Abstract
The small GTPases RalA and RalB are activated downstream of oncogenic Ras. While activation of RalA is critically important for tumor initiation and growth of Ras-driven cancers, the highly similar small GTPase RalB is implicated in cell survival and metastasis. This difference in function between these two related proteins maps to the C-terminus, a 30 amino acid region that regulates subcellular localization and contains several potential phosphorylation sites. Here we discuss our recent evidence that phosphorylation by the mitotic kinase Aurora A promotes RalA relocalization to mitochondrial membranes, where it recruits the effector RalBP1 and the large dynamin-related GTPase Drp1 to promote mitochondrial fission. As upregulation of both RalA and Aurora A have been observed in human tumors, and phosphorylation of RalA at the site targeted by Aurora A promotes tumorigenesis, it is possible that regulation of mitochondrial fission is one mechanism by which RalA promotes cancer.
Mutations in the RAS gene are present in nearly one third of all human cancers and up to 90% of pancreatic cancers.Citation1 Constitutively active Ras proteins promote tumorigenesis through the engagement of downstream effector proteins, including phosphatidylinositol-3 kinase (PI3K),Citation2 the Raf family of serine/threonine kinases,Citation3-Citation6 and the RalGEF family of guanine nucleotide exchange factors.Citation7-Citation9 Of these three, the RalGEF pathway is the least well understood, although mounting evidence points to a role for this pathway in multiple aspects of tumorigenesis.Citation10-Citation18 RalGEFs comprise a family of six guanine nucleotide exchange factors (RalGDS, RGL, RGL2/Rlf, RGL3, RalGPS1 and RalGPS2) of which all but RalGPS1 and RalGPS2 are directly activated by Ras.Citation19 These proteins promote the activation of two nearly identical small GTPases, RalA and RalB, which in turn engage a diverse set of downstream effector molecules, including the exocyst components Sec5Citation20,Citation21 and Exo84,Citation22 the large multifunctional RalBP1 (RLIP76/RLIP1/RIP1),Citation23 the actin crosslinking protein filamin,Citation24 and the transcription factor ZONAB.Citation25 Despite 80% sequence identity, and near 100% identity in their effector binding domains,Citation26 RalA and RalB promote distinct aspects of tumor growth downstream of oncogenic Ras. While RalA has been shown to be important for tumor initiation and growth, RalB seems to play more of a role in promoting survival and metastasis.Citation13,Citation17,Citation27,Citation28 This difference in function between RalA and RalB maps to the 30 amino acid hypervariable C-terminus of the protein, as replacing this region in RalB with that of RalA is sufficient to impart both the polarized membrane delivery function, as well as transforming activity to RalB.Citation13,Citation29
The hypervariable regions of RalA and RalB are both highly basic and have a conserved CAAX motif that targets the protein for prenylation, but they otherwise share little sequence identity.Citation26 Both sequence analysis and targeted mutational analysis of these regions have identified several putative phosphorylation sites that are unique to either RalA or RalB.Citation12,Citation13,Citation30,Citation31 These sites are of particular interest, as analysis of the related small GTPase KRas suggests that phosphorylation of the hypervariable domain can have a profound impact on subcellular localization and protein function.Citation32 Of the two serine residues in the RalA C-terminus (S183 and S194) and five threonine or serine residues in the RalB C-terminus (T178, S182, S192, S193 and S198), several have been shown to be targets of phosphorylation,Citation12,Citation30,Citation31 with the best-characterized being S194 of RalA. S194 is phosphorylated by Aurora A, a kinase activated in mitosis that is known to be important for mitotic entry, centrosome duplication and maturation, bipolar spindle assembly and cytokinesis,Citation31,Citation33 and can be dephosphorylated by the phosphatase PP2A.Citation30 Phosphorylation of S194 leads to an increase in active, GTP-bound RalA and redistributes the protein from the plasma membrane to internal membranes.Citation30,Citation31,Citation34 Furthermore, Aurora A cooperates with RalA to promote tumorigenesis, and mutating S194 to alanine or dephosphorylating S194 by PP2A inhibits RalA transforming ability.Citation30,Citation34
In order to better understand the role of this phosphorylation, we examined the specific subcellular localization of RalA in the presence and absence of ectopic Aurora A. Similar to what was found for KRas following its phosphorylation by PKC,Citation32 immunofluorescence analysis of GFP-tagged RalA and immunoblot analysis of biochemically fractionated cells revealed that a portion of RalA localizes at mitochondria or membranes tightly associated with this organelle. Furthermore, expression of an active (T288D) mutant of Aurora A or mutation of RalA-S194 to Aspartic acid to mimic phosphorylation increased the amount of RalA in the mitochondria while inhibition of Aurora A, or mutation of RalA-S194 to Alanine had the opposite effect.Citation35
Analysis of the localization of RalA at mitochondria also revealed a striking difference in the morphology of mitochondria in cells with activated Aurora A. While the majority of cells expressing a vector control exhibited a mixture of long interconnected mitochondria and short punctate mitochondria, cells expressing active Aurora AT288D exhibited a large percentage of small punctate mitochondria. Cells expressing the kinase-inactive AuroraK162R, on the other hand, exhibited a large percentage of long interconnected mitochondria.
