Abstract
The investigation of Rho-family GTPases has uncovered mechanisms for spatiotemporal control of cellular processes such as cell polarization, movement, morphogenesis, and cell division. Now Rho GTPase plays another leading role in the discovery of a new signaling mechanism for auxin, a multi-functional hormone that regulates pattern formation in plants. Arabidopsis leaf epidermal pavement cells (PCs) develop the puzzle-piece cell shape with interlocking lobes and indentations via interdigitated cellular growth.1 Through the ABP1 (Auxin Binding Protein 1) cell surface receptor, auxin coordinately activates two mutually exclusive Rho GTPase signaling pathways that are activated in the complementary lobing and indenting sides of adjacent cells: the ROP2 pathway for lobe formation and the ROP6 pathway for promoting indentation. This new signaling mechanism also involves ROP2-dependent polar accumulation of PIN1 in the plasma membrane, a member of the PIN auxin efflux carrier family that is critical for the formation of various developmental patterns including the PC interdigitation pattern. This Rho-dependent auxin signaling mechanism explains how interdigitated cellular growth is coordinated. In this extra view, we propose that the same mechanism can also explain how a uniform auxin signal initiates the formation of the interdigitated pattern.
Introduction
In the late 19th century, Charles and Francis Darwin demonstrated phototropic bending of coleoptiles is mediated by a diffusible molecule,Citation2 which was later shown to be auxin (indole acetic acid). Since Darwin's finding, this small molecule hormone has been in the center stage of the study of hormone-mediated morphogenetic patterns in plants. Auxin participates in the modulation of nearly every aspect of growth and development in plants such as embryogenesis,Citation3 tissue differentiation,Citation4,Citation5 formation of shoot and root primordia and their patterning,Citation6,Citation7 and environmentmodulated growth behaviors such as gravitropic response and shade avoidance.Citation8,Citation9 How could such a small molecule orchestrate so many diverse processes? One common underlying mechanism appears to be auxin regulation of gene expression through the TIR1/AFB-AUX/IAA auxin signaling system.Citation10–Citation12 Transport Inhibitor resistant 1 (TIR1)/auxin-related F-box (AFB) proteins are F-box proteins that target the AUX/IAA transcriptional repressors for ubiquitinylation-dependent degradation.Citation13,Citation14 Auxin directly binds to TIR1/AFBs,Citation15–Citation17 and acts like a glue to stabilize the binding of substrate AUX/IAA proteins.Citation18 Degradation of AUX/IAA proteins results in the release of ARF transcription factors that are repressed in the presence of AUX/IAA proteins.
Another common mechanism for the versatile auxin actions is its polar transport to generate its localized accumulation, concentration gradients and directional flow.Citation19,Citation20 In this regard, auxin shares some of the properties of morphogens involved in pattern formation in animals.Citation21 Polar auxin transport (PAT) largely relies on a family of auxin efflux carriers named PINs (pin-formed), which are polarly localized to the plasma membrane (PM) via their asymmetric endocytosis and recycling.Citation22–Citation24 Molecular mechanisms underlying PIN polarization are poorly understood, but auxin itself has been implicated in the regulation of PIN distribution via a TIR1/AFB-independent pathway.Citation25,Citation26
ABP1 was originally identified from maize coleoptiles as a protein having a capacity to bind to radio-labeled auxin.Citation27 Cells in Arabidopsis abp1 knockout embryos fail to undergo polar expansion, leading to early embryo-lethality.Citation28 ABP1 has also been implicated in the regulation of rapid auxin responses such as activation of H+ pump ATPases, transient increase of calcium inside cells and swelling of protoplasts as well as the modulation of cell division and cell expansion.Citation29–Citation34 Although the majority of ABP1 is present in ER, a fraction of ABP1 is found in the outer face of the PM through its association with a GPI-anchored protein,Citation35–Citation38 suggesting that ABP1 could be a cell surface auxin receptor for the activation of non-transcriptional auxin responses.Citation32 This notion is strongly supported by our recent work demonstrating that ABP1 is required for auxin-dependent rapid activation of plant Rho-family members, ROP2 and ROP6, which are localized to and activated at the plasma membrane.Citation1,Citation39 ROP2 activation by auxin also requires PIN1, which is localized to the plasma