Abstract
For directional cell migration to occur cells must interpret guiding cues present in their environment. Chemotaxis based on negative or positive signals has been long thought as the main driving force of guided cell migration. However during collective cell migration cells do receive information from external signals but also upon interactions with their direct neighbours. These multiple inputs must be translated into intracellular reorganisation in order to promote efficient directional migration. Small GTPases, being involved in establishing cell polarity and regulating protrusive activity, are likely to play a central role in signal integration. Indeed, recent findings from our laboratory indicate that Contact-Inhibition of Locomotion controlled by N-Cadherin and chemotaxis dependent on Sdf1/Cxcr4 signalling converge towards regulation of the localized activity of RhoA and Rac1. All together they establish cell polarity and select well-oriented cell protrusions to ensure directional cell migration.
Despite the fact that collective cell migration and chemotaxis are recognized as major mode and means of cell migrationCitation1–Citation5 the question of how large cell population make sense of multiple inputs remains unstudied. We recently addressed the respective roles of cell-cell interactions and chemotaxis during collective cell migration using Xenopus neural crest cells as a model.Citation6 We found that neural crest cells were strongly attracted by the Stromal cell-derived factor-1 (Sdf1),Citation6 a widely studied chemoattractant (reviewed in ref. Citation7). Importantly, chemotaxis was highly dependent on cell interactions. Cell dissociation completely abolished the response to Sdf1 while increasing cell density progressively rescued chemotaxis to control levels. We have recently shown that directional migration of neural crest is dependent on Contact Inhibition of Locomotion (CIL),Citation8 the process by which a cell collapses its protrusions and changes its direction of migration upon contact with another cell.Citation9,Citation10 Thus, if neural crest cells are surrounded by other neural crest cells, as is the case at the origin of neural crest migration, they can not move as each cell is surrounded by other cells. However, cells at the free edge only experience CIL at their back and can therefore produce protrusions in the direction of the free space and move in that direction. This process can generate directional migration of groups of cells during collective cell migration.Citation10 In our recent paperCitation6 we identified N-Cadherin as a cell-cell adhesion molecule involved in CIL. A mild N-Cadherin inhibition, unable to dissociate the cells, was sufficient to impair chemotaxis toward Sdf1.Citation6 Following N-Cadherin inhibition, cells lost the ability to sense each other and did not exhibit CIL. They formed protrusions on top of each other and failed to repolarize upon collisions with other cells. By contrast, we found that Sdf1 was unable to efficiently polarize the cells but could stabilize cell protrusions of previously polarized cells. Interestingly, we showed that both cell contact and Sdf1 effects can be integrated into precise regulation of Rac1 activity levels and distribution throughout the cell.Citation6 These results are discussed below alongside recent publications on other migratory cell populations.
Cell-Cell Contact: The Making of the Back
We showed that in neural crest cells N-Cadherin is localized at the cell contact where it colocalizes with p120- and β-catenin.Citation6 In addition, using FRET probes we found that Rac1 activity is lower at the cell contact than in other regions of the cell, such as the lamellipodium at the free edge that exhibits the highest level of Rac1 activity. By contrast, in single cells several high spots of Rac1 activity were observed around the cell and small unstable cell protrusions could form in any direction. In groups, blocking N-Cadherin led to an increase of Rac1 levels at the cell contact and ectopic cell protrusions in between the cells were generated. This indicates that N-Cadherin is required for contact-specific Rac1 inhibition and that Rac1 inhibition is required to prevent the formation of cell protrusions between the cells.
The direct link between N-Cadherin and Rac1 inhibition in neural crest cells has not been demonstrated but several mechanisms are possible. We recently demonstrated that Xenopus neural crest cells exhibit CIL.Citation8 Neural crest cells collapse cell protrusions upon cell contact through activation of RhoA downstream of the non-canonical Wnt/PCP pathway.Citation8,Citation11 As Rac1 and RhoA antagonize each other,Citation12–Citation14 activation of RhoA would lead to an inhibition of Rac1. We have shown that N-Cadherin is required for CILCitation6 but its precise role in the process remains to be elucidated. Noren and colleaguesCitation15 showed that cytosolic p120-catenin can bind to and inhibit RhoA and activate Vav2, a Rac1 activator. They proposed a model in which formation of cell adhesion complexes would recruit p120 and consequently release its inhibitory effect on RhoA which in turn would block Rac1 activation at the cell contact.Citation15 In addition, Charasse et al.Citation16 demonstrated that single cells plated on N-Cadherin show a dramatic increase of RhoA activity reinforcing the idea that signaling downstream of N-Cadherin is sufficient to activate RhoA even in absence of cell contact.Citation16 Whatever the mechanism involved, our results indicate that N-Cadherin/CIL imposes a strong back identity by inducing RhoA activity at the cell contact. In turn, Rac1 activity is restricted to the free edge where lamellipodia form.