Mitochondrial morphology is maintained through a balance of the opposing processes of fusion and fission and it has become clear in recent years that maintaining this balance is critical to a number of cellular processes and implicated in a number of human diseases.Citation36-Citation38 Given that Aurora A activity promoted the mitochondrial localization of RalA and affected mitochondrial morphology, we performed both loss-of-function and gain-of-function analysis and found that RalA was required for normal mitochondrial dynamics. Specifically, Aurora A promoted mitochondrial division in a RalA and S194 dependent fashion, and expression of the phosphomimetic RalAS194D was sufficient to induce mitochondrial fragmentation. Furthermore, phosphorylation of RalA by Aurora A promoted mitochondrial fission, as opposed to blocking fusion, and was accompanied by an increase in the levels of the large, fission-promoting GTPase Drp1 at the mitochondria.Citation35
Phosphorylation of RalA on S194 was previously shown to promote the binding of RalA to RalBP1 over Sec5 and Exo84.Citation34 Consistent with this, shRNA-mediated knockdown of RalBP1 phenocopied the effects of RalA knockdown on mitochondrial morphology, promoting long interconnected networks. Additionally, expression of active Aurora AT288D resulted in an increase in the levels of RalBP1 associated with the mitochondrial fraction. This increase in mitochondrial RalBP1 was abrogated by knockdown of RalA, suggesting that RalA recruits RalBP1 to mitochondria to promote mitochondrial fission. Indeed fusion of the C-terminus of RalA in the S194D phosphomimetic configuration to RalBP1 was sufficient to promote both mitochondrial localization and mitochondrial fission.Citation35
Mitochondrial fission increases during mitosis, and is dependent on both the mitochondrial recruitment and phosphorylation of Drp1.Citation39-Citation41 As Aurora A is active during mitosis,Citation42 we tested and found that the levels of S194-phosphorylated RalA were increased in mitotic HeLa cell extracts when compared with unsynchronized cells and that this phosphorylation was coincident with increased recruitment of RalA, RalBP1, and Drp1 to mitochondrial membranes at mitotis. Importantly, knockdown of RalA, RalBP1 or Aurora A led to a decrease in Drp1 recruitment to mitochondria during mitosis, suggesting that this pathway is critical for the mitotic mitochondrial recruitment of Drp1.
During mitosis, Drp1 is also phosphorylated on S616 by the mitotic kinase Cyclin B/CDK1.Citation31 Previous studies have shown that RalBP1 binds to Cyclin B/CDK1 and promotes the phosphorylation of the protein Epsin to suppress receptor-mediated endocytosis.Citation43 We confirmed that RalBP1 bound to Cyclin B and associated with CDK1 kinase activity in mitotic extracts. Furthermore, similar to its role with Epsin, RalBP1 promoted CDK1 phosphorylation of Drp1 in vitro, and loss of RalBP1, but not RalA, decreased the levels of S616 phosphorylated Drp1 in mitotic extracts. Importantly, we detected a complex including RalA, RalBP1, Drp1 and Cyclin B on biochemically enriched mitochondrial membranes during mitosis, suggesting a model whereby RalBP1 forms a complex with Drp1 and Cyclin B/CDK1 to promote Drp1 phosphorylation and this complex is recruited to mitochondrial membranes through the binding to S194-phosphorylated RalA ().