membrane region where ROP2 is activated.Citation1 Since PIN1 is involved in auxin export, this result further supports the hypothesis that ABP1-dependent auxin perception that activates ROP2 occurs on the cell surface. The action of ABP1 at the cell surface has also been demonstrated for its role in the regulation of PIN1 endocytosis.Citation26 Taken together these findings established a non-transcriptional auxin signaling system, which occurs at the junction of the cell surface and the plasma membrane and underlies the ROP-mediated interdigitated cell growth in leaf epidermal pavement cells.Citation1,Citation39 However, these findings do not exclude a role for ER-localized ABP1 in mediating auxin signaling, given ER accumulation of a large amount of ABP1.Citation35,Citation40,Citation41 Recent work has also implicated PIN5 in the auxin transport from the cytosol to ER lumen.Citation42 Interestingly SPIKE1, a putative RhoGEF, was detected in ER.Citation43 These observations raise an interesting possibility that ABP1 might also mediate auxin signaling through other ROPs in ER.Citation44
Importantly the work by Xu and colleagues also reveal a ROP GTPase-dependent connection between PIN polarization and ABP1 in these cells.Citation1 The regulation of PIN distribution by the cell surface ABP1 is also demonstrated in another recent report.Citation26 Furthermore, the ICR1/RIP1 ROP-interacting protein has been shown to regulate PIN distribution through its promotion of PIN recycling.Citation39,Citation45 These findings support the notion that the new Rho GTPase-dependent auxin signaling system participates in the modulation of PIN-dependent pattern formation in plants.
In this extra view, we will discuss how uniform auxin could activate the interdigitation patterning of PCs through its coordination of the two counteracting ABP1-ROP pathways that are also used to coordinate interdigitated growth in PCs.Citation1
A Novel Auxin Signaling Pathway at the PM
Recent studies established a new auxin signaling mechanism that occurs at the cell surface.Citation1,Citation26 Different from the TIR1/AFB1 pathway that regulates gene expression, this new auxin signaling system directly regulates cytoplamsic events through ABP1 and ROP signaling pathways.Citation1,Citation26 The rapid ABP1-dependant activation of ROPs by auxin, which increases one fold within 30 sec, implies that the binding of auxin to ABP1 may directly activates transmembrane signaling that further activates ROPs. Secreted ABP1 is associated with a GPI-anchored protein on the outer surface of the PM,Citation38 which does not directly participate in transmembrane signaling. We speculate that a transmembrane partner is required for the transmission of extracellular auxin/ABP1 signal to the cytoplasm. Activation of both ROP2 and ROP6 by auxin, but with different kinetics, supports the notion of the co-receptors for ABP1. Receptor-like kinases (RLKs), a group of transmembrane proteins that can transmit extracellular signals to intracellular events, are good candidates for the coreceptors of ABP1. Recent studies indicate that RLKs can regulate RhoGEF activity through their direct interactions.Citation46,Citation47 Some RLKs could act together with ABP1 to perceive auxin signal, and then transmit signals to RopGEFs.
Auxin Coordinates Interdigitated Growth through Complementary ROP2 and ROP6 in PCs
In leaf PCs, ROP2 and ROP6 are locally activated at the opposing sides of the cell wall and are mutually exclusive along the PM within a PC.Citation48–Citation50 Therefore, localized extracellular auxin is expected to activate ROP2 and ROP6 at the opposing sites of the adjacent cells (lobes and indentations). We speculate the existence of mechanisms for the generation and maintenance of the local accumulation of extracellular auxin. PIN1 is localized to the lobe tips and this localization requires localized ROP2, suggesting a localized auxin-ROP2-PIN1-auxin positive feedback loop could be the mechanism for the generation and maintenance of the localized extracellular auxin.Citation1 However, auxin is highly diffusible, and it would be impossible to maintain the localized positive feedback loop. Therefore, we propose that mutual inhibition of the ROP2 and ROP6 pathways could dynamically maintain the localized positive feedback loop to generate sustained local auxin accumulation along the cell wall. As such local extracellular auxin coordinately activates both ROP2 at the lobing side and ROP6 at the complementary indenting side. This model can explain how extracellular auxin coordinates lobe and indentation development at the steady-state, once the interdigitation pattern has been initiated.
