Abstract
Scribble is a potential tumour suppressor protein, whose loss is a frequent event in late stage cancer development. In both Drosophila and mammalian model systems, Scribble has been shown capable of regulating cell polarity, cell proliferation and apoptosis. Although several interacting partners, including βPiX, have been identified that help to explain how Scribble can regulate cell polarity and migration, little is known about how Scribble can control cell proliferation. Recent work from our laboratory has shown that Scribble can directly regulate the ERK signaling pathway. This is mediated by a direct protein-protein interaction between Scribble and ERK, which has two components. In the first, Scribble appears to anchor ERK at membrane-bound sites, with the loss of Scribble enhancing ERK nuclear translocation. In the second, Scribble can decrease the levels of active phosphorylated ERK, a function that is dependent upon the ability of Scribble to bind ERK directly. One of the consequences of this activity of Scribble is the inhibition of EJ-ras induced cell transformation. These results provide some of the first direct mechanistic information on how Scribble can regulate cell proliferation and, furthermore, they provide indications as to the identity of other signaling intermediates that may be recruited by Scribble to directly regulate mitogenic signaling pathways.
The first evidence linking cell polarity and cell proliferation control came from studies in Drosophila and Caenorhabditis elegans, and provided some of the first indications of the potential functional relevance of this link in the development of human tumors. These studies identified three multi-protein complexes as central to the control of this pathway: the Par complex, the Scribble complex and the Crumbs complex.Citation1–Citation4 In Drosophila these proteins cooperate in the regulation of polarity and proliferation.Citation1,Citation5,Citation6 In humans, the functions of these proteins are less clear, although several studies have reported that the loss or overexpression of certain components of the network is linked to cancer development.Citation7,Citation8 As we will see in the following discussion, the molecular basis for some of these observations, are beginning to be unraveled. Studies with human tumor viruses have provided further compelling evidence of potential tumor suppressor functions for the cell polarity regulatory module. For example, the high-risk Human Papillomaviruses (HPVs), which are the causative agents of cervical cancer, specifically target the Scribble complex;Citation9 with the HPV-16 E6 oncoprotein degrading Scribble, whereas the HPV-18 E6 oncoprotein degrades Discs Large (Dlg), another component of the complex.Citation10 Perhaps most importantly, HPV-16 is the cause of over 60% of all cervical cancers,Citation11 and this particular link to Scribble is therefore intriguing. Interestingly, in the evolutionarily-related Rhesus Papillomavirus (RhPV), which causes cervical cancer in the Rhesus macaque, this complex is also targeted, but in this case Par3 is the viral substrate.Citation12 These studies suggest that the pathways of polarity and proliferation control that are regulated by the Scribble and Par complexes are functionally relevant for Papillomavirus-induced cervical cancers.
The Scribble protein is depicted schematically in , and it comprises 1,630 amino acids. It is classified as a LAP family member, and contains a series of 16 leucine rich repeats (LRRs) at the N terminus, which are required for correct localization of Scribble to the basolateral membrane, and these are followed by 4 PDZ (PSD95/Dlg/ZO-1) domains that function as sites of protein-protein interactions.Citation13,Citation14 Indeed, Scribble is localized primarily at sites of cell-cell contact, where it is proposed to act as a molecular scaffold. Loss of Scribble expression has been reported in colon, breast and uterine tumors,Citation15–Citation17 and the human equivalent can functionally replace the Drosophila homologue, resulting in a restoration of the normal polarity and proliferation controls that are lost in Scribble-null flies.Citation18 One of the major functions of Scribble in human cells is the regulation of cell polarity and motility, which seems to be achieved, in part, through its association with the βPiX.Citation19 βPix is a Guanine nucleotide exchange factor (GEF) for the Rac/Cdc42 small GTPase complex,Citation20 and thereby directly regulates actin remodeling in response to various stimulatory cues.Citation21–Citation23 Through this interaction, Scribble controls Cdc42 localisation during cell migration.Citation24 Scribble also has the capacity to regulate apoptotic signaling in mammary epithelial cells and in Drosophila eye imaginal discs, where, depending on the particular experimental setting, it can exhibit both pro-and anti-apoptotic potential.Citation25,Citation26
We were intrigued by previous studies that had linked Scribble to the control of mitogenic signaling pathways; with loss of Scribble clearly contributing to oncogenic Ras-induced cell transformation, both in Drosophila and in mammalian cells.Citation26–Citation28 Analysis of the Scribble sequence revealed the existence of two perfect kinase interaction motifs (KIM sites; see ), suggesting that Scribble had the propensity to interact with extracellular signal-related kinase (ERK). In our study, we showed that Scribble is a strong substrate for phosphorylation by ERK and also by protein kinase A (PKA), consistent with recent studies showing that Scribble is heavily phosphorylated at these sites, and also elsewhere in the molecule.Citation29 Most importantly, however, we found that Scribble forms a strong direct interaction with ERK that is absolutely dependent upon the integrity of the two identified KIM sites, although the interaction with the carboxy terminal site is much stronger. In a number of different experimental settings, we verified that Scribble could directly regulate ERK activation, as determined by the level of active phosphorylated ERK expressed in cells in the presence or absence of Scribble; and we demonstrated the importance of the KIM sites for this regulation. Thus, under circumstances where Scribble can no longer interact with ERK, we observed an increase both in the levels of ERK nuclear translocation, and in the levels of phosphorylated active ERK. This activity of Scribble also correlated with the capacity of Scribble to inhibit oncogenic Ras-induced cell transformation in an oncogene cooperation assay.