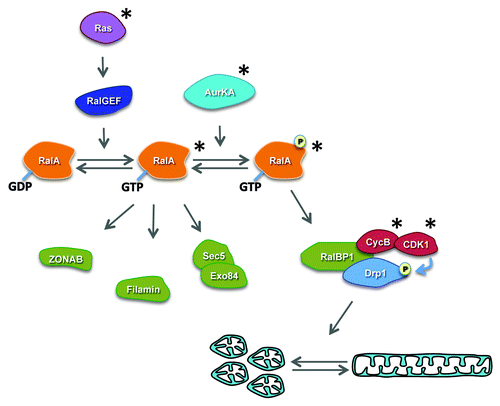
Figure 1. Ras activation of RalA leads to engagement of multiple effector pathways. The RalGEF-Ral signaling pathway is a key effector pathway downstream of activated Ras. Activation of RalA in turn leads to activation of several downstream signaling pathways affecting diverse cellular processes, including exocytosis, endocytosis, actin dynamics and transcription. New evidence also indicates that following phosphorylation by Aurora A, RalA and its effector RalBP1 play a key role in the regulation of mitochondrial fission during mitosis by regulating the phosphorylation of Drp1 and its recruitment to mitochondrial membranes. “*” denotes proteins whose expression or activity have been reported to be elevated in human cancer.
This model predicts that mitotic mitochondrial fission will be disrupted in cells lacking either RalA or RalBP1. Indeed, while the mitochondria of scramble control cells underwent fission during metaphase, mitochondria remained interconnected in cells in which either RalA or RalBP1 had been knocked down by shRNA. The effects of RalA knockdown were rescued by expression of an shRNA-resistant wild-type RalA, but not RalAS194A, underscoring the importance of this phosphorylation site for mitochondrial fission.
The loss of mitotic mitochondrial fission in RalA and RalBP1 knockdown cells often resulted in mitochondrial bridges between daughter cells during cytokinesis, which was associated with a failure to complete cytokinesis and unequal distribution of mitochondria to the two daughter cells. Additionally, knockdown cells exhibited signs of mitochondrial dysfunction, with decreased levels of ATP and reduced cell growth over time.Citation35
Notably, upregulation of Aurora A protein levelsCitation44,Citation45 and RalA activityCitation10,Citation11,Citation13,Citation46,Citation47 has been observed in human cancer cells. Furthermore, knockdown or inhibition of Aurora A kinase activity impairs tumor growth,Citation48,Citation49 and knockdown or inhibition of RalA reduces Ras-induced transformation and tumor growth of multiple cancer cell lines derived from a diverse set of cancers.Citation10,Citation11,Citation13,Citation30,Citation46,Citation47,Citation50-Citation52 Importantly, the loss of Ras-induced tumor formation following RalA knockdown can be rescued in most cells by wildtype, but not an S194A mutant of RalA, confirming the importance of Aurora A phosphorylation for this process.Citation30,Citation31,Citation34 Maintenance of a dynamic mitochondrial network has been shown to be important for several cellular processes that are also known to be critical for tumorigenesis, including autophagy and the cargo-specific clearance of mitochondria known as mitophagy,Citation53-Citation56 apoptosisCitation57-Citation59 and maintenance of metabolic function.Citation37,Citation60,Citation61 We have shown that metabolic function is compromised in cells lacking RalA and RalBP1, ultimately leading to diminished cellular growth.Citation35 Taken together, these data suggest the intriguing possibility that aberrant activation and phosphorylation of RalA promote tumor growth, at least in part, through the regulation of mitochondrial fission. The finding that the tumor suppressor p53 regulates mitochondrial fission through down-regulation of miR-499, which negatively regulates Drp1 expression,Citation62 suggests that dysregulated mitochondrial dynamics might be a general feature of cancer cells and indeed, analysis of cancer cell lines by electron microscopy has revealed abnormal mitochondrial morphologies.Citation63
It will be interesting to test whether, in addition to metabolic function, autophagy and apoptosis are also affected by loss of RalA and RalBP1, and whether their restoration can rescue the loss of tumorigenesis phenotype characteristic of RalA inhibition. Intriguingly, RalB has been shown to directly impact both autophagyCitation64 and apoptosis,Citation28 through engagement of Exo84 and Sec5, respectively, however it is not known how these two related small GTPases use different effector pathways to potentially converge on the same cellular processes and what impact this convergence may have on Ras-driven tumorigenesis.