Uniform Auxin may Initiate Interdigitation Pattern in Pavement Cells
Application of uniform auxin promotes the formation of the interdigitated cell pattern in leaf pavement cells. In a yuc mutant, which contains lower concentration of auxin, the interdigitation in PCs is inhibited.Citation1 Auxin treatment re-activated the interdigitation pattern in yuc mutant, which indicates that uniform auxin activates the interdigitation patterning in PCs. How does uniformly applied auxin lead to the initial establishment of cell cortical regions that define lobe—or indentation—forming sites to activate the interdigitation pattern? In other cell types, a uniform field of signal activates cell polarization through a stochastic local activation of the positive feedback loop at the leading edge and its interaction with an antagonistic pathway in the trailing edge.Citation25,Citation45,Citation51,Citation52 Similarly, we speculate that a uniform auxin field could transform into a localized signal through a stochastic local activation of the ROP2-PIN1 positive feedback loop and its interaction with the antagonistic ROP6 pathway. Given stochastic local activation of an intracellular ROP2 signaling component, the positive feedback loop would be transiently initiated locally only at one side of but not simultaneously at both of the opposing sides of a cell wall region. Given a local activation of the ROP2-PIN1 loop activated at a random site in cell 1, ROP6 would be suppressed at this site due to the mutual inhibition of the ROP2 and ROP6 pathways in this cell ().Citation48–Citation50 The feedback loop would rapidly increase the level of extracellular auxin, which would preferentially activate the ROP6 pathway at the complementary site in cell 2, because at these higher levels, auxin causes greater ROP6 activation compared with ROP2.Citation1 Once ROP6 were preferentially activated at the complementary site in cell 2, ROP2 would be inhibited at this site again due to the mutual inhibition of the ROP2 and ROP6 pathways. Therefore, we propose a stochastic local activation of the ROP2-PIN1-axuin positive feedback loop, the differential ROP2 and ROP6 activation, and the mutual inhibition between the ROP2 and ROP6 pathways within one PC collectively provide a mechanism for the transformation of uniform auxin into local extracellular auxin and for subsequent sustained activation of the ROP2 and ROP6 pathways at the two opposing sides of the extracellular auxin. Consequently uniform auxin could initiate the formation of the interdigitated pattern of ROP2 and ROP6 activation and the formation of interdigitated lobes and indentations between neighboring cells. This coordination of cell polarity within and between pavement cells through the regulation of two mutually excluding Rho GTPase signaling pathways by an uniform auxin signal remarkably resembles the Rho GTPase-dependent formation of front and back end cell polarity mediated by a uniform signal in animal neutrophil cells.Citation51 Similar interactions of multiple Rho GTPase signaling pathways could also be used to explain the coordination of PIN protein polarization required for the formation of various plant developmental and morphological patterns discussed below.
