell division site. J Cell Sci 2008; 121:1151 - 1158
- Piekny AJ, Glotzer M. Anillin is a scaffold protein that links RhoA, actin, and myosin during cytokinesis. Curr Biol 2008; 18:30 - 36
- Gregory SL, Ebrahimi S, Milverton J, Jones WM, Bejsovec A, Saint R. Cell division requires a direct link between microtubule-bound RacGAP and Anillin in the contractile ring. Curr Biol 2008; 18:25 - 29
- Bement WM, Benink HA, von Dassow G. A microtubule-dependent zone of active RhoA during cleavage plane specification. J Cell Biol 2005; 170:91 - 101
- Mishima M, Kaitna S, Glotzer M. Central spindle assembly and cytokinesis require a kinesin-like protein/RhoGAP complex with microtubule bundling activity. Dev Cell 2002; 2:41 - 54
- Powers J, Bossinger O, Rose D, Strome S, Saxton W. A nematode kinesin required for cleavage furrow advancement. Curr Biol 1998; 8:1133 - 1136
- Raich WB, Moran AN, Rothman JH, Hardin J. Cytokinesis and midzone microtubule organization in Caenorhabditis elegans require the kinesin-like protein ZEN-4. Mol Biol Cell 1998; 9:2037 - 2049
- Adams RR, Tavares AA, Salzberg A, Bellen HJ, Glover DM. pavarotti encodes a kinesin-like protein required to organize the central spindle and contractile ring for cytokinesis. Genes Dev 1998; 12:1483 - 1494
- Nislow C, Lombillo VA, Kuriyama R, McIntosh JR. A plus-end-directed motor enzyme that moves antiparallel microtubules in vitro localizes to the interzone of mitotic spindles. Nature 1992; 359:543 - 547
- Fontijn RD, Goud B, Echard A, Jollivet F, van Marle J, Pannekoek H, et al. The human kinesin-like protein RB6K is under tight cell cycle control and is essential for cytokinesis. Mol Cell Biol 2001; 21:2944 - 2955
- Toure A, Dorseuil O, Morin L, Timmons P, Jegou B, Reibel L, et al. MgcRacGAP, a new human GTPase-activating protein for Rac and Cdc42 similar to Drosophila rotundRacGAP gene product, is expressed in male germ cells. J Biol Chem 1998; 273:6019 - 6023
- D'Avino PP, Savoian MS, Capalbo L, Glover DM. RacGAP50C is sufficient to signal cleavage furrow formation during cytokinesis. J Cell Sci 2006; 119:4402 - 4408
- Hutterer A, Glotzer M, Mishima M. Clustering of centralspindlin is essential for its accumulation to the central spindle and the midbody. Curr Biol 2009; 19:2043 - 2049
- Zavortink M, Contreras N, Addy T, Bejsovec A, Saint R. Tum/RacGAP50C provides a critical link between anaphase microtubules and the assembly of the contractile ring in Drosophila melanogaster. J Cell Sci 2005; 118:5381 - 5392
- Zhao WM, Fang G. MgcRacGAP controls the assembly of the contractile ring and the initiation of cytokinesis. Proc Natl Acad Sci U S A 2005; 102:13158 - 13163
- Yuce O, Piekny A, Glotzer M. An ECT2-centralspindlin complex regulates the localization and function of RhoA. J Cell Biol 2005; 170:571 - 582
- Nishimura Y, Yonemura S. Centralspindlin regulates ECT2 and RhoA accumulation at the equatorial cortex during cytokinesis. J Cell Sci 2006; 119:104 - 114
- Kamijo K, Ohara N, Abe M, Uchimura T, Hosoya H, Lee JS, et al. Dissecting the role of Rho-mediated signaling in contractile ring formation. Mol Biol Cell 2006; 17:43 - 55
- Leptin M. Gastrulation in Drosophila: the logic and the cellular mechanisms. EMBO J 1999; 18:3187 - 3192
- Gryzik T, Muller HA. FGF8-like1 and FGF8-like2 encode putative ligands of the FGF receptor Htl and are required for mesoderm migration in the Drosophila gastrula. Curr Biol 2004; 14:659 - 667