If the control of mitochondrial dynamics proves to be important for Ras-driven tumorigenesis, it could represent an important step towards discovering a more effective way to treat cancer. While inhibition of Ras or other small GTPases has proven to be problematic as a therapeutic strategy,Citation65 perhaps targeted disruption of mitochondrial dynamics will prove more pharmacologically feasible. Small molecule inhibitors of the mitochondrial fusion and fission machinery, including the recently described Drp1 inhibitor Mdivi-1,Citation66 are under development,Citation67 and it will be enlightening to test them in mouse tumor models to see if they have a clinical effect on tumor growth.
In conclusion, the finding that phosphorylation of RalA promotes mitochondrial fission through the recruitment of RalBP1 and Drp1 to mitochondria represents an important step forward in our understanding of RalA function, in the functional differences between RalA and RalB, and provides potential insight into how RalA regulates Ras-mediated tumorigenesis.
Acknowledgments
We thank Donita Brady for review of the manuscript. This work was supported by NIH grant CA94184 (C.M.C.). D.F.K. is a Leukemia and Lymphoma Society Fellow.
References
- Bos JL. ras oncogenes in human cancer: a review. Cancer Res 1989; 49:4682 - 9; PMID: 2547513
- Rodriguez-Viciana P, Warne PH, Dhand R, Vanhaesebroeck B, Gout I, Fry MJ, et al. Phosphatidylinositol-3-OH kinase as a direct target of Ras. Nature 1994; 370:527 - 32; http://dx.doi.org/10.1038/370527a0; PMID: 8052307
- Zhang XF, Settleman J, Kyriakis JM, Takeuchi-Suzuki E, Elledge SJ, Marshall MS, et al. Normal and oncogenic p21ras proteins bind to the amino-terminal regulatory domain of c-Raf-1. Nature 1993; 364:308 - 13; http://dx.doi.org/10.1038/364308a0; PMID: 8332187
- Han M, Golden A, Han Y, Sternberg PW. C. elegans lin-45 raf gene participates in let-60 ras-stimulated vulval differentiation. Nature 1993; 363:133 - 40; http://dx.doi.org/10.1038/363133a0; PMID: 8483497
- Warne PH, Viciana PR, Downward J. Direct interaction of Ras and the amino-terminal region of Raf-1 in vitro. Nature 1993; 364:352 - 5; http://dx.doi.org/10.1038/364352a0; PMID: 8332195
- Kyriakis JM, App H, Zhang XF, Banerjee P, Brautigan DL, Rapp UR, et al. Raf-1 activates MAP kinase-kinase. Nature 1992; 358:417 - 21; http://dx.doi.org/10.1038/358417a0; PMID: 1322500
- Hofer F, Fields S, Schneider C, Martin GS. Activated Ras interacts with the Ral guanine nucleotide dissociation stimulator. Proc Natl Acad Sci USA 1994; 91:11089 - 93; http://dx.doi.org/10.1073/pnas.91.23.11089; PMID: 7972015
- Spaargaren M, Bischoff JR. Identification of the guanine nucleotide dissociation stimulator for Ral as a putative effector molecule of R-ras, H-ras, K-ras, and Rap. Proc Natl Acad Sci USA 1994; 91:12609 - 13; http://dx.doi.org/10.1073/pnas.91.26.12609; PMID: 7809086
- Kikuchi A, Demo SD, Ye ZH, Chen YW, Williams LT. ralGDS family members interact with the effector loop of ras p21. Mol Cell Biol 1994; 14:7483 - 91; PMID: 7935463
- Zipfel PA, Brady DC, Kashatus DF, Ancrile BD, Tyler DS, Counter CM. Ral activation promotes melanomagenesis. Oncogene 2010; 29:4859 - 64; http://dx.doi.org/10.1038/onc.2010.224; PMID: 20562921