The ABP1 Activation of Two Mutually Exclusive ROP Pathways could Coordinate Polarization of PINs Required for Auxin Gradient Formation and Auxin Flow
The mechanism for auxin promotion of PC interdigitated growth through the coordination of the two mutually exclusive ROP2 and ROP6 pathways might also apply to the coordination of PIN polarization in other cell systems such as in roots and shoot apex. In PCs, the auxin-activated ROP2 pathway is essential for PIN1 polar localization at the lobe apex by inhibiting its internalization.Citation1 We also speculate that the ROP6 pathway could regulate PIN1 localization on the PM by promoting its internalization at the indentation site. In root cells, ABP1 is reported to mediate the auxin inhibition of clathrin-dependent endocytosis, which further regulates PIN1 localization.Citation26 However, the molecular mechanism about this regulation is still not clear, although some evidence also support a role for ROP signaling in PIN localization in roots. Overexpression of ROP2 leads to the enhanced polar accumulation of PIN2, while expression of a dominant negative (DN) form of ROP2 causeed a delay in gravitropic response, which is mediated by PIN2.Citation53 ICR1, a downstream effector of ROPs, modulates PIN localization in both root cells and embryos apparently by regulating PIN protein recycling to the PM.Citation45,Citation54 These data hints the presence of ABP1-ROP mediated auxin signaling pathways in the regulation of PIN polarization in root cells or other systems. We speculate that a ABP1-ROP signaling system coordinates PIN polarization in root or other cells in a manner similar to ROP2- and ROP6-coordination of PIN localization in pavement cells (). For example, in root stele cells where PIN1 is localized basally, a ROP2 or ROP2-like pathway could be activated by auxin at the basal site, which would then inhibit PIN1 endocytosis locally, generating a positive feedback loop as in PCs. At this site, a ROP6 or ROP6-like pathway would be inhibited by the ROP2 pathway. At the opposing side of neighboring cells involved in the auxin transport in the same direction, however, the ROP6 or ROP6-like pathway could be activated and could inhibit the ROP2 pathway to exclude PIN1 localization at this side (). This is a tantalizing speculation that would be worthy of future investigation.
Future Perspectives
Auxin regulation of multiple coordinating and counteracting Rho GTPase signaling pathways provides an elegant mechanism underlying interdigitated cell growth in leaf PCs and may also serve to initiate the formation of the interdigitated cell pattern and coordination of PIN protein polarization in various plant systems. It would be interesting to see if the analogy of such elegant mechanisms could be extended to moving cells in animal systems. Future experimental and theoretical studies should generate unifying principles and paradigms for the coordination of cell polarization processes by the highly conserved Rho family of small GTPases.
Figures and Tables
Figure 1 A working model for the auxin regulation of the pavement cell interdigitation pattern. (A) Auxin is proposed to modulate PC interdigitation through activating mutually exclusive ROP2 and ROP6 pathways, which are localized to the lobing and indenting side and promote lobe protrusion and neck indentation, respectively. ROP2 promotes PIN1 localization to the apex of lobe that further activates an auxin-ROP2-PIN1-auxin positive feedback loop. (B) Auxin activates ROP2 and ROP6 with different kinetics. ROP2 and ROP6 have similar response when the auxin level is low as 1 nM, but ROP6 activation has higher saturation level at 1 µM while ROP2 activation saturated at around 100 nM.
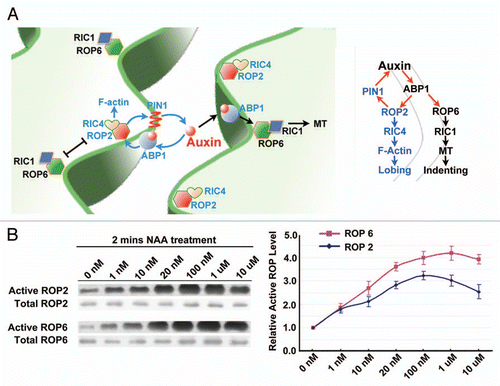
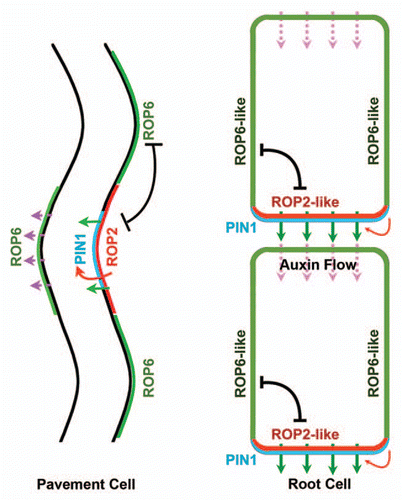
Figure 2 ROP GTPase regulation of PIN protein localization in different cells. In leaf PCs, ROP2 promotes PIN1 PM localization at the lobe tip and further initiates a positive feedback at this region. The mutual inhibition between ROP2 and ROP6 help to restrict the localization of PIN1 at the lobe site. In root vascular cells, ROP2 or ROP2-like pathway is speculated to participate in a positive feedback at the basal site that continuously promotes PIN1 localization and auxin flow. In other sides, a ROP6 or ROP6-like pathway is speculated to inhibit the ROP2 pathway that further restricts PIN1 localization to the basal site.