- Stathopoulos A, Tam B, Ronshaugen M, Frasch M, Levine M. pyramus and thisbe: FGF genes that pattern the mesoderm of Drosophila embryos. Genes Dev 2004; 18:687 - 699
- Klingseisen A, Clark IB, Gryzik T, Muller HA. Differential and overlapping functions of two closely related Drosophila FGF8-like growth factors in mesoderm development. Development 2009; 136:2393 - 2402
- Kadam S, McMahon A, Tzou P, Stathopoulos A. FGF ligands in Drosophila have distinct activities required to support cell migration and differentiation. Development 2009; 136:739 - 747
- Shishido E, Higashijima S, Emori Y, Saigo K. Two FGF-receptor homologues of Drosophila: one is expressed in mesodermal primordium in early embryos. Development 1993; 117:751 - 761
- Beiman M, Shilo BZ, Volk T. Heartless, a Drosophila FGF receptor homolog, is essential for cell migration and establishment of several mesodermal lineages. Genes Dev 1996; 10:2993 - 3002
- Gisselbrecht S, Skeath JB, Doe CQ, Michelson AM. heartless encodes a fibroblast growth factor receptor (DFR1/DFGF-R2) involved in the directional migration of early mesodermal cells in the Drosophila embryo. Genes Dev 1996; 10:3003 - 3017
- Schumacher S, Gryzik T, Tannebaum S, Muller HA. The RhoGEF Pebble is required for cell shape changes during cell migration triggered by the Drosophila FGF receptor Heartless. Development 2004; 131:2631 - 2640
- Smallhorn M, Murray MJ, Saint R. The epithelial-mesenchymal transition of the Drosophila mesoderm requires the Rho GTP exchange factor Pebble. Development 2004; 131:2641 - 2651
- Munro E, Nance J, Priess JR. Cortical flows powered by asymmetrical contraction transport PAR proteins to establish and maintain anterior-posterior polarity in the early C. elegans embryo. Dev Cell 2004; 7:413 - 424
- Cheeks RJ, Canman JC, Gabriel WN, Meyer N, Strome S, Goldstein B. C. elegans PAR proteins function by mobilizing and stabilizing asymmetrically localized protein complexes. Curr Biol 2004; 14:851 - 862
- Cuenca AA, Schetter A, Aceto D, Kemphues K, Seydoux G. Polarization of the C. elegans zygote proceeds via distinct establishment and maintenance phases. Development 2003; 130:1255 - 1265
- Motegi F, Sugimoto A. Sequential functioning of the ECT-2 RhoGEF, RHO-1 and CDC-42 establishes cell polarity in Caenorhabditis elegans embryos. Nat Cell Biol 2006; 8:978 - 985
- Schonegg S, Hyman AA. CDC-42 and RHO-1 coordinate acto-myosin contractility and PAR protein localization during polarity establishment in C. elegans embryos. Development 2006; 133:3507 - 3516
- Jenkins N, Saam JR, Mango SE. CYK-4/GAP provides a localized cue to initiate anteroposterior polarity upon fertilization. Science (New York, NY) 2006; 313:1298 - 1301
- Govek EE, Newey SE, Van Aelst L. The role of the Rho GTPases in neuronal development. Genes Dev 2005; 19:1 - 49
- Koh CG. Rho GTPases and their regulators in neuronal functions and development. Neurosignals 2006; 15:228 - 237
- Lowery LA, Van Vactor D. The trip of the tip: understanding the growth cone machinery. Nat Rev Mol Cell Biol 2009; 10:332 - 343
- Shamah SM, Lin MZ, Goldberg JL, Estrach S, Sahin M, Hu L, et al. EphA receptors regulate growth cone dynamics through the novel guanine nucleotide exchange factor ephexin. Cell 2001; 105:233 - 244
- Swiercz JM, Kuner R, Behrens J, Offermanns S. Plexin-B1 directly interacts with PDZ-RhoGEF/LARG to regulate RhoA and growth cone morphology. Neuron 2002; 35:51 - 63