- Martin TD, Samuel JC, Routh ED, Der CJ, Yeh JJ. Activation and involvement of Ral GTPases in colorectal cancer. Cancer Res 2011; 71:206 - 15; http://dx.doi.org/10.1158/0008-5472.CAN-10-1517; PMID: 21199803
- Wang H, Owens C, Chandra N, Conaway MR, Brautigan DL, Theodorescu D. Phosphorylation of RalB is important for bladder cancer cell growth and metastasis. Cancer Res 2010; 70:8760 - 9; http://dx.doi.org/10.1158/0008-5472.CAN-10-0952; PMID: 20940393
- Lim K-H, Baines AT, Fiordalisi JJ, Shipitsin M, Feig LA, Cox AD, et al. Activation of RalA is critical for Ras-induced tumorigenesis of human cells. Cancer Cell 2005; 7:533 - 45; http://dx.doi.org/10.1016/j.ccr.2005.04.030; PMID: 15950903
- Chien Y, White MA. RAL GTPases are linchpin modulators of human tumour-cell proliferation and survival. EMBO Rep 2003; 4:800 - 6; http://dx.doi.org/10.1038/sj.embor.embor899; PMID: 12856001
- González-García A, Pritchard CA, Paterson HF, Mavria G, Stamp G, Marshall CJ. RalGDS is required for tumor formation in a model of skin carcinogenesis. Cancer Cell 2005; 7:219 - 26; http://dx.doi.org/10.1016/j.ccr.2005.01.029; PMID: 15766660
- Jiménez M, Pérez de Castro I, Benet M, García JF, Inghirami G, Pellicer A. The Rgr oncogene induces tumorigenesis in transgenic mice. Cancer Res 2004; 64:6041 - 9; http://dx.doi.org/10.1158/0008-5472.CAN-03-3389; PMID: 15342385
- Bodemann BO, White MA. Ral GTPases and cancer: linchpin support of the tumorigenic platform. Nat Rev Cancer 2008; 8:133 - 40; http://dx.doi.org/10.1038/nrc2296; PMID: 18219307
- Hamad NM, Elconin JH, Karnoub AE, Bai W, Rich JN, Abraham RT, et al. Distinct requirements for Ras oncogenesis in human versus mouse cells. Genes Dev 2002; 16:2045 - 57; http://dx.doi.org/10.1101/gad.993902; PMID: 12183360
- Neel NF, Martin TD, Stratford JK, Zand TP, Reiner DJ, Der CJ. The RalGEF-Ral effector signaling network: The road less traveled for anti-Ras drug discovery. Genes Cancer 2011; 2:275 - 87; http://dx.doi.org/10.1177/1947601911407329; PMID: 21779498
- Moskalenko S, Henry DO, Rosse C, Mirey G, Camonis JH, White MA. The exocyst is a Ral effector complex. Nat Cell Biol 2002; 4:66 - 72; http://dx.doi.org/10.1038/ncb728; PMID: 11740492
- Sugihara K, Asano S, Tanaka K, Iwamatsu A, Okawa K, Ohta Y. The exocyst complex binds the small GTPase RalA to mediate filopodia formation. Nat Cell Biol 2002; 4:73 - 8; http://dx.doi.org/10.1038/ncb720; PMID: 11744922
- Moskalenko S, Tong C, Rosse C, Mirey G, Formstecher E, Daviet L, et al. Ral GTPases regulate exocyst assembly through dual subunit interactions. J Biol Chem 2003; 278:51743 - 8; http://dx.doi.org/10.1074/jbc.M308702200; PMID: 14525976
- Cantor SB, Urano T, Feig LA. Identification and characterization of Ral-binding protein 1, a potential downstream target of Ral GTPases. Mol Cell Biol 1995; 15:4578 - 84; PMID: 7623849
- Ohta Y, Suzuki N, Nakamura S, Hartwig JH, Stossel TP. The small GTPase RalA targets filamin to induce filopodia. Proc Natl Acad Sci USA 1999; 96:2122 - 8; http://dx.doi.org/10.1073/pnas.96.5.2122; PMID: 10051605