Acknowledgements
We thank members of the Yang laboratory for stimulating discussions. This work is supported by grants from the US. National Institute of General Medical Sciences to Z.Y. (GM081451).
Extra View to: Xu T, Wen M, Nagawa S, Fu Y, Chen JG, Wu MJ, et al. Cell surface- and Rho GTPase-based auxin signaling controls cellular interdigitation in Arabidopsis. Cell 2010; 143:99 - 110; PMID: 20887895; http://dx.doi.org/10.1016/j.cell.2010.09.003
References
- Xu T, Wen M, Nagawa S, Fu Y, Chen JG, Wu MJ, et al. Cell Surface- and Rho GTPase-Based Auxin Signaling Controls Cellular Interdigitation in Arabidopsis. Cell 2010; 143:99 - 110; PMID: 20887895; http://dx.doi.org/10.1016/j.cell.2010.09.003
- Darwin C, Darwin F. The power of movement in plants Das Bewegungsvermögen der Pflanze 13 (Schweizerbart'sche Verlagsbuchhandlung 1881)
- Friml J, et al. Efflux-dependent auxin gradients establish the apical-basal axis of Arabidopsis. Nature 2003; 426:147 - 153; PMID: 14614497; http://dx.doi.org/10.1038/nature02085
- Hardtke CS, Berleth T. The Arabidopsis gene MONOPTEROS encodes a transcription factor mediating embryo axis formation and vascular development. EMBO J 1998; 17:1405 - 1411; PMID: 9482737; http://dx.doi.org/10.1093/emboj/17.5.1405
- Scarpella E, Marcos D, Friml J, Berleth T. Control of leaf vascular patterning by polar auxin transport. Genes Dev 2006; 20:1015 - 1027; PMID: 16618807; http://dx.doi.org/10.1101/gad.1402406
- Benková E. Local, efflux-dependent auxin gradients as a common module for plant organ formation. Cell 2003; 115:591 - 602; PMID: 14651850; http://dx.doi.org/10.1016/S0092-8674(03)00924-3
- Reinhardt D. Phyllotaxis—a new chapter in an old tale about beauty and magic numbers. Curr Opin Plant Biol 2005; 8:487 - 493; PMID: 16054863; http://dx.doi.org/10.1016/j.pbi.2005.07.012
- Esmon CA, Pedmale UV, Liscum E. Plant tropisms: providing the power of movement to a sessile organism. Int J Dev Biol 2005; 49:665 - 674; PMID: 16096973; http://dx.doi.org/10.1387/ijdb.052028ce
- Tao Y, et al. Rapid synthesis of auxin via a new tryptophan-dependent pathway is required for shade avoidance in plants. Cell 2008; 133:164 - 176; PMID: 18394996; http://dx.doi.org/10.1016/j.cell.2008.01.049
- Chapman EJ, Estelle M. Mechanism of Auxin-Regulated Gene Expression in Plants. Annu Rev Genet 2009; 43:265 - 285; PMID: 19686081; http://dx.doi.org/10.1146/annurev-genet-102108-134148
- Calderon-Villalobos LI, Tan X, Zheng N, Estelle M. Auxin perception—structural insights. Cold Spring Harb Perspect Biol 2010; 2:a005546; PMID: 20504967; http://dx.doi.org/10.1101/cshperspect.a005546
- Perrot-Rechenmann C. Cellular Responses to Auxin: Division versus Expansion. Csh Perspect Biol 2010;
- Ruegger M, et al. The TIR1 protein of Arabidopsis functions in auxin response and is related to human SKP2 and yeast Grr1p. Genes Dev 1998; 12:198 - 207; PMID: 9436980; http://dx.doi.org/10.1101/gad.12.2.198
- Gray WM, Kepinski S, Rouse D, Leyser O, Estelle M. Auxin regulates SCFTIR1-dependent degradation of AUX/IAA proteins. Nature 2001; 414:271 - 276; PMID: 11713520; http://dx.doi.org/10.1038/35104500