- Sahin M, Greer PL, Lin MZ, Poucher H, Eberhart J, Schmidt S, et al. Eph-dependent tyrosine phosphorylation of ephexin1 modulates growth cone collapse. Neuron 2005; 46:191 - 204
- Shi L, Butt B, Ip FC, Dai Y, Jiang L, Yung WH, et al. Ephexin1 is required for structural maturation and neurotransmission at the neuromuscular junction. Neuron 2010; 65:204 - 216
- Habets GG, Scholtes EH, Zuydgeest D, van der Kammen RA, Stam JC, Berns A, et al. Identification of an invasion-inducing gene, Tiam-1, that encodes a protein with homology to GDP-GTP exchangers for Rho-like proteins. Cell 1994; 77:537 - 549
- Michiels F, Habets GG, Stam JC, van der Kammen RA, Collard JG. A role for Rac in Tiam1-induced membrane ruffling and invasion. Nature 1995; 375:338 - 340
- Hoshino M, Sone M, Fukata M, Kuroda S, Kaibuchi K, Nabeshima Y, et al. Identification of the stef gene that encodes a novel guanine nucleotide exchange factor specific for Rac1. J Biol Chem 1999; 274:17837 - 17844
- Sone M. Still life, a protein in synaptic terminals of Drosophila homologous to GDP-GTP exchangers. Science (New York, NY) 1997; 275:1405
- Ng J, Luo L. Rho GTPases regulate axon growth through convergent and divergent signaling pathways. Neuron 2004; 44:779 - 793
- Ehler E, van Leeuwen F, Collard JG, Salinas PC. Expression of Tiam-1 in the developing brain suggests a role for the Tiam-1-Rac signaling pathway in cell migration and neurite outgrowth. Mol Cell Neurosci 1997; 9:1 - 12
- Yoshizawa M, Hoshino M, Sone M, Nabeshima Y. Expression of stef, an activator of Rac1, correlates with the stages of neuronal morphological development in the mouse brain. Mech Dev 2002; 113:65 - 68
- Leeuwen FN, Kain HE, Kammen RA, Michiels F, Kranenburg OW, Collard JG. The guanine nucleotide exchange factor Tiam1 affects neuronal morphology; opposing roles for the small GTPases Rac and Rho. J Cell Biol 1997; 139:797 - 807
- Kunda P, Paglini G, Quiroga S, Kosik K, Caceres A. Evidence for the involvement of Tiam1 in axon formation. J Neurosci 2001; 21:2361 - 2372
- Matsuo N, Hoshino M, Yoshizawa M, Nabeshima Y. Characterization of STEF, a guanine nucleotide exchange factor for Rac1, required for neurite growth. J Biol Chem 2002; 277:2860 - 2868
- Malliri A, van der Kammen RA, Clark K, van der Valk M, Michiels F, Collard JG. Mice deficient in the Rac activator Tiam1 are resistant to Ras-induced skin tumours. Nature 2002; 417:867 - 871
- Ueda S, Fujimoto S, Hiramoto K, Negishi M, Katoh H. Dock4 regulates dendritic development in hippocampal neurons. J Neurosci Res 2008; 86:3052 - 3061
- Watabe-Uchida M, John KA, Janas JA, Newey SE, Van Aelst L. The Rac activator DOCK7 regulates neuronal polarity through local phosphorylation of stathmin/Op18. Neuron 2006; 51:727 - 739
- Shin EY, Shin KS, Lee CS, Woo KN, Quan SH, Soung NK, et al. Phosphorylation of p85 beta PIX, a Rac/Cdc42-specific guanine nucleotide exchange factor, via the Ras/ERK/PAK2 pathway is required for basic fibroblast growth factor-induced neurite outgrowth. J Biol Chem 2002; 277:44417 - 44430
- Schmid RS, Midkiff BR, Kedar VP, Maness PF. Adhesion molecule L1 stimulates neuronal migration through Vav2-Pak1 signaling. Neuroreport 2004; 15:2791 - 2794
- Cowan CW, Shao YR, Sahin M, Shamah SM, Lin MZ, Greer PL, et al. Vav family GEFs link activated Ephs to endocytosis and axon guidance. Neuron 2005; 46:205 - 217
- Nishimura T, Yamaguchi T, Kato K, Yoshizawa M, Nabeshima Y, Ohno S, et al. PAR-6-PAR-3 mediates Cdc42-induced Rac activation through the Rac GEFs STEF/Tiam1. Nat Cell Biol 2005; 7:270 - 277