- Frankel P, Aronheim A, Kavanagh E, Balda MS, Matter K, Bunney TD, et al. RalA interacts with ZONAB in a cell density-dependent manner and regulates its transcriptional activity. EMBO J 2005; 24:54 - 62; http://dx.doi.org/10.1038/sj.emboj.7600497; PMID: 15592429
- Chardin P, Tavitian A. Coding sequences of human ralA and ralB cDNAs. Nucleic Acids Res 1989; 17:4380; http://dx.doi.org/10.1093/nar/17.11.4380; PMID: 2662142
- Lim K-H, O'Hayer K, Adam SJ, Kendall SD, Campbell PM, Der CJ, et al. Divergent roles for RalA and RalB in malignant growth of human pancreatic carcinoma cells. Curr Biol 2006; 16:2385 - 94; http://dx.doi.org/10.1016/j.cub.2006.10.023; PMID: 17174914
- Chien Y, Kim S, Bumeister R, Loo YM, Kwon SW, Johnson CL, et al. RalB GTPase-mediated activation of the IkappaB family kinase TBK1 couples innate immune signaling to tumor cell survival. Cell 2006; 127:157 - 70; http://dx.doi.org/10.1016/j.cell.2006.08.034; PMID: 17018283
- Shipitsin M, Feig LA. RalA but not RalB enhances polarized delivery of membrane proteins to the basolateral surface of epithelial cells. Mol Cell Biol 2004; 24:5746 - 56; http://dx.doi.org/10.1128/MCB.24.13.5746-5756.2004; PMID: 15199131
- Sablina AA, Chen W, Arroyo JD, Corral L, Hector M, Bulmer SE, et al. The tumor suppressor PP2A Abeta regulates the RalA GTPase. Cell 2007; 129:969 - 82; http://dx.doi.org/10.1016/j.cell.2007.03.047; PMID: 17540176
- Wu J-C, Chen T-Y, Yu C-TR, Tsai S-J, Hsu J-M, Tang M-J, et al. Identification of V23RalA-Ser194 as a critical mediator for Aurora-A-induced cellular motility and transformation by small pool expression screening. J Biol Chem 2005; 280:9013 - 22; http://dx.doi.org/10.1074/jbc.M411068200; PMID: 15637052
- Bivona TG, Quatela SE, Bodemann BO, Ahearn IM, Soskis MJ, Mor A, et al. PKC regulates a farnesyl-electrostatic switch on K-Ras that promotes its association with Bcl-XL on mitochondria and induces apoptosis. Mol Cell 2006; 21:481 - 93; http://dx.doi.org/10.1016/j.molcel.2006.01.012; PMID: 16483930
- Vader G, Lens S. The Aurora kinase family in cell division and cancer. Biochim Biophys Acta 2008; 1786:60 - 72
- Lim K-H, Brady DC, Kashatus DF, Ancrile BB, Der CJ, Cox AD, et al. Aurora-A phosphorylates, activates, and relocalizes the small GTPase RalA. Mol Cell Biol 2010; 30:508 - 23; http://dx.doi.org/10.1128/MCB.00916-08; PMID: 19901077
- Kashatus DF, Lim K-H, Brady DC, Pershing NLK, Cox AD, Counter CM. RALA and RALBP1 regulate mitochondrial fission at mitosis. Nat Cell Biol 2011; 13:1 - 10; http://dx.doi.org/10.1038/ncb2310; PMID: 21173798
- Liesa M, Palacín M, Zorzano A. Mitochondrial dynamics in mammalian health and disease. Physiol Rev 2009; 89:799 - 845; http://dx.doi.org/10.1152/physrev.00030.2008; PMID: 19584314
- Otera H, Mihara K. Molecular mechanisms and physiologic functions of mitochondrial dynamics. J Biochem 2011; 149:241 - 51; http://dx.doi.org/10.1093/jb/mvr002; PMID: 21233142
- Westermann B. Mitochondrial fusion and fission in cell life and death. Nat Rev Mol Cell Biol 2010; 11:872 - 84; http://dx.doi.org/10.1038/nrm3013; PMID: 21102612
- Arakaki N, Nishihama T, Owaki H, Kuramoto Y, Suenaga M, Miyoshi E, et al. Dynamics of mitochondria during the cell cycle. Biol Pharm Bull 2006; 29:1962 - 5; http://dx.doi.org/10.1248/bpb.29.1962; PMID: 16946518