- Dharmasiri N, Dharmasiri S, Estelle M. The F-box protein TIR1 is an auxin receptor. Nature 2005; 435:441 - 445; PMID: 15917797; http://dx.doi.org/10.1038/nature03543
- Dharmasiri N, Dharmasiri S, Weijers D, Lechner E, Yamada M, Hobbie L, et al. Plant development is regulated by a family of auxin receptor F box proteins. Dev Cell 2005; 9:109 - 119; PMID: 15992545; http://dx.doi.org/10.1016/j.devcel.2005.05.014
- Kepinski S, Leyser O. The Arabidopsis F-box protein TIR1 is an auxin receptor. Nature 2005; 435:446 - 451; PMID: 15917798; http://dx.doi.org/10.1038/nature03542
- Tan X, Calderon-Villalobos LIA, Sharon M, Zheng C, Robinson CV, Estelle M, et al. Mechanism of auxin perception by the TIR1 ubiquitin ligase. Nature 2007; 446:640 - 645; PMID: 17410169; http://dx.doi.org/10.1038/nature05731
- Boutté Y, Ikeda Y, Grebe M. Mechanisms of auxin-dependent cell and tissue polarity. Curr Opin Plant Biol 2007; 10:616 - 623; PMID: 17720615; http://dx.doi.org/10.1016/j.pbi.2007.07.008
- Vanneste S, Friml J. Auxin: a trigger for change in plant development. Cell 2009; 136:1005 - 1016; PMID: 19303845; http://dx.doi.org/10.1016/j.cell.2009.03.001
- Wartlick O, Kicheva A, Gonzalez-Gaitan M. Morphogen gradient formation. Cold Spring Harb Perspect Biol 2009; 1:1255; PMID: 20066104; http://dx.doi.org/10.1101/cshperspect.a001255
- Gälweiler L, Guan C, Müller A, Wisman E, Mendgen K, Yephremov A, et al. Regulation of polar auxin transport by AtPIN1 in Arabidopsis vascular tissue. Science 1998; 282:2226 - 2230; PMID: 9856939; http://dx.doi.org/10.1126/science.282.5397.2226
- Geldner N, Anders N, Wolters H, Keicher J, Kornberger W, Muller P, et al. The Arabidopsis GNOM ARF-GEF mediates endosomal recycling, auxin transport and auxin-dependent plant growth. Cell 2003; 112:219 - 230; PMID: 12553910; http://dx.doi.org/10.1016/S0092-8674(03)00003-5
- Dhonukshe P, Tanaka H, Goh T, Ebine K, Mähönen AP, Prasad K, et al. Generation of cell polarity in plants links endocytosis, auxin distribution and cell fate decisions. Nature 2008; 456:962 - 966; PMID: 18953331; http://dx.doi.org/10.1038/nature07409
- Paciorek T, Zazímalová E, Ruthardt N, Petrásek J, Stierhof YD, Kleine-Vehn J, et al. Auxin inhibits endocytosis and promotes its own efflux from cells. Nature 2005; 435:1251 - 1256; PMID: 15988527; http://dx.doi.org/10.1038/nature03633
- Robert S, Kleine-Vehn J, Barbez E, Sauer M, Paciorek T, Baster P, et al. ABP1 mediates auxin inhibition of clathrin-dependent endocytosis in Arabidopsis. Cell 2010; 143:111 - 121; PMID: 20887896; http://dx.doi.org/10.1016/j.cell.2010.09.027
- Hertel R, Russo VEA, Thomson KS. In-vitro auxin binding to particulate cell fractions from corn coleoptiles. Planta 1972; 107:325; http://dx.doi.org/10.1007/BF00386394
- Chen JG, Ullah H, Young JC, Sussman MR, Jones AM. ABP1 is required for organized cell elongation and division in Arabidopsis embryogenesis. Genes Dev 2001; 15:902 - 911; PMID: 11297513; http://dx.doi.org/10.1101/gad.866201
- Thiel G, Blatt MR, Fricker MD, White IR, Millner P. Modulation of K+ Channels in Vicia Stomatal Guard-Cells by Peptide Homologs to the Auxin-Binding Protein-C Terminus. Proc Natl Acad Sci USA 1993; 90:11493 - 11497; PMID: 8265579; http://dx.doi.org/10.1073/pnas.90.24.11493
