Abstract
After endocytosis, a selective endocytic recycling process returns many endocytosed molecules back to the plasma membrane. The RAB-10/Rab10 GTPase is known to be a key recycling regulator for specific cargo molecules. New evidence, focused on C. elegans RAB-10 in polarized epithelia, points to a key role of RAB-10 in the regulation of endosomal phosphatidylinositol-4,5-bisphosphate (PI(4,5)P2) levels. In turn, PI(4,5)P2 levels strongly influence the recruitment of many peripheral membrane proteins, including those important for vesicle budding through their membrane bending activities. Part of the effect of RAB-10 on endosomal PI(4,5)P2 is through its newly identified effector CNT-1, a predicted GTPase activating protein (GAP) of the small GTPase ARF-6/Arf6. In mammals PI(4,5)P2 generating enzymes are known Arf6 effectors. In C. elegans we found that RAB-10, CNT-1 and ARF-6 are present on the same endosomes, that RAB-10 recruits CNT-1 to endosomes, and that loss of CNT-1 or RAB-10 leads to overaccumulation of endosomal PI(4,5)P2, presumably via hyperactivation of endosomal ARF-6. In turn this leads to over-recruitment of PI(4,5)P2-dependent membrane-bending proteins RME-1/Ehd and SDPN-1/Syndapin/PACSIN. Conversely, in arf-6 mutants, endosomal PI(4,5)P2 levels were reduced and endosomal recruitment of RME-1 and SDPN-1 failed. This work makes an unexpected link between distinct classes of small GTPases that control endocytic recycling, and provides insight into how this interaction affects endosome function at the level of lipid phosphorylation.
In response to interactions between cells, a constantly changing extracellular environment, and changes in intracellular physiology, cells optimize their fitness by strictly regulating the composition of their plasma membranes. The steady-state molecular composition of the plasma membrane depends heavily upon the relative rates and cargo selectivity of the secretory, endocytosis and endocytic recycling pathways. Here we focus on recent advances that allow insight into the interplay between members of the Rab and Arf small GTPase families that control the endocytic recycling process, and in particular their effects on phosphoinositide lipids and the recruitment of membrane-bending proteins.
After internalization by endocytosis, cargo is sorted for delivery to several destinations. Endocytic recycling pathways can return endocytosed molecules to the plasma membrane directly, indirectly via the recycling endosome, or to the Golgi via retrograde transport. Cargo can also be actively sorted for degradation in the lysosome. Endocytic recycling pathways play key roles in diverse processes such as nutrient uptake, cell polarity, cell migration, cytokinesis, synaptic plasticity, immune response, control of blood sugar levels and growth factor receptor modulation.Citation1 Thus recycling contributes essential functions to many aspects of cellular and organismal development and homeostasis, and understanding the molecular mechanisms of endocytic recycling is a major goal of modern cell biology. Toward this end we have developed the intestinal epithelial cells of the microscopic nematode C. elegans as a simple model system, within the intact animal, to elucidate general mechanistic principles of the recycling process. This system also allows insight into how such pathways are coordinated in the context of a polarized cell, where the presence of two distinct plasma membrane domains necessitates a more elaborate sorting process.
A variety of small GTPases have been implicated in endocytic recycling, including Rab4, Rab5, Rab8, Rab10, Rab11, Rab35 and Arf6.Citation2-Citation4 The requirement for RAB-10/Rab10 in recycling was first demonstrated in the C. elegans intestine, where RAB-10 was shown to function in basolateral recycling, with the most pronounced effects on cargos lacking AP-2 and clathrin sorting sequences.Citation5,Citation6 RAB-10 was shown to be physically associated with endosomes along the recycling pathway in the polarized intestinal epithelium, and loss of RAB-10 appeared to block recycling prior to the entry of cargo into RME-1-positive recycling tubules. Work in C. elegans also identified RAB-10 as a key regulator of AMPA-type glutamate receptor GLR-1 in postsynaptic membranes of the nervous system.Citation7 Studies in MDCK cells identified requirements for Rab10 in basolateral recycling, and linked Rab10 to the common recycling endosome, a shared sorting station for cargo endocytosed from both basolateral and apical plasma membrane domains.Citation8 Despite these advances, the detailed mechanism(s) by which RAB-10 promotes recycling remained elusive.