- Mitra K, Wunder C, Roysam B, Lin G, Lippincott-Schwartz J. A hyperfused mitochondrial state achieved at G1-S regulates cyclin E buildup and entry into S phase. Proc Natl Acad Sci USA 2009; 106:11960 - 5; http://dx.doi.org/10.1073/pnas.0904875106; PMID: 19617534
- Taguchi N, Ishihara N, Jofuku A, Oka T. Mitotic phosphorylation of dynamin-related GTPase Drp1 participates in mitochondrial fission. J Biol Chem 2007; 282:11521 - 9; http://dx.doi.org/10.1074/jbc.M607279200; PMID: 17301055
- Bischoff JR, Anderson L, Zhu Y, Mossie K, Ng L, Souza B, et al. A homologue of Drosophila aurora kinase is oncogenic and amplified in human colorectal cancers. EMBO J 1998; 17:3052 - 65; http://dx.doi.org/10.1093/emboj/17.11.3052; PMID: 9606188
- Rossé C, L'Hoste S, Offner N, Picard A, Camonis J. RLIP, an effector of the Ral GTPases, is a platform for Cdk1 to phosphorylate epsin during the switch off of endocytosis in mitosis. J Biol Chem 2003; 278:30597 - 604; http://dx.doi.org/10.1074/jbc.M302191200; PMID: 12775724
- Fukushige S, Waldman FM, Kimura M, Abe T, Furukawa T, Sunamura M, et al. Frequent gain of copy number on the long arm of chromosome 20 in human pancreatic adenocarcinoma. Genes Chromosomes Cancer 1997; 19:161 - 9; http://dx.doi.org/10.1002/(SICI)1098-2264(199707)19:3<161::AID-GCC5>3.0.CO;2-W; PMID: 9218997
- Li D, Zhu J, Firozi PF, Abbruzzese JL, Evans DB, Cleary K, et al. Overexpression of oncogenic STK15/BTAK/Aurora A kinase in human pancreatic cancer. Clin Cancer Res 2003; 9:991 - 7; PMID: 12631597
- Bodempudi V, Yamoutpoor F, Pan W, Dudek AZ, Esfandyari T, Piedra M, et al. Ral overactivation in malignant peripheral nerve sheath tumors. Mol Cell Biol 2009; 29:3964 - 74; http://dx.doi.org/10.1128/MCB.01153-08; PMID: 19414599
- Yu Y, Feig LA. Involvement of R-Ras and Ral GTPases in estrogen-independent proliferation of breast cancer cells. Oncogene 2002; 21:7557 - 68; http://dx.doi.org/10.1038/sj.onc.1205961; PMID: 12386818
- Okada T, Sawada T, Osawa T, Adachi M, Kubota K. MK615 inhibits pancreatic cancer cell growth by dual inhibition of Aurora A and B kinases. World J Gastroenterol 2008; 14:1378 - 82; http://dx.doi.org/10.3748/wjg.14.1378; PMID: 18322951
- Hata T, Furukawa T, Sunamura M, Egawa S, Motoi F, Ohmura N, et al. RNA interference targeting aurora kinase a suppresses tumor growth and enhances the taxane chemosensitivity in human pancreatic cancer cells. Cancer Res 2005; 65:2899 - 905; http://dx.doi.org/10.1158/0008-5472.CAN-04-3981; PMID: 15805292
- Vigil D, Martin TD, Williams F, Yeh JJ, Campbell SL, Der CJ. Aberrant overexpression of the Rgl2 Ral small GTPase-specific guanine nucleotide exchange factor promotes pancreatic cancer growth through Ral-dependent and Ral-independent mechanisms. J Biol Chem 2010; 285:34729 - 40; http://dx.doi.org/10.1074/jbc.M110.116756; PMID: 20801877
- Yin J, Pollock C, Tracy K, Chock M, Martin P, Oberst M, et al. Activation of the RalGEF/Ral pathway promotes prostate cancer metastasis to bone. Mol Cell Biol 2007; 27:7538 - 50; http://dx.doi.org/10.1128/MCB.00955-07; PMID: 17709381
- Sowalsky AG, Alt-Holland A, Shamis Y, Garlick JA, Feig LA. RalA function in dermal fibroblasts is required for the progression of squamous cell carcinoma of the skin. Cancer Res 2011; 71:758 - 67; http://dx.doi.org/10.1158/0008-5472.CAN-10-2756; PMID: 21159665