- Bauly JM, Sealy IM, Macdonald H, Brearley J, Dröge S, Hillmer S, et al. Overexpression of auxin-binding protein enhances the sensitivity of guard cells to auxin. Plant Physiol 2000; 124:1229 - 1238; PMID: 11080299; http://dx.doi.org/10.1104/pp.124.3.1229
- Yamagami M, Haga K, Napier RM, Iino M. Two distinct signaling pathways participate in auxin-induced swelling of pea epidermal protoplasts. Plant Physiol 2004; 134:735 - 747; PMID: 14764902; http://dx.doi.org/10.1104/pp.103.031294
- Badescu GO, Napier RM. Receptors for auxin: will it all end in TIRs?. Trends Plant Sci 2006; 11:217 - 223; PMID: 16564202; http://dx.doi.org/10.1016/j.tplants.2006.03.001
- Braun N, Wyrzykowska J, Muller P, David K, Couch D, Perrot-Rechenmann C, et al. Conditional repression of AUXIN BINDING PROTEIN1 reveals that it coordinates cell division and cell expansion during postembryonic shoot development in Arabidopsis and tobacco. Plant Cell 2008; 20:2746 - 2762; PMID: 18952781; http://dx.doi.org/10.1105/tpc.108.059048
- Tromas A, Braun N, Muller P, Khodus T, Paponov IA, Palme K, et al. The AUXIN BINDING PROTEIN 1 is required for differential auxin responses mediating root growth. PLoS ONE 2009; 4:6648; PMID: 19777056; http://dx.doi.org/10.1371/journal.pone.0006648
- Jones AM, Herman E. KDEL-containing, auxin-binding protein is secreted to the plasma membrane and cell wall. Plant Physiol 1993; 101:595 - 606; PMID: 12231715
- Diekmann W, Venis MA, Robinson DG. Auxins Induce Clustering of the Auxin-Binding Protein at the Surface of Maize Coleoptile Protoplasts. Proc Natl Acad Sci USA 1995; 92:3425 - 3429; PMID: 11607527; http://dx.doi.org/10.1073/pnas.92.8.3425
- Henderson J, Bauly JM, Ashford DA, Oliver SC, Hawes CR, Lazarus CM, et al. Retention of maize auxin-binding protein in the endoplasmic reticulum: Quantifying escape and the role of auxin. Planta 1997; 202:313 - 323; PMID: 9232903; http://dx.doi.org/10.1007/s004250050133
- Shimomura S. Identification of a glycosylphosphatidylinositol-anchored plasma membrane protein interacting with the C-terminus of auxin-binding protein 1: A photoaffinity crosslinking study. Plant Mol Biol 2006; 60:663 - 677; PMID: 16649105; http://dx.doi.org/10.1007/s11103-005-5471-1
- Nagawa S, Xu T, Yang Z. RHO GTPase in plants: Conservation and invention of regulators and effectors. Small GTPases 2010; 1:78 - 88; PMID: 21686259; http://dx.doi.org/10.4161/sgtp.1.2.14544
- Tian H, Klämbt D, Jones AM. Auxin-binding protein 1 does not bind auxin within the endoplasmic reticulum despite this being the predominant subcellular location for this hormone receptor. J Biol Chem 1995; 270:26962 - 26969; PMID: 7592943; http://dx.doi.org/10.1074/jbc.270.45.26962
- Leblanc N, David K, Grosclaude J, Pradier JM, Barbier-Brygoo H, Labiau S, et al. A novel immunological approach establishes that the auxin-binding protein, Nt-abp1, is an element involved in auxin signaling at the plasma membrane. J Biol Chem 1999; 274:28314 - 28320; PMID: 10497189; http://dx.doi.org/10.1074/jbc.274.40.28314