- Debant A, Serra-Pages C, Seipel K, O'Brien S, Tang M, Park SH, et al. The multidomain protein Trio binds the LAR transmembrane tyrosine phosphatase, contains a protein kinase domain, and has separate rac-specific and rho-specific guanine nucleotide exchange factor domains. Proc Natl Acad Sci USA 1996; 93:5466 - 5471
- Kawai T, Sanjo H, Akira S. Duet is a novel serine/threonine kinase with Dbl-Homology (DH) and Pleckstrin-Homology (PH) domains. Gene 1999; 227:249 - 255
- Johnson RC, Penzes P, Eipper BA, Mains RE. Isoforms of kalirin, a neuronal Dbl family member, generated through use of different 5'- and 3'-ends along with an internal translational initiation site. J Biol Chem 2000; 275:19324 - 19333
- Steven R, Kubiseski TJ, Zheng H, Kulkarni S, Mancillas J, Ruiz Morales A, et al. UNC-73 activates the Rac GTPase and is required for cell and growth cone migrations in C. elegans. Cell 1998; 92:785 - 795
- Blangy A, Vignal E, Schmidt S, Debant A, Gauthier-Rouviere C, Fort P. TrioGEF1 controls Rac- and Cdc42-dependent cell structures through the direct activation of rhoG. J Cell Sci 2000; 113:Pt 4 729 - 739
- Bellanger JM, Lazaro JB, Diriong S, Fernandez A, Lamb N, Debant A. The two guanine nucleotide exchange factor domains of Trio link the Rac1 and the RhoA pathways in vivo. Oncogene 1998; 16:147 - 152
- O'Brien SP, Seipel K, Medley QG, Bronson R, Segal R, Streuli M. Skeletal muscle deformity and neuronal disorder in Trio exchange factor-deficient mouse embryos. Proc Natl Acad Sci USA 2000; 97:12074 - 12078
- Ma XM, Kiraly DD, Gaier ED, Wang Y, Kim EJ, Levine ES, et al. Kalirin-7 is required for synaptic structure and function. J Neurosci 2008; 28:12368 - 12382
- Cahill ME, Xie Z, Day M, Barbolina MV, Miller CA, Weiss C, et al. Kalirin regulates cortical spine morphogenesis and disease-related behavioral phenotypes. Proc Natl Acad Sci USA 2009; 106:13058 - 13063
- Spencer AG, Orita S, Malone CJ, Han M. A RHO GTPase-mediated pathway is required during P cell migration in Caenorhabditis elegans. Proc Natl Acad Sci USA 2001; 98:13132 - 13137
- Steven R, Zhang L, Culotti J, Pawson T. The UNC-73/Trio RhoGEF-2 domain is required in separate isoforms for the regulation of pharynx pumping and normal neurotransmission in C. elegans. Genes Dev 2005; 19:2016 - 2029
- Williams SL, Lutz S, Charlie NK, Vettel C, Ailion M, Coco C, et al. Trio's Rho-specific GEF domain is the missing Galpha q effector in C. elegans. Genes Dev 2007; 21:2731 - 2746
- McPherson CE, Eipper BA, Mains RE. Genomic organization and differential expression of Kalirin isoforms. Gene 2002; 284:41 - 51
- Portales-Casamar E, Briancon-Marjollet A, Fromont S, Triboulet R, Debant A. Identification of novel neuronal isoforms of the Rho-GEF Trio. Biol Cell 2006; 98:183 - 193
- Awasaki T, Saito M, Sone M, Suzuki E, Sakai R, Ito K, et al. The Drosophila trio plays an essential role in patterning of axons by regulating their directional extension. Neuron 2000; 26:119 - 131
- Newsome TP, Schmidt S, Dietzl G, Keleman K, Asling B, Debant A, et al. Trio combines with dock to regulate Pak activity during photoreceptor axon pathfinding in Drosophila. Cell 2000; 101:283 - 294
- Liebl EC, Forsthoefel DJ, Franco LS, Sample SH, Hess JE, Cowger JA, et al. Dosage-sensitive, reciprocal genetic interactions between the Abl tyrosine kinase and the putative GEF trio reveal trio's role in axon pathfinding. Neuron 2000; 26:107 - 118