- Gomes LC, Benedetto GD, Scorrano L. During autophagy mitochondria elongate, are spared from degradation and sustain cell viability. Nat Cell Biol 2011; 13:589 - 98; http://dx.doi.org/10.1038/ncb2220; PMID: 21478857
- Rambold AS, Kostelecky B, Elia N, Lippincott-Schwartz J. Tubular network formation protects mitochondria from autophagosomal degradation during nutrient starvation. Proc Natl Acad Sci USA 2011; 108:10190 - 5; http://dx.doi.org/10.1073/pnas.1107402108; PMID: 21646527
- Youle RJ, Narendra DP. Mechanisms of mitophagy. Nat Rev Mol Cell Biol 2011; 12:9 - 14; http://dx.doi.org/10.1038/nrm3028; PMID: 21179058
- Chen H, Chan DC. Mitochondrial dynamics--fusion, fission, movement, and mitophagy--in neurodegenerative diseases. Hum Mol Genet 2009; 18:R169 - 76; http://dx.doi.org/10.1093/hmg/ddp326; PMID: 19808793
- Sheridan C, Martin SJ. Mitochondrial fission/fusion dynamics and apoptosis. Mitochondrion 2010; 10:640 - 8; http://dx.doi.org/10.1016/j.mito.2010.08.005; PMID: 20727425
- Wasilewski M, Scorrano L. The changing shape of mitochondrial apoptosis. Trends Endocrinol Metab 2009; 20:287 - 94; http://dx.doi.org/10.1016/j.tem.2009.03.007; PMID: 19647447
- Youle RJ, Karbowski M. Mitochondrial fission in apoptosis. Nat Rev Mol Cell Biol 2005; 6:657 - 63; http://dx.doi.org/10.1038/nrm1697; PMID: 16025099
- Campello S, Scorrano L. Mitochondrial shape changes: orchestrating cell pathophysiology. EMBO Rep 2010; 11:678 - 84; http://dx.doi.org/10.1038/embor.2010.115; PMID: 20725092
- Parone PA, Da Cruz S, Tondera D, Mattenberger Y, James DI, Maechler P, et al. Preventing mitochondrial fission impairs mitochondrial function and leads to loss of mitochondrial DNA. PLoS ONE 2008; 3:e3257; http://dx.doi.org/10.1371/journal.pone.0003257; PMID: 18806874
- Wang J-X, Jiao J-Q, Li Q, Long B, Wang K, Liu J-P, et al. miR-499 regulates mitochondrial dynamics by targeting calcineurin and dynamin-related protein-1. Nat Med 2011; 17:71 - 8; http://dx.doi.org/10.1038/nm.2282; PMID: 21186368
- Arismendi-Morillo G. Electron microscopy morphology of the mitochondrial network in gliomas and their vascular microenvironment. Biochim Biophys Acta 2011; 1807:602 - 8
- Bodemann BO, Orvedahl A, Cheng T, Ram RR, Ou Y-H, Formstecher E, et al. RalB and the exocyst mediate the cellular starvation response by direct activation of autophagosome assembly. Cell 2011; 144:253 - 67; http://dx.doi.org/10.1016/j.cell.2010.12.018; PMID: 21241894
- Cox AD. Ras history: The saga continues. Small GTPases 2010; 1:2 - 27; http://dx.doi.org/10.4161/sgtp.1.1.12178; PMID: 21686117
- Cassidy-Stone A, Chipuk JE, Ingerman E, Song C, Yoo C, Kuwana T, et al. Chemical inhibition of the mitochondrial division dynamin reveals its role in Bax/Bak-dependent mitochondrial outer membrane permeabilization. Dev Cell 2008; 14:193 - 204; http://dx.doi.org/10.1016/j.devcel.2007.11.019; PMID: 18267088
- Zorzano A, Sebastín D, Segalés J, Palacín M. The molecular machinery of mitochondrial fusion and fission: An opportunity for drug discovery?. Curr Opin Drug Discov Devel 2009; 12:597 - 606; PMID: 19736619