- Mravec J, Skupa P, Bailly A, Hoyerová K, Krecek P, Bielach A, et al. Subcellular homeostasis of phytohormone auxin is mediated by the ER-localized PIN5 transporter. Nature 2009; 459:1136 - 1140; PMID: 19506555; http://dx.doi.org/10.1038/nature08066
- Zhang C, Kotchoni SO, Samuels AL, Szymanski DB. SPIKE1 signals originate from and assemble specialized domains of the endoplasmic reticulum. Curr Biol 2010; 20:2144 - 2149; PMID: 21109438; http://dx.doi.org/10.1016/j.cub.2010.11.016
- Wu HM, Hazak O, Cheung AY, Yalovsky S. RAC/ROP GTPases and Auxin Signaling. Plant Cell 2011; 23:4 1208 - 1218; PMID: 21478442; http://dx.doi.org/10.1105/tpc.111.083907
- Hazak O, Bloch D, Poraty L, Sternberg H, Zhang J, Friml J, et al. A rho scaffold integrates the secretory system with feedback mechanisms in regulation of auxin distribution. PLoS Biol 2010; 8:1000282; PMID: 20098722; http://dx.doi.org/10.1371/journal.pbio.1000282
- Kaothien P, Ok SH, Shuai B, Wengier D, Cotter R, Kelley D, et al. Kinase partner protein interacts with the LePRK1 and LePRK2 receptor kinases and plays a role in polarized pollen tube growth. Plant J 2005; 42:492 - 503; PMID: 15860008; http://dx.doi.org/10.1111/j.1365-313X.2005.02388.x
- Zhang Y, McCormick S. A distinct mechanism regulating a pollen-specific guanine nucleotide exchange factor for the small GTPase Rop in Arabidopsis thaliana. Proc Natl Acad Sci USA 2007; 104:18830 - 18835; PMID: 18000057; http://dx.doi.org/10.1073/pnas.0705874104
- Fu Y, Gu Y, Zheng Z, Wasteneys G, Yang Z. Arabidopsis interdigitating cell growth requires two antagonistic pathways with opposing action on cell morphogenesis. Cell 2005; 120:687 - 700; PMID: 15766531; http://dx.doi.org/10.1016/j.cell.2004.12.026
- Yang Z. Cell polarity signaling in Arabidopsis. Annu Rev Cell Dev Biol 2008; 24:551 - 575; PMID: 18837672; http://dx.doi.org/10.1146/annurev.cellbio.23.090506.123233
- Fu Y, Xu T, Zhu L, Wen M, Yang Z. A ROP GTPase signaling pathway controls cortical microtubule ordering and cell expansion in Arabidopsis. Curr Biol 2009; 19:1827 - 1832; PMID: 19818614; http://dx.doi.org/10.1016/j.cub.2009.08.052
- Xu J, Wang F, Van Keymeulen A, Herzmark P, Straight A, Kelly K, et al. Divergent Signals and Cytoskeletal Assemblies Regulate Self-Organizing Polarity in Neutrophils. Cell 2003; 114:201 - 214; PMID: 12887922; http://dx.doi.org/10.1016/S0092-8674(03)00555-5
- Van Keymeulen A, Wong K, Knight ZA, Govaerts C, Hahn KM, Shokat KM, et al. To stabilize neutrophil polarity, PIP3 and Cdc42 augment RhoA activity at the back as well as signals at the front. J Cell Biol 2006; 174:437 - 445; PMID: 16864657; http://dx.doi.org/10.1083/jcb.200604113
- Li L, Xu J, Xu ZH, Xue HW. Brassinosteroids stimulate plant tropisms through modulation of polar auxin transport in Brassica and Arabidopsis. Plant Cell 2005; 17:2738 - 2753; PMID: 16141452; http://dx.doi.org/10.1105/tpc.105.034397
- Bloch D, Hazak O, Lavy M, Yalovsky S. A novel ROP/RAC GTPase effector integrates plant cell form and pattern formation. Plant Signal Behav 2008; 3:41 - 43; PMID: 19704766; http://dx.doi.org/10.4161/psb.3.1.4838