These studies now raise a number of interesting issues. Obviously, the prevalence of potential regulatory phosphoacceptor sites on Scribble is worthy of further investigation. It was intriguing that we found an apparent link between the two very closely located ERK and PKA phospho-sites at S1445 and S1448, respectively. Under no circumstances were we able to observe dual phosphorylation of both sites in vivo, suggesting that one is mutually exclusive of the other. This suggests an important regulatory function, and our current studies are using proteomic approaches to analyse changes in the capacity of the different phosphorylated forms of Scribble to interact with different cellular targets. However, phosphorylation seems to have an effect on the pattern of Scribble expression within cells, suggesting that differential phosphorylation at these sites may affect Scribble's localization, and hence its function. In addition, it has been shown that the activation of Cdc42, which controls many aspects of cell polarization at the leading edge,Citation21,Citation30,Citation31 in turn requires the Scribble PDZ domains (implying βPiX involvement) and the Scribble carboxy-terminal region.Citation24 This suggests that the association of Scribble with ERK through the carboxy terminal KIM site may contribute both to the regulation of directional cell migration, and to the maintenance of cellular polarity. Finally, these studies also raise important questions from an HPV E6 point-of-view. We had previously shown that Dlg was rendered more susceptible to HPV E6 targeting as a result of certain phospho-modifications.Citation32 Studies are now ongoing to determine whether similar modifications of Scribble might also affect its susceptibility to HPV oncoprotein targeting, which might have important implications for the capacity of the virus to induce malignancy.
Since potential regulation of the ERK signaling cascade by Scribble has been reported in Drosophila and in mammalian cells, it is worth considering the degree of evolutionary conservation in the Scribble KIM sites and the corresponding phospho-acceptor sites. As can be seen from , the carboxy terminal KIM site is conserved through mammals to birds. However, this site is absent in Drosophila, as is the corresponding ERK phosphoacceptor site. Conversely, the N terminal KIM site is conserved in mammals, but is absent in birds, and there is only a weak consensus KIM site in Drosophila. The loss of Scribble is known to cooperate with an activated ERK signaling pathway to induce cell transformation in Drosophila. However the differences between flies and mammals suggest that this may not occur through the same mechanisms as are seen in mammalian cells. In Drosophila the loss of Scribble affecting the levels of JNK activation may be the more likely cause of these phenotypes.Citation26,Citation33 Finally, it is also worth noting that the PKA phospho-acceptor site is unique to human Scribble, and this suggests a further fine-tuning of Scribble function in human cells, which does not occur in other organisms. How relevant this may be to potential tumor suppressor mechanisms remains to be determined, but this is an interesting possibility.
Our studies have demonstrated that Scribble can inhibit the activation of ERK. We proposed two possible mechanisms by which Scribble might achieve this: either by inhibition of ERK phosphorylation by upstream kinases, or by recruitment of a protein phosphatase that can directly inhibit ERK phosphorylation. We favor the latter hypothesis, as we consistently observe that Scribble can reduce ERK phospho-levels below base-line, suggesting an active de-phosphorylation of ERK, as opposed to inhibition of ERK phosphorylation. Studies are currently under way to identify potential phosphatase-bound partners of Scribble, and our preliminary proteomic analyses have identified several such candidates. Thus, one could propose that Scribble can interact with ERK at defined sites within the cell, and at the same time recruit a protein phosphatase which, in turn, can regulate levels of ERK activation; a schematic model of this is shown in . Should this prove to be the mechanism by which Scribble can control ERK activation, this also has potentially important implications for some of Scribble's other interacting partners, a number of which are listed in . Perhaps one of the most important of these is βPix, which is itself intimately linked to the Ras/ERK signaling pathway.Citation34 Therefore Scribble could conceivably act as a platform for phosphatase recruitment to different signaling components, and thus exert modulatory activities in a direct fashion, rather than acting in the rather static scaffolding manner that has often been proposed. Further studies will be aimed at further defining the role of Scribble in these signaling complexes.