War of the Fronts: Survival of the Fittest
Different mechanisms have been proposed to explain how cells could make sense of external signals. The most popular model postulates that cells first probe their surrounding and form cell protrusions towards the highest chemoattractant concentration. An alternative model proposes that cells would autonomously produce randomly oriented protrusions, whose stability will be positively or negatively regulated by external cues (reviewed in ref. Citation4). Our findings strongly support the latter.
Comparing individual and clustered cells we showed that neural crest cells have an intrinsic ability to produce cell protrusions in all directions without the need of any external stimulus to do so. However, orientation, size and stability of cell protrusions are directly influenced by cell-cell interactions.Citation6 Cell-cell contacts restrict protrusions to the free edge of the cell, increasing their size and stability. By contrast, Sdf1 signaling through its Cxcr4 G-protein coupled receptor (GPCR) do not affect either the size or the orientation of cell protrusions, but it is able to stabilize them. This protrusion stabilizing effect was stronger on clustered cells than single cells providing an explanation as to why clustered cells chemotax better than single cells.Citation6 Altogether these data support the idea that cells must first be polarized and therefore the formation of cell protrusions would be a requirement for chemotaxis.
How can chemoattractants such as Sdf1 stabilize protrusions? Rac1 is one the main players in protrusion stabilityCitation17 and several downstream targets of the Sdf1/Cxcr4 axis modulate Rac1 activityCitation18 (). For instance Cxcr4 can activate the Phosphatidylinositol 3 Kinases (PI3K) pathway.Citation19,Citation20 All PI3K isoforms can activate Rac1 Citation21 and they are both involved in a positive feedback loop.Citation2,Citation22,Citation23 This positive feedback is likely to involve activation of guanine nucleotide exchange factors (GEFs) downstream of PI3K. PI3K phosphorylates Phosphatidylinositol-2-Phosphate (PIP2) into Phosphatidylinositol-3-Phosphate (PIP3). PIP3 acts as a docking site for proteins containing a Plekstrin Homologue (PH) domain and promotes their activation. Proteins containing a PH domain are plentiful but of particular interest are GEFs such as Vav proteins and P-Rex1 (PIP3-dependent Rac Exchanger 1) which convert inactive GDP-bound Rac1 into active GTP-bound Rac1.Citation24,Citation25 Both Vav and P-Rex1 were found to be activated through PI3K pathway downstream of Sdf1.Citation24,Citation26 In addition, in T Lymphocytes Cxcr4 can be relocated into lipid rafts where it specifically co-localizes with Rac1 and facilitates its GTP loading.Citation27 Interestingly, this Cxcr4 relocation into the lipid rafts was shown to be Sdf1 independent and Cxcr4 association with Rac1 improved cell response to Sdf1.Citation27 This further supports the idea that specific regulation of Rac1 activity is a prerequisite for efficient chemotaxis. Requirement of PI3K in this process was not assessed.
Cxcr4 signaling can also lead to the ERK and p38 MAPK pathways activationCitation18,Citation19,Citation28–Citation30 which can be further amplified by Rac1 activity.Citation28,Citation31 Moreover, Cxcr4 can activate NFκB pathway directly or through ERK/MAPK (reviewed in ref. Citation19 and Citation32) which can in turn promote Cxcr4 expression.Citation33 However, if an increase of Cxcr4 expression downstream of Cxcr4 signaling could improve the cell response in the long run it is unlikely to be involved in short-term regulation of protrusion stability. More importantly, ERK signaling has been shown involved in inhibiting Rho and ROCK (Rho-associated kinase) signaling allowing focal adhesions to disassemble, promoting cell migration.Citation34 Therefore ERK activation downstream of Cxcr4 might maintain low RhoA activity levels helping to keep a dominant Rac1 activity at the front.