- Bateman J, Shu H, Van Vactor D. The guanine nucleotide exchange factor trio mediates axonal development in the Drosophila embryo. Neuron 2000; 26:93 - 106
- Forsthoefel DJ, Liebl EC, Kolodziej PA, Seeger MA. The Abelson tyrosine kinase, the Trio GEF and Enabled interact with the Netrin receptor Frazzled in Drosophila. Development 2005; 132:1983 - 1994
- Honigberg L, Kenyon C. Establishment of left/right asymmetry in neuroblast migration by UNC-40/DCC, UNC-73/Trio and DPY-19 proteins in C. elegans. Development 2000; 127:4655 - 4668
- Briancon-Marjollet A, Ghogha A, Nawabi H, Triki I, Auziol C, Fromont S, et al. Trio mediates netrin-1-induced Rac1 activation in axon outgrowth and guidance. Mol Cell Biol 2008; 28:2314 - 2323
- Yang L, Bashaw GJ. Son of sevenless directly links the Robo receptor to rac activation to control axon repulsion at the midline. Neuron 2006; 52:595 - 607
- Bashaw GJ, Hu H, Nobes CD, Goodman CS. A novel Dbl family RhoGEF promotes Rho-dependent axon attraction to the central nervous system midline in Drosophila and overcomes Robo repulsion. J Cell Biol 2001; 155:1117 - 1122
- Pawson C, Eaton BA, Davis GW. Formin-dependent synaptic growth: evidence that Dlar signals via Diaphanous to modulate synaptic actin and dynamic pioneer microtubules. J Neurosci 2008; 28:11111 - 11123
- Henson PM. Engulfment: ingestion and migration with Rac, Rho and TRIO. Curr Biol 2005; 15:R29 - R30
- Lucanic M, Kiley M, Ashcroft N, L'Etoile N, Cheng HJ. The Caenorhabditis elegans P21-activated kinases are differentially required for UNC-6/netrin-mediated commissural motor axon guidance. Development 2006; 133:4549 - 4559
- Watari-Goshima N, Ogura K, Wolf FW, Goshima Y, Garriga G. C. elegans VAB-8 and UNC-73 regulate the SAX-3 receptor to direct cell and growth-cone migrations. Nat Neurosci 2007; 10:169 - 176
- Levy-Strumpf N, Culotti JG. VAB-8, UNC-73 and MIG-2 regulate axon polarity and cell migration functions of UNC-40 in C. elegans. Nat Neurosci 2007; 10:161 - 168
- Vanderzalm PJ, Pandey A, Hurwitz ME, Bloom L, Horvitz HR, Garriga G. C. elegans CARMIL negatively regulates UNC-73/Trio function during neuronal development. Development 2009; 136:1201 - 1210
- Lamaze C, Chuang TH, Terlecky LJ, Bokoch GM, Schmid SL. Regulation of receptor-mediated endocytosis by Rho and Rac. Nature 1996; 382:177 - 179
- Montell DJ. Border-cell migration: the race is on. Nat Rev Mol Cell Biol 2003; 4:13 - 24
- Rorth P. Initiating and guiding migration: lessons from border cells. Trends Cell Biol 2002; 12:325 - 331
- Bianco A, Poukkula M, Cliffe A, Mathieu J, Luque CM, Fulga TA, et al. Two distinct modes of guidance signalling during collective migration of border cells. Nature 2007; 448:362 - 365
- Prasad M, Montell DJ. Cellular and molecular mechanisms of border cell migration analyzed using timelapse live-cell imaging. Dev Cell 2007; 12:997 - 1005
- Fulga TA, Rorth P. Invasive cell migration is initiated by guided growth of long cellular extensions. Nat Cell Biol 2002; 4:715 - 719
- Duchek P, Rorth P. Guidance of cell migration by EGF receptor signaling during Drosophila oogenesis. Science (New York, NY) 2001; 291:131 - 133
- Duchek P, Somogyi K, Jekely G, Beccari S, Rorth P. Guidance of cell migration by the Drosophila PDGF/VEGF receptor. Cell 2001; 107:17 - 26
- McDonald JA, Pinheiro EM, Montell DJ. PVF1, a PDGF/VEGF homolog, is sufficient to guide border cells and interacts genetically with Taiman. Development 2003; 130:3469 - 3478
- McDonald JA, Pinheiro EM, Kadlec L, Schupbach T, Montell DJ. Multiple EGFR ligands participate in guiding migrating border cells. Dev Biol 2006; 296:94 - 103
- Hasegawa H, Kiyokawa E, Tanaka S, Nagashima K, Gotoh N, Shibuya M, et al. DOCK180, a major CRK-binding protein, alters cell morphology upon translocation to the cell membrane. Mol Cell Biol 1996; 16:1770 - 1776
- Wu YC, Horvitz HR. C. elegans phagocytosis and cell-migration protein CED-5 is similar to human DOCK180. Nature 1998; 392:501 - 504
- Postner MA, Miller KG, Wieschaus EF. Maternal effect mutations of the sponge locus affect actin cytoskeletal rearrangements in Drosophila melanogaster embryos. J Cell Biol 1992; 119:1205 - 1218
- Yajnik V, Paulding C, Sordella R, McClatchey AI, Saito M, Wahrer DC, et al. DOCK4, a GTPase activator, is disrupted during tumorigenesis. Cell 2003; 112:673 - 684
- Cote JF, Vuori K. GEF what? Dock180 and related proteins help Rac to polarize cells in new ways. Trends Cell Biol 2007; 17:383 - 393
- Rushton E, Drysdale R, Abmayr SM, Michelson AM, Bate M. Mutations in a novel gene, myoblast city, provide evidence in support of the founder cell hypothesis for Drosophila muscle development. Development 1995; 121:1979 - 1988
- Erickson MR, Galletta BJ, Abmayr SM. Drosophila myoblast city encodes a conserved protein that is essential for myoblast fusion, dorsal closure, and cytoskeletal organization. J Cell Biol 1997; 138:589 - 603
- Nolan KM, Barrett K, Lu Y, Hu KQ, Vincent S, Settleman J. Myoblast city, the Drosophila homolog of DOCK180/CED-5, is required in a Rac signaling pathway utilized for multiple developmental processes. Genes Dev 1998; 12:3337 - 3342
- Ishimaru S, Ueda R, Hinohara Y, Ohtani M, Hanafusa H. PVR plays a critical role via JNK activation in thorax closure during Drosophila metamorphosis. EMBO J 2004; 23:3984 - 3994
- Brugnera E, Haney L, Grimsley C, Lu M, Walk SF, Tosello-Trampont AC, et al. Unconventional Rac-GEF activity is mediated through the Dock180-ELMO complex. Nat Cell Biol 2002; 4:574 - 582
- Murphy AM, Montell DJ. Cell type-specific roles for Cdc42, Rac, and RhoL in Drosophila oogenesis. J Cell Biol 1996; 133:617 - 630
- Mayer BJ, Hamaguchi M, Hanafusa H. A novel viral oncogene with structural similarity to phospholipase C. Nature 1988; 332:272 - 275
- Galletta BJ, Niu XP, Erickson MR, Abmayr SM. Identification of a Drosophila homologue to vertebrate Crk by interaction with MBC. Gene 1999; 228:243 - 252
- Balagopalan L, Chen MH, Geisbrecht ER, Abmayr SM. The CDM superfamily protein MBC directs myoblast fusion through a mechanism that requires phosphatidylinositol 3,4,5-triphosphate binding but is independent of direct interaction with DCrk. Mol Cell Biol 2006; 26:9442 - 9455
- Franca-Koh J, Kamimura Y, Devreotes PN. Leading-edge research: PtdIns(3,4,5)P3 and directed migration. Nat Cell Biol 2007; 9:15 - 17
- Kobayashi S, Shirai T, Kiyokawa E, Mochizuki N, Matsuda M, Fukui Y. Membrane recruitment of DOCK180 by binding to PtdIns(3,4,5)P3. Biochem J 2001; 354:73 - 78
- Cote JF, Motoyama AB, Bush JA, Vuori K. A novel and evolutionarily conserved PtdIns(3,4,5)P3-binding domain is necessary for DOCK180 signalling. Nat Cell Biol 2005; 7:797 - 807
- Reddien PW, Horvitz HR. CED-2/CrkII and CED-10/Rac control phagocytosis and cell migration in Caenorhabditis elegans. Nat Cell Biol 2000; 2:131 - 136
- Gumienny TL, Brugnera E, Tosello-Trampont AC, Kinchen JM, Haney LB, Nishiwaki K, et al. CED-12/ELMO, a novel member of the CrkII/Dock180/Rac pathway, is required for phagocytosis and cell migration. Cell 2001; 107:27 - 41
- Zhou Z, Caron E, Hartwieg E, Hall A, Horvitz HR. The C. elegans PH domain protein CED-12 regulates cytoskeletal reorganization via a Rho/Rac GTPase signaling pathway. Dev Cell 2001; 1:477 - 489
- Wu YC, Tsai MC, Cheng LC, Chou CJ, Weng NY. C. elegans CED-12 acts in the conserved crkII/DOCK180/Rac pathway to control cell migration and cell corpse engulfment. Dev Cell 2001; 1:491 - 502
- Wang X, Wang J, Gengyo-Ando K, Gu L, Sun CL, Yang C, et al. C. elegans mitochondrial factor WAH-1 promotes phosphatidylserine externalization in apoptotic cells through phospholipid scramblase SCRM-1. Nat Cell Biol 2007; 9:541 - 549
- Wang X, Wu YC, Fadok VA, Lee MC, Gengyo-Ando K, Cheng LC, et al. Cell corpse engulfment mediated by C. elegans phosphatidylserine receptor through CED-5 and CED-12. Science (New York, NY) 2003; 302:1563 - 1566
- deBakker CD, Haney LB, Kinchen JM, Grimsley C, Lu M, Klingele D, et al. Phagocytosis of apoptotic cells is regulated by a UNC-73/TRIO-MIG-2/RhoG signaling module and armadillo repeats of CED-12/ELMO. Curr Biol 2004; 14:2208 - 2216
- van den Eijnde SM, Boshart L, Baehrecke EH, De Zeeuw CI, Reutelingsperger CP, Vermeij-Keers C. Cell surface exposure of phosphatidylserine during apoptosis is phylogenetically conserved. Apoptosis 1998; 3:9 - 16
- Fadok VA, Bratton DL, Rose DM, Pearson A, Ezekewitz RA, Henson PM. A receptor for phosphatidylserine-specific clearance of apoptotic cells. Nature 2000; 405:85 - 90
- Li MO, Sarkisian MR, Mehal WZ, Rakic P, Flavell RA. Phosphatidylserine receptor is required for clearance of apoptotic cells. Science (New York, NY) 2003; 302:1560 - 1563
- Albert ML, Kim JI, Birge RB. alphavbeta5 integrin recruits the CrkII-Dock180-rac1 complex for phagocytosis of apoptotic cells. Nat Cell Biol 2000; 2:899 - 905
- Fullard JF, Kale A, Baker NE. Clearance of apoptotic corpses. Apoptosis 2009; 14:1029 - 1037
- Pollard TD, Borisy GG. Cellular motility driven by assembly and disassembly of actin filaments. Cell 2003; 112:453 - 465
- Bompard G, Caron E. Regulation of WASP/WAVE proteins: making a long story short. J Cell Biol 2004; 166:957 - 962
- Insall RH, Machesky LM. Actin dynamics at the leading edge: from simple machinery to complex networks. Dev Cell 2009; 17:310 - 322
- Wallar BJ, Alberts AS. The formins: active scaffolds that remodel the cytoskeleton. Trends Cell Biol 2003; 13:435 - 446
- Peng J, Wallar BJ, Flanders A, Swiatek PJ, Alberts AS. Disruption of the Diaphanous-related formin Drf1 gene encoding mDia1 reveals a role for Drf3 as an effector for Cdc42. Curr Biol 2003; 13:534 - 545
- Yasuda S, Oceguera-Yanez F, Kato T, Okamoto M, Yonemura S, Terada Y, et al. Cdc42 and mDia3 regulate microtubule attachment to kinetochores. Nature 2004; 428:767 - 771
- Ji P, Jayapal SR, Lodish HF. Enucleation of cultured mouse fetal erythroblasts requires Rac GTPases and mDia2. Nat Cell Biol 2008; 10:314 - 321
- Pruyne D, Evangelista M, Yang C, Bi E, Zigmond S, Bretscher A, et al. Role of formins in actin assembly: nucleation and barbed-end association. Science (New York, NY) 2002; 297:612 - 615
- Schirenbeck A, Bretschneider T, Arasada R, Schleicher M, Faix J. The Diaphanous-related formin dDia2 is required for the formation and maintenance of filopodia. Nat Cell Biol 2005; 7:619 - 625
- Williams MJ, Habayeb MS, Hultmark D. Reciprocal regulation of Rac1 and Rho1 in Drosophila circulating immune surveillance cells. J Cell Sci 2007; 120:502 - 511
- Leung T, Chen XQ, Manser E, Lim L. The p160 RhoA-binding kinase ROK alpha is a member of a kinase family and is involved in the reorganization of the cytoskeleton. Mol Cell Biol 1996; 16:5313 - 5327
- Riento K, Ridley AJ. Rocks: multifunctional kinases in cell behaviour. Nat Rev Mol Cell Biol 2003; 4:446 - 456
- Amano M, Ito M, Kimura K, Fukata Y, Chihara K, Nakano T, et al. Phosphorylation and activation of myosin by Rho-associated kinase (Rho-kinase). J Biol Chem 1996; 271:20246 - 20249
- Kimura K, Ito M, Amano M, Chihara K, Fukata Y, Nakafuku M, et al. Regulation of myosin phosphatase by Rho and Rho-associated kinase (Rho-kinase). Science (New York, NY) 1996; 273:245 - 248
- Kawano Y, Fukata Y, Oshiro N, Amano M, Nakamura T, Ito M, et al. Phosphorylation of myosin-binding subunit (MBS) of myosin phosphatase by Rho-kinase in vivo. J Cell Biol 1999; 147:1023 - 1038
- Ohashi K, Nagata K, Maekawa M, Ishizaki T, Narumiya S, Mizuno K. Rho-associated kinase ROCK activates LIM-kinase 1 by phosphorylation at threonine 508 within the activation loop. J Biol Chem 2000; 275:3577 - 3582
- Fukata Y, Oshiro N, Kinoshita N, Kawano Y, Matsuoka Y, Bennett V, et al. Phosphorylation of adducin by Rho-kinase plays a crucial role in cell motility. J Cell Biol 1999; 145:347 - 361
- Matsui T, Maeda M, Doi Y, Yonemura S, Amano M, Kaibuchi K, et al. Rho-kinase phosphorylates COOH-terminal threonines of ezrin/radixin/moesin (ERM) proteins and regulates their head-to-tail association. J Cell Biol 1998; 140:647 - 657
- Maekawa M, Ishizaki T, Boku S, Watanabe N, Fujita A, Iwamatsu A, et al. Signaling from Rho to the actin cytoskeleton through protein kinases ROCK and LIM-kinase. Science (New York, NY) 1999; 285:895 - 898
- Bernard O. Lim kinases, regulators of actin dynamics. Int J Biochem Cell Biol 2007; 39:1071 - 1076
- Edwards DC, Sanders LC, Bokoch GM, Gill GN. Activation of LIM-kinase by Pak1 couples Rac/Cdc42 GTPase signalling to actin cytoskeletal dynamics. Nat Cell Biol 1999; 1:253 - 259
- Matsuoka Y, Li X, Bennett V. Adducin: structure, function and regulation. Cell Mol Life Sci 2000; 57:884 - 895
- Hughes SC, Fehon RG. Understanding ERM proteins—the awesome power of genetics finally brought to bear. Curr Opin Cell Biol 2007; 19:51 - 56
- Eva A, Aaronson SA. Isolation of a new human oncogene from a diffuse B-cell lymphoma. Nature 1985; 316:273 - 275
- Hart MJ, Eva A, Evans T, Aaronson SA, Cerione RA. Catalysis of guanine nucleotide exchange on the CDC42Hs protein by the dbl oncogene product. Nature 1991; 354:311 - 314
- Hart MJ, Eva A, Zangrilli D, Aaronson SA, Evans T, Cerione RA, et al. Cellular transformation and guanine nucleotide exchange activity are catalyzed by a common domain on the dbl oncogene product. J Biol Chem 1994; 269:62 - 65
- Bos JL, Rehmann H, Wittinghofer A. GEFs and GAPs: critical elements in the control of small G proteins. Cell 2007; 129:865 - 877
- Schmidt A, Hall A. Guanine nucleotide exchange factors for Rho GTPases: turning on the switch. Genes Dev 2002; 16:1587 - 1609
- Snyder JT, Rossman KL, Baumeister MA, Pruitt WM, Siderovski DP, Der CJ, et al. Quantitative analysis of the effect of phosphoinositide interactions on the function of Dbl family proteins. J Biol Chem 2001; 276:45868 - 45875
- Yang J, Zhang Z, Roe SM, Marshall CJ, Barford D. Activation of Rho GTPases by DOCK exchange factors is mediated by a nucleotide sensor. Science (New York, NY) 2009; 325:1398 - 1402