Since Rab-GTPases generally regulate membrane transport through their effectors, we sought to identify the mechanism by which RAB-10 controls recycling by identifying RAB-10 effectors. In a yeast two-hybrid screen we identified RAB-10(GTP) binding protein CNT-1, the only C. elegans homolog of mammalian Arf6 GTPase-activating proteins (Arf GAP) ACAP1 and ACAP2. Arf6 is an important regulator of endocytic recycling, membrane lipid modifications, and nucleation of membrane associated actin polymerization at the plasma membrane and endosomes.Citation9,Citation10 Notably, Arf6 can activate PIP5-kinase (PIP5KI) to generate phosphatidylinositol-4,5-bisphosphate (PI(4,5)P2).Citation11,Citation12 ACAPs are known to function in endocytic recycling in mammals, but their precise roles and functional connections with other recycling regulators remain poorly defined. Our work indicates that the C-terminal ANK repeats of C. elegans CNT-1/ACAP bind to RAB-10, and that RAB-10 is required to recruit CNT-1 to endosomes of the C. elegans intestinal epithelia.Citation13 This was similar to other recent results in mammals showing that Rab35 binds and recruits ACAP2, with the ACAP2 ANK repeats acting as the Rab35 binding surface.Citation14 In fact we found that in vitro C. elegans CNT-1 can also bind to RAB-8 and RAB-35; although for RAB-8 the effects on CNT-1 were modest, and no effect was found for RAB-35 on CNT-1. The interaction of RAB-35 with CNT-1 may be important in C. elegans in other cell-types or developmental times that were not examined. Both studies indicated that certain Rabs can regulate recycling by controlling the activation state of ARF-6/Arf6. Rab35-mediated regulation of Arf6 via ACAP2 was important for promoting the NGF-induced outgrowth of neurites in cultured mammalian cells.Citation15 The work in C. elegans extended this analysis to a physiologically relevant intact animal system rather than cultured cells, and directly determined the effects on endosomal PI(4,5)P2. Furthermore our study documented loss of recruitment of PI(4,5)P2-binding membrane bending proteins RME-1/Ehd and SDPN-1/Syndapin/PACSIN upon loss of ARF-6, and over-recruitment of both membrane bending proteins upon loss of CNT-1 or RAB-10. Finally we correlated these effects with the trapping of specific classes of recycling transmembrane cargo proteins when RAB-10, CNT-1 or ARF-6 were missing.
Because each of these proteins is encoded by only a single gene in C. elegans, and because we used true knockout mutants of rab-10, cnt-1 and arf-6, rather than using less rigorous RNAi-based approaches, we were able to show that the loss of RAB-10 induced a stronger augmentation in PI(4,5)P2 level than did loss of CNT-1, and found that loss of ARF-6 could alleviate only part of the increased level of endosomal PI(4,5)P2 in rab-10 mutants. These results indicated that there must be an additional mechanism by which RAB-10 affects PI(4,5)P2 levels, in addition to that mediated by CNT-1 and ARF-6. Thus it seems quite likely that RAB-10 can also affect other PI-kinases and/or PI-phosphatases either directly or through additional effectors, possibly even via other Arfs. Identifying this additional mechanism will be an important goal for the future.
In mammals ACAP1 has been reported to be part of a clathrin complex that functions on endosomes to promote the recycling of the insulin responsive glucose transporter Glut4, and the adhesion molecule β-integrin.Citation16 Our in vivo data also provide indirect evidence of a clathrin and CNT-1/ACAP interaction. However, previous studies never established if the functional interaction of ACAP and endosomal clathrin requires Arf6. Furthermore, it remains unclear what the role of clathrin coats would be in endocytic recycling. Since Arf proteins most commonly promote membrane traffic by recruiting vesicle coat proteins, such as clathrin adaptors, loss of a recycling promoting Arf might be expected to reduce the recruitment of endosomal clathrin.Citation17,Citation18 However, endosomal clathrin levels increased upon the loss of ARF-6 in our studies. Another potential model for the functional relevance of a CNT-1/ACAP interaction with endosomal clathrin could be at the interface of the recycling and degradative pathways. Clathrin lattices on endosomes have been suggested to form degradative subdomains on endosomes together with HRS and ESCRT proteins, facilitating the sorting of proteins destined for lysosomal degradation.Citation19-Citation21 Thus CNT-1/ACAPs could function with clathrin on the endosomal subdomains to maintain the proper balance between recycling and degradative traffic. It will therefore be worthwhile to test functional involvement of CNT-1 in the retrograde transport and degradation pathway which is very sensitive to changes in such clathrin-coated subdomains.Citation21 Additionally, CNT-1 and ACAPs harbors N-terminal BAR domains. Some BAR domains are thought to be membrane curvature sensors and often display membrane remodeling capability.Citation22-Citation24 Thus through their BAR domains, CNT-1 and ACAPs could help to form functional endosomal subdomains.