Finally, Sdf1/Cxcr4 impact on Rac1 could also be modulated by Syndecan-4. Both Sdf1 and Syndecan-4 bind to Fibronectin (Fn)Citation11,Citation35,Citation36 and bind to each other.Citation37 In addition, Syndecans have been described as co-receptors of GPCR.Citation35 Interestingly, we recently showed that Syndecan-4/Fn signaling participates in Rac1 inhibitionCitation11 possibly through its interaction with Wnt/PCP. Therefore one can hypothesize that Syndecan-4 could have a dual role during neural crest cell migration. In absence of Sdf1, Syndecan-4/Fn would inhibit Rac1 while upon Sdf1 expression Syndecan-4 could be recruited to improve Sdf1 presentation to Cxcr4 consequently promoting Rac1 activation.
Directionality by Numbers
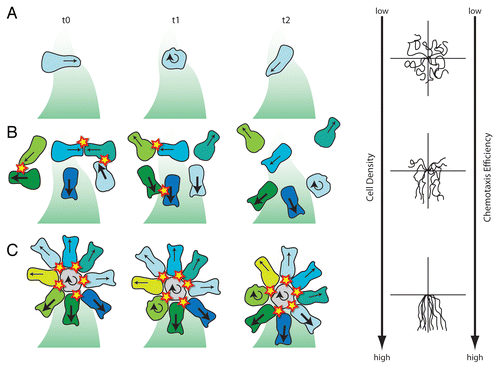
How can high cell density help cells to chemotax properly? Cells migrate by alternation of straight movements and reorientation phases with no net progression respectively called run and tumble.Citation38 In a single cell situation, a protrusion will be randomly generated and the cell will migrate in that orientation for some time. After a while this protrusion will collapse and a new one will be formed in a different orientation (). As Sdf1 is unable to dictate the orientation of cell protrusions the stabilization of cell protrusions in all directions will be the same. In this case the advantage given by a well-oriented protrusion is poor. However, when considering a cell population, cell protrusions would have to pass a first filter based on cell-cell interactions before being biased by the chemoattractant. All protrusions oriented towards another cell will be eliminated upon cell collisions (due to N-Cadherin/CIL-dependent RhoA activation leading to Rac1 inhibition) while protrusions oriented toward a cell-free region will last longer and may be stabilized further by the chemoattractant (Rac1 activation). Such system removes most of the badly oriented protrusions beforehand allowing a stronger impact of the chemoattractant on cell behaviour (). Finally in a cell cluster, cells have long-lasting protrusions and have a steady polarity based on their cell contact (RhoA)—free edge (Rac1) axis. Such group will exhibit a radial polarity with outer cells equally polarized towards the free space. In this situation, protrusions facing high concentrations of chemoattractant will be further stabilized. The resulting driving force will then lead to directional migration toward the source of the chemoattractant. Even in clusters, cells keep alternating between phases of run and tumble (see above). When a cell collapses its protrusion, the balance of forces around the group is modified and the cluster rotates accordingly (). All these observations made in the course of our recent workCitation6 show that collective chemotaxis improves as cell density increases. This is mainly due to the fact that a high cell density leads to a high probability of cell collisions which then eliminates wrongly oriented cell protrusions upon cell contact. This reinforces the idea that cell coordination during collective cell migration is highly contact-inhibition dependent.
Seeking New Directions
Most of the mechanisms and signaling pathways discussed here and summarized in have yet to be studied in neural crest cells. Previous works on small GTPases have clearly emphasized the fact that the specific roles of these molecules are highly cell type and context dependent. Their functions are likely to differ greatly when considering the formation of epithelial junctions, transient contacts between mesenchymal cells or protrusive activity in isolated or clustered cells. Further work is needed to better understand how migratory neural crest cells actually integrate multiple inputs into useful temporal and spatial regulation of RhoGTPases in order to achieve directional collective cell migration in vivo. A challenging goal since neural crest cells as a mesenchymal population have their cell-cell interactions constantly remodelled and migrate through their surrounding tissues in hundreds in just a few hours.
Figures and Tables
Figure 1 Signal integration through RhoGTPases during collective chemotaxis in neural crest cells. (A) Representation of the border of a neural crest cell group. Cells are polarized according to their cell contacts by a mechanism dependent on CIL.Citation8,Citation10 RhoA and Rac1 activity are shown in red and green respectively. Only outer cells have a free edge and exhibit a clear front-back polarity. Inner cells remain unpolarized. (B) Intracellular signaling integrating inputs from contact-inhibition of locomotion and chemotaxis through small GTPases. At the cell contact N-cadherin/Syndecan-4/PCP (Frizzled-Dsh)/CIL signaling leads to a strong RhoA activity restricting Rac1 at the free-edge. At the back, RhoA/ROCK signaling controls stress fibers formation and cell body contraction in part through regulation of myosin light chain (MLC) phosphorylation. At the front, Rac1 activity controls WAVE/Arp2/3 cascade leading to actin branching and lamellipodium formation/stabilization and antagonizes RhoA impact on MLC via activation of PAK. In addition, Rac1 activity may be amplified by a PI3K/GEFs/Rac1 positive feedback loop downstream of Cxcr4. (C) Summary of the main players involved in establishing and maintaining front and back cell identities. Arp2/3, Actin-related proteins 2/3; Dsh, Dishevelled; PAK, p21-activated kinases; ROCK, Rho-Associated Kinase; WAVE, WASP family verprolin-homologous. Other abbreviations have been described in the text.
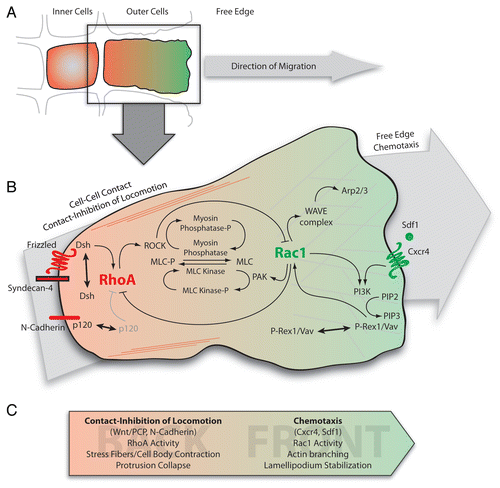
Figure 2 Different migratory behaviours of neural crest cells placed in a gradient of Sdf1. (A) Single cells that are isolated from other neural crest cells fail to polarize according to the Sdf1 gradient and therefore show poor chemotaxis. (B) Single cells that experience transient contacts are polarized upon collisions and chemotax more or less efficiently according to cell density. (C) Cell clusters show a radial symmetry with cells polarized along their cell contact—free edge axis. Front cell protrusions are stabilized and generate a driving force towards Sdf1. Orientation and size of the arrows indicate the direction and stability of cell protrusions. Round arrows mark tumbling and non-polarized cells. Cells are colour coded to help follow their behaviour from one time point to the other. (D) Typical cell tracks obtained in each situation showing that chemotaxis improves as cell density increases. Cell collisions are shown as stars. Shades of green represent Sdf1 gradient. Based on data from reference Citation6.
Extra View to: Theveneau E, Marchant L, Kuriyama S, Gull M, Moepps B, Parsons M, Mayor R. Collective chemotaxis requires contact-dependent cell polarity. Dev Cell 2010; 19:39 - 53; PMID: 20643349; http://dx.doi.org/10.1016/j.devcel.2010.06.012
References
- Andrew N, Insall RH. Chemotaxis in shallow gradients is mediated independently of PtdIns 3-kinase by biased choices between random protrusions. Nat Cell Biol 2007; 9:193 - 200
- Charest PG, Firtel RA. Feedback signaling controls leading-edge formation during chemotaxis. Curr Opin Genet Dev 2006; 16:339 - 347
- Friedl P, Gilmour D. Collective cell migration in morphogenesis, regeneration and cancer. Nat Rev Mol Cell Biol 2009; 10:445 - 457
- Insall RH. Understanding eukaryotic chemotaxis: a pseudopod-centred view. Nat Rev Mol Cell Biol 2010; 11:453 - 458
- Rorth P. Collective cell migration. Annu Rev Cell Dev Biol 2009; 25:407 - 429
- Theveneau E, Marchant L, Kuriyama S, Gull M, Moepps B, Parsons M, et al. Collective chemotaxis requires contact-dependent cell polarity. Dev Cell 2010; 19:39 - 53
- Raz E, Mahabaleshwar H. Chemokine signaling in embryonic cell migration: a fisheye view. Development 2009; 136:1223 - 1229
- Carmona-Fontaine C, Matthews HK, Kuriyama S, Moreno M, Dunn GA, Parsons M, et al. Contact inhibition of locomotion in vivo controls neural crest directional migration. Nature 2008; 456:957 - 961
- Abercrombie M, Heaysman JE, Karthauser HM. Social behaviour of cells in tissue culture. III. Mutual influence of sarcoma cells and fibroblasts. Exp Cell Res 1957; 13:276 - 291
- Mayor R, Carmona-Fontaine C. Keeping in touch with contact inhibition of locomotion. Trends Cell Biol 2010; 20:319 - 328
- Matthews HK, Marchant L, Carmona-Fontaine C, Kuriyama S, Larrain J, Holt MR, et al. Directional migration of neural crest cells in vivo is regulated by Syndecan-4/Rac1 and non-canonical Wnt signaling/RhoA. Development 2008; 135:1771 - 1780
- Sander EE, ten Klooster JP, van Delft S, van der Kammen RA, Collard JG. Rac downregulates Rho activity: reciprocal balance between both GTPases determines cellular morphology and migratory behavior. J Cell Biol 1999; 147:1009 - 1022
- Yamaguchi Y, Katoh H, Yasui H, Mori K, Negishi M. RhoA inhibits the nerve growth factor-induced Rac1 activation through Rho-associated kinase-dependent pathway. J Biol Chem 2001; 276:18977 - 18983
- van Leeuwen FN, van Delft S, Kain HE, van der Kammen RA, Collard JG. Rac regulates phosphorylation of the myosin-II heavy chain, actinomyosin disassembly and cell spreading. Nat Cell Biol 1999; 1:242 - 248
- Noren NK, Liu BP, Burridge K, Kreft B. p120 catenin regulates the actin cytoskeleton via Rho family GTPases. J Cell Biol 2000; 150:567 - 580
- Charrasse S, Meriane M, Comunale F, Blangy A, Gauthier-Rouviere C. N-cadherin-dependent cell-cell contact regulates Rho GTPases and beta-catenin localization in mouse C2C12 myoblasts. J Cell Biol 2002; 158:953 - 965
- Ridley AJ, Schwartz MA, Burridge K, Firtel RA, Ginsberg MH, Borisy G, et al. Cell migration: integrating signals from front to back. Science 2003; 302:1704 - 1709
- Busillo JM, Benovic JL. Regulation of CXCR4 signaling. Biochim Biophys Acta 2007; 1768:952 - 963
- Ganju RK, Brubaker SA, Meyer J, Dutt P, Yang Y, Qin S, et al. The alpha-chemokine, stromal cell-derived factor-1alpha, binds to the transmembrane G-protein-coupled CXCR-4 receptor and activates multiple signal transduction pathways. J Biol Chem 1998; 273:23169 - 23175
- Sotsios Y, Whittaker GC, Westwick J, Ward SG. The CXC chemokine stromal cell-derived factor activates a Gi-coupled phosphoinositide 3-kinase in T lymphocytes. J Immunol 1999; 163:5954 - 5963
- Papakonstanti EA, Zwaenepoel O, Bilancio A, Burns E, Nock GE, Houseman B, et al. Distinct roles of class IA PI3K isoforms in primary and immortalised macrophages. J Cell Sci 2008; 121:4124 - 4133
- Srinivasan S, Wang F, Glavas S, Ott A, Hofmann F, Aktories K, et al. Rac and Cdc42 play distinct roles in regulating PI(3,4,5)P3 and polarity during neutrophil chemotaxis. J Cell Biol 2003; 160:375 - 385
- Weiner OD, Neilsen PO, Prestwich GD, Kirschner MW, Cantley LC, Bourne HR. A PtdInsP(3)- and Rho GTPase-mediated positive feedback loop regulates neutrophil polarity. Nat Cell Biol 2002; 4:509 - 513
- Garcia-Bernal D, Wright N, Sotillo-Mallo E, Nombela-Arrieta C, Stein JV, Bustelo XR, et al. Vav1 and Rac control chemokine-promoted T lymphocyte adhesion mediated by the integrin alpha4beta1. Mol Biol Cell 2005; 16:3223 - 3235
- Welch HC, Coadwell WJ, Ellson CD, Ferguson GJ, Andrews SR, Erdjument-Bromage H, et al. P-Rex1, a PtdIns(3,4,5)P3- and Gbetagamma-regulated guanine-nucleotide exchange factor for Rac. Cell 2002; 108:809 - 821
- Carretero-Ortega J, Walsh CT, Hernandez-Garcia R, Reyes-Cruz G, Brown JH, Vazquez-Prado J. Phosphatidylinositol 3,4,5-triphosphate-dependent Rac exchanger 1 (P-Rex-1), a guanine nucleotide exchange factor for Rac, mediates angiogenic responses to stromal cell-derived factor-1/chemokine stromal cell derived factor-1 (SDF-1/CXCL-12) linked to Rac activation, endothelial cell migration and in vitro angiogenesis. Mol Pharmacol 2010; 77:435 - 442
- Wysoczynski M, Reca R, Ratajczak J, Kucia M, Shirvaikar N, Honczarenko M, et al. Incorporation of CXCR4 into membrane lipid rafts primes homing-related responses of hematopoietic stem/progenitor cells to an SDF-1 gradient. Blood 2005; 105:40 - 48
- Shin I, Kim S, Song H, Kim HR, Moon A. H-Ras-specific activation of Rac-MKK3/6-p38 pathway: its critical role in invasion and migration of breast epithelial cells. J Biol Chem 2005; 280:14675 - 14683
- Alsayed Y, Ngo H, Runnels J, Leleu X, Singha UK, Pitsillides CM, et al. Mechanisms of regulation of CXCR4/SDF-1 (CXCL12)-dependent migration and homing in multiple myeloma. Blood 2007; 109:2708 - 2717
- Huang CY, Lee CY, Chen MY, Yang WH, Chen YH, Chang CH, et al. Stromal cell-derived factor-1/CXCR4 enhanced motility of human osteosarcoma cells involves MEK1/2, ERK and NFkappaB-dependent pathways. J Cell Physiol 2009; 221:204 - 212
- Zhong B, Jiang K, Gilvary DL, Epling-Burnette PK, Ritchey C, Liu J, et al. Human neutrophils utilize a Rac/Cdc42-dependent MAPK pathway to direct intracellular granule mobilization toward ingested microbial pathogens. Blood 2003; 101:3240 - 3248
- Rehman AO, Wang CY. SDF-1alpha promotes invasion of head and neck squamous cell carcinoma by activating NFkappaB. J Biol Chem 2008; 283:19888 - 19894
- Kukreja P, Abdel-Mageed AB, Mondal D, Liu K, Agrawal KC. Upregulation of CXCR4 expression in PC-3 cells by stromal-derived factor-1alpha (CXCL12) increases endothelial adhesion and transendothelial migration: role of MEK/ERK signaling pathway-dependent NFkappaB activation. Cancer Res 2005; 65:9891 - 9898
- Pullikuth AK, Catling AD. Scaffold mediated regulation of MAPK signaling and cytoskeletal dynamics: a perspective. Cell Signal 2007; 19:1621 - 1632
- Carey DJ. Syndecans: multifunctional cell-surface co-receptors. Biochem J 1997; 327:1 - 16
- Pelletier AJ, van der Laan LJ, Hildbrand P, Siani MA, Thompson DA, Dawson PE, et al. Presentation of chemokine SDF-1alpha by fibronectin mediates directed migration of T cells. Blood 2000; 96:2682 - 2690
- Charnaux N, Brule S, Hamon M, Chaigneau T, Saffar L, Prost C, et al. Syndecan-4 is a signaling molecule for stromal cell-derived factor-1 (SDF-1)/CXCL12. FEBS J 2005; 272:1937 - 1951
- Polin M, Tuval I, Drescher K, Gollub JP, Goldstein RE. Chlamydomonas swims with two “gears” in a eukaryotic version of run-and-tumble locomotion. Science 2009; 325:487 - 490