Figures and Tables
Figure 1 Schematic diagram showing the human Scribble protein. The major functional domains on Scribble are shown including the Leucine Rich Repeats (LRRS) and the 4 PDZ domains. Also shown are the N and C terminal KIM sites and their corresponding phospho-acceptor sites (S853 and S1448), together with the location of the PKA phospho-acceptor site (S1445).
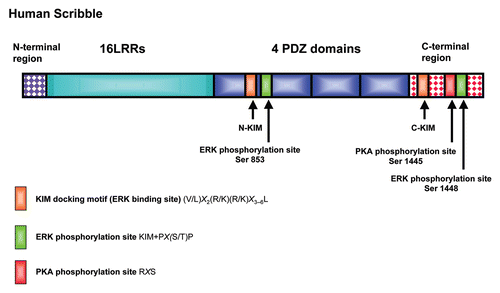
Figure 2 Scribble integrates signaling pathways to control polarized cell migration, cell proliferation and cell invasion. Scribble interacts directly with ERK and βPix. It controls directional migration through regulating the activity of Cdc42 via βPix and also controls the ERK signaling cascade through controlling ERK activity and localisation. This activity of Scribble has implications for controlling cell proliferation and invasion, and we propose that Scribble regulation of ERK is achieved by the direct recruitment of a phosphatase by Scribble. The consequences of ERK phosphorylation of Scribble at S853 and S1448 are currently unknown.
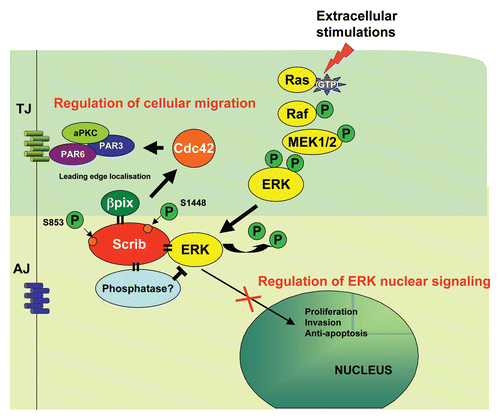
Table 1 Sequence alignments of the scribble protein from different species
Table 2 Known interacting partners of scribble and their sites of interaction on the scribble protein
Acknowledgements
Work in the LB laboratory is supported by research grants from the Associazione Italiana per la Ricerca sul Cancro, Association for International Cancer Research and Telethon. K.N. was supported by the Yoshida (YKK) Scholarships Foundation. We are grateful to Miranda Thomas for comments on the manuscript.
Extra View to: Nagasaka K, Pim D, Massimi P, Thomas M, Tomaic V, Subbaiah VK, et al. The cell polarity regulator hScrib controls ERK activation through a KIM site-dependent interaction. Oncogene 2010; 29:5311 - 5321; PMID: 20622900; http://dx.doi.org/10.1038/onc.2010.265
References
- Bilder D, Li M, Perrimon N. Cooperative regulation of cell polarity and growth by Drosophila tumor suppressors. Science 2000; 289:113 - 116
- Kemphues KJ, Kusch M, Wolf N. Maternal-effect lethal mutations on linkage group II of Caenorhabditis elegans. Genetics 1988; 120:977 - 986
- Tepass U, Knust E. Crumbs and stardust act in a genetic pathway that controls the organization of epithelia in Drosophila melanogaster. Dev Biol 1993; 159:311 - 326
- Tepass U, Theres C, Knust E. crumbs encodes an EGF-like protein expressed on apical membranes of Drosophila epithelial cells and required for organization of epithelia. Cell 1990; 61:787 - 799
- Bilder D. Epithelial polarity and proliferation control: links from the Drosophila neoplastic tumor suppressors. Genes Dev 2004; 18:1909 - 1925
- Zeitler J, Hsu CP, Dionne H, Bilder D. Domains controlling cell polarity and proliferation in the Drosophila tumor suppressor Scribble. J Cell Biol 2004; 167:1137 - 1146
- Aranda V, Haire T, Nolan ME, Calarco JP, Rosenberg AZ, Fawcett JP, et al. Par6-aPKC uncouples ErbB2 induced disruption of polarized epithelial organization from proliferation control. Nat Cell Biol 2006; 8:1235 - 1245
- Humbert PO, Grzeschik NA, Brumby AM, Galea R, Elsum I, Richardson HE. Control of tumourigenesis by the Scribble/Dlg/Lgl polarity module. Oncogene 2008; 27:6888 - 6907
- Thomas M, Narayan N, Pim D, Tomaic V, Massimi P, et al. Human papillomaviruses, cervical cancer and cell polarity. Oncogene 2008; 27:7018 - 7030
- Thomas M, Massimi P, Navarro C, Borg JP, Banks L. The hScrib/Dlg apico-basal control complex is differentially targeted by HPV-16 and HPV-18 E6 proteins. Oncogene 2005; 24:6222 - 6230
- zur Hausen H. Papillomaviruses and cancer: from basic studies to clinical application. Nat Rev Cancer 2002; 2:342 - 350
- Tomaic V, Gardiol D, Massimi P, Ozbun M, Myers M, Banks L. Human and primate tumour viruses use PDZ binding as an evolutionarily conserved mechanism of targeting cell polarity regulators. Oncogene 2009; 28:1 - 8
- Fanning AS, Anderson JM. PDZ domains: fundamental building blocks in the organization of protein complexes at the plasma membrane. J Clin Invest 1999; 103:767 - 772
- Santoni MJ, Pontarotti P, Birnbaum D, Borg JP. The LAP family: a phylogenetic point of view. Trends Genet 2002; 18:494 - 497
- Nakagawa S, Yano T, Nakagawa K, Takizawa S, Suzuki Y, Yasugi T, et al. Analysis of the expression and localisation of a LAP protein, human scribble, in the normal and neoplastic epithelium of uterine cervix. Br J Cancer 2004; 90:194 - 199
- Gardiol D, Zacchi A, Petrera F, Stanta G, Banks L. Human discs large and scrib are localized at the same regions in colon mucosa and changes in their expression patterns are correlated with loss of tissue architecture during malignant progression. Int J Cancer 2006; 119:1285 - 1290
- Navarro C, Nola S, Audebert S, Santoni MJ, Arsanto JP, Ginestier C, et al. Junctional recruitment of mammalian Scribble relies on E-cadherin engagement. Oncogene 2005; 24:4330 - 4339
- Dow LE, Brumby AM, Muratore R, Coombe ML, Sedelies KA, et al. hScrib is a functional homologue of the Drosophila tumour suppressor Scribble. Oncogene 2003; 22:9335 - 9340
- Lahuna O, Quellari M, Achard C, Nola S, Meduri G, Navarro C, et al. Thyrotropin receptor trafficking relies on the hScrib-betaPIX-GIT1-ARF6 pathway. EMBO J 2005; 24:1364 - 1374
- Audebert S, Navarro C, Nourry C, Chasserot-Golaz S, Lecine P, Bellaiche Y, et al. Mammalian Scribble forms a tight complex with the betaPIX exchange factor. Curr Biol 2004; 14:987 - 995
- Etienne-Manneville S, Hall A. Rho GTPases in cell biology. Nature 2002; 420:629 - 635
- Raftopoulou M, Hall A. Cell migration: Rho GTPases lead the way. Dev Biol 2004; 265:23 - 32
- Yamazaki D, Kurisu S, Takenawa T. Regulation of cancer cell motility through actin reorganization. Cancer Sci 2005; 96:379 - 386
- Osmani N, Vitale N, Borg JP, Etienne-Manneville S. Scrib controls Cdc42 localization and activity to promote cell polarization during astrocyte migration. Curr Biol 2006; 16:2395 - 2405
- Zhan L, Rosenberg A, Bergami KC, Yu M, Xuan Z, Jaffe AB, et al. Deregulation of scribble promotes mammary tumorigenesis and reveals a role for cell polarity in carcinoma. Cell 2008; 135:865 - 878
- Brumby AM, Richardson HE. scribble mutants cooperate with oncogenic Ras or Notch to cause neoplastic overgrowth in Drosophila. EMBO J 2003; 22:5769 - 5779
- Pagliarini RA, Xu T. A genetic screen in Drosophila for metastatic behavior. Science 2003; 302:1227 - 1231
- Dow LE, Elsum IA, King CL, Kinross KM, Richardson HE, Humbert PO. Loss of human Scribble cooperates with H-Ras to promote cell invasion through deregulation of MAPK signalling. Oncogene 2008; 27:5988 - 6001
- Olsen JV, Vermeulen M, Santamaria A, Kumar C, Miller ML, Jensen LJ, et al. Quantitative phosphoproteomics reveals widespread full phosphorylation site occupancy during mitosis. Sci Signal 3:3
- Etienne-Manneville S, Hall A. Integrin-mediated activation of Cdc42 controls cell polarity in migrating astrocytes through PKCzeta. Cell 2001; 106:489 - 498
- Ridley AJ, Schwartz MA, Burridge K, Firtel RA, Ginsberg MH, Borisy G, et al. Cell migration: integrating signals from front to back. Science 2003; 302:1704 - 1709
- Massimi P, Narayan N, Cuenda A, Banks L. Phosphorylation of the discs large tumour suppressor protein controls its membrane localisation and enhances its susceptibility to HPV E6-induced degradation. Oncogene 2006; 25:4276 - 4285
- Uhlirova M, Jasper H, Bohmann D. Non-cellautonomous induction of tissue overgrowth by JNK/Ras cooperation in a Drosophila tumor model. Proc Natl Acad Sci USA 2005; 102:13123 - 13128
- Shin EY, Shin KS, Lee CS, Woo KN, Quan SH, Soung NK, et al. Phosphorylation of p85beta PIX, a Rac/Cdc42-specific guanine nucleotide exchange factor, via the Ras/ERK/PAK2 pathway is required for basic fibroblast growth factor-induced neurite outgrowth. J Biol Chem 2002; 277:44417 - 44430
- Nagasaka K, Pim D, Massimi P, Thomas M, Tomaic V, Subbaiah VK, et al. The cell polarity regulator hScrib controls ERK activation through a KIM site-dependent interaction. Oncogene 2010; In press
- Nakagawa S, Huibregtse JM. Human scribble (Vartul) is targeted for ubiquitin-mediated degradation by the high-risk papillomavirus E6 proteins and the E6AP ubiquitin-protein ligase. Mol Cell Biol 2000; 20:8244 - 8253
- Sun Y, Aiga M, Yoshida E, Humbert PO, Bamji SX. Scribble interacts with beta-catenin to localize synaptic vesicles to synapses. Mol Biol Cell 2009; 20:3390 - 3400
- Nagasaka K, Nakagawa S, Yano T, Takizawa S, Matsumoto Y, Tsuruga T, et al. Human homolog of Drosophila tumor suppressor Scribble negatively regulates cell cycle progression from G1 to S phase by localizing at the basolateral membrane in epithelial cells. Cancer Sci 2006; 97:1217 - 1225
- Takizawa S, Nagasaka K, Nakagawa S, Yano T, Nakagawa K, Yasugi T, et al. Human scribble, a novel tumor suppressor identified as a target of high-risk HPV E6 for ubiquitin-mediated degradation, interacts with adenomatous polyposis coli. Genes Cells 2006; 11:453 - 464
- Petit MM, Meulemans SM, Alen P, Ayoubi TA, Jansen E, Van de Ven WJ. The tumor suppressor Scrib interacts with the zyxin-related protein LPP, which shuttles between cell adhesion sites and the nucleus. BMC Cell Biol 2005; 6:1
- Metais JY, Navarro C, Santoni MJ, Audebert S, Borg JP. hScrib interacts with ZO-2 at the cell-cell junctions of epithelial cells. FEBS Lett 2005; 579:3725 - 3730
- Phua DC, Humbert PO, Hunziker W. Vimentin regulates scribble activity by protecting it from proteasomal degradation. Mol Biol Cell 2009; 20:2841 - 2855
- Werme K, Wigerius M, Johansson M. Tick-borne encephalitis virus NS5 associates with membrane protein scribble and impairs interferon-stimulated JAKSTAT signalling. Cell Microbiol 2008; 10:696 - 712
- Liu H, Golebiewski L, Dow EC, Krug RM, Javier RT, Rice AP. The ESEV PDZ Binding-Motif of the Avian Influenza A Virus NS1 Protein Protects Infected Cells from Apoptosis through Directly Targeting Scribble. J Virol 2010; In press
- Kallay LM, McNickle A, Brennwald PJ, Hubbard AL, Braiterman LT. Scribble associates with two polarity proteins, Lgl2 and Vangl2, via distinct molecular domains. J Cell Biochem 2006; 99:647 - 664