Our in vitro and in vivo assays also suggested the interplay between CNT-1 and RAB-8,Citation13 it is important to note that Rab8 has been suggested to function in the secretory pathway in mammalian cells,Citation25,Citation26 and this delivery process may use the recycling endosomes as an intermediate.Citation27 Studies in C. elegans and in mammalian cell culture indicate that RAB-8/Rab8 and RAB-10/Rab10 function redundantly under certain circumstances for membrane protein secretion/recycling, perhaps by sharing effectors such as CNT-1 and EHBP-1.Citation6,Citation28 Unlike RAB-10 endosomal recruitment through interaction with EHBP-1,Citation6 mammalian MICAL-L1 has been reported to be important for the association of Rab8 with endosomes.Citation29 Follow-up studies further suggested that Arf6 can recruit MICAL-L1 onto tubular endosomes,Citation30 suggesting that Arf6 can regulate Rab8 localization and function indirectly via MICAL-L1.
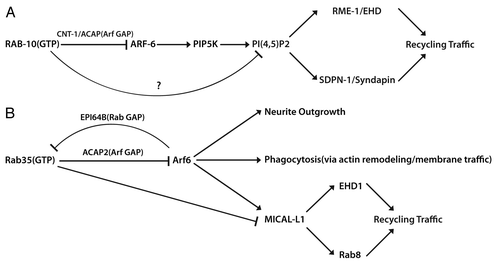
Many recent reports indicate coordinate Rab/Arf GTPase influencing numerous cellular processes.Citation15,Citation30-Citation32 Our studies provide an example of a Rab-to-Arf regulatory cascade, with RAB-10 and ARF-6 acting in sequential steps to regulate endosomal PI(4,5)P2 levels, thus regulating endosomal recruitment of PI(4,5)P2-binding proteins implicated in membrane bending and membrane fission. In addition to the Rab-to-Arf regulatory loops, an Arf-to-Rab regulatory cascade has also been recently reported. In cultured human cells, Arf6(GTP) can interact with the Rab35 GAP EPI64B, negatively regulating Rab35 activity.Citation32 This work indicates that Arf6-to-Rab35 regulation is required for endocytic pathways important for cytokinesis. Taken together, the discoveries of Rab-to-Arf and Arf-to-Rab regulatory cascades suggest that signaling between these classes of small GTPases represent an evolutionally conserved mechanism that coordinates membrane trafficking events. These cascades are likely to be important for many higher-level physiological processes that require regulated endocytic recycling pathways ().
Figure 1. Coordinate Rab/Arf GTPase cascades influence multiple cellular processes. (A) In C. elegans, RAB-10 and ARF-6 act in sequential steps via CNT-1/Arf GAP to regulate endosomal PI(4,5)P2 levels and membrane recruitment of recycling traffic related PI(4,5)P2-binding proteins RME-1 and SDPN-1. (B) In mammalian systems, Rab35(GTP) recruits ACAP2/Arf GAP and thus regulates Arf6 activity. Rab35-mediated regulation of Arf6 via ACAP2 is important for NGF-induced outgrowth of neurite and phagocytosis. Recent studies suggested that Arf6 can recruit MICAL-L1 onto endosomes and regulate EHD1 and Rab8 localization/function indirectly. Arf6(GTP) also interacts with the Rab35 GAP EPI64B, negatively regulating Rab35 activity. This Arf6-to-Rab35 cascade is necessary for endocytic trafficking during cytokinesis.
Acknowledgments
This work was supported by NIH Grant GM067237 to B.D.G.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- Grant BD, Donaldson JG. Pathways and mechanisms of endocytic recycling. Nat Rev Mol Cell Biol 2009; 10:597 - 608; http://dx.doi.org/10.1038/nrm2755; PMID: 19696797
- Sönnichsen B, De Renzis S, Nielsen E, Rietdorf J, Zerial M. Distinct membrane domains on endosomes in the recycling pathway visualized by multicolor imaging of Rab4, Rab5, and Rab11. J Cell Biol 2000; 149:901 - 14; http://dx.doi.org/10.1083/jcb.149.4.901; PMID: 10811830
- Grant B, Zhang Y, Paupard MC, Lin SX, Hall DH, Hirsh D. Evidence that RME-1, a conserved C. elegans EH-domain protein, functions in endocytic recycling. Nat Cell Biol 2001; 3:573 - 9; http://dx.doi.org/10.1038/35078549; PMID: 11389442
- Lin SX, Grant B, Hirsh D, Maxfield FR. Rme-1 regulates the distribution and function of the endocytic recycling compartment in mammalian cells. Nat Cell Biol 2001; 3:567 - 72; http://dx.doi.org/10.1038/35078543; PMID: 11389441
- Chen CC, Schweinsberg PJ, Vashist S, Mareiniss DP, Lambie EJ, Grant BD. RAB-10 is required for endocytic recycling in the Caenorhabditis elegans intestine. Mol Biol Cell 2006; 17:1286 - 97; http://dx.doi.org/10.1091/mbc.E05-08-0787; PMID: 16394106
- Shi A, Chen CC, Banerjee R, Glodowski D, Audhya A, Rongo C, et al. EHBP-1 functions with RAB-10 during endocytic recycling in Caenorhabditis elegans. Mol Biol Cell 2010; 21:2930 - 43; http://dx.doi.org/10.1091/mbc.E10-02-0149; PMID: 20573983
- Glodowski DR, Chen CC, Schaefer H, Grant BD, Rongo C. RAB-10 regulates glutamate receptor recycling in a cholesterol-dependent endocytosis pathway. Mol Biol Cell 2007; 18:4387 - 96; http://dx.doi.org/10.1091/mbc.E07-05-0486; PMID: 17761527
- Babbey CM, Ahktar N, Wang E, Chen CC, Grant BD, Dunn KW. Rab10 regulates membrane transport through early endosomes of polarized Madin-Darby canine kidney cells. Mol Biol Cell 2006; 17:3156 - 75; http://dx.doi.org/10.1091/mbc.E05-08-0799; PMID: 16641372
- Donaldson JG, Honda A. Localization and function of Arf family GTPases. Biochem Soc Trans 2005; 33:639 - 42; http://dx.doi.org/10.1042/BST0330639; PMID: 16042562
- D’Souza-Schorey C, Chavrier P. ARF proteins: roles in membrane traffic and beyond. Nat Rev Mol Cell Biol 2006; 7:347 - 58; http://dx.doi.org/10.1038/nrm1910; PMID: 16633337
- Honda A, Nogami M, Yokozeki T, Yamazaki M, Nakamura H, Watanabe H, et al. Phosphatidylinositol 4-phosphate 5-kinase alpha is a downstream effector of the small G protein ARF6 in membrane ruffle formation. Cell 1999; 99:521 - 32; http://dx.doi.org/10.1016/S0092-8674(00)81540-8; PMID: 10589680
- Yin HL, Janmey PA. Phosphoinositide regulation of the actin cytoskeleton. Annu Rev Physiol 2003; 65:761 - 89; http://dx.doi.org/10.1146/annurev.physiol.65.092101.142517; PMID: 12471164
- Shi A, Liu O, Koenig S, Banerjee R, Chen CC, Eimer S, et al. RAB-10-GTPase-mediated regulation of endosomal phosphatidylinositol-4,5-bisphosphate. Proc Natl Acad Sci U S A 2012; 109:E2306 - 15; http://dx.doi.org/10.1073/pnas.1205278109; PMID: 22869721
- Kanno E, Ishibashi K, Kobayashi H, Matsui T, Ohbayashi N, Fukuda M. Comprehensive screening for novel rab-binding proteins by GST pull-down assay using 60 different mammalian Rabs. Traffic 2010; 11:491 - 507; http://dx.doi.org/10.1111/j.1600-0854.2010.01038.x; PMID: 20070612
- Kobayashi H, Fukuda M. Rab35 regulates Arf6 activity through centaurin-β2 (ACAP2) during neurite outgrowth. J Cell Sci 2012; 125:2235 - 43; http://dx.doi.org/10.1242/jcs.098657; PMID: 22344257
- Li J, Peters PJ, Bai M, Dai J, Bos E, Kirchhausen T, et al. An ACAP1-containing clathrin coat complex for endocytic recycling. J Cell Biol 2007; 178:453 - 64; http://dx.doi.org/10.1083/jcb.200608033; PMID: 17664335
- Krauss M, Kinuta M, Wenk MR, De Camilli P, Takei K, Haucke V. ARF6 stimulates clathrin/AP-2 recruitment to synaptic membranes by activating phosphatidylinositol phosphate kinase type Igamma. J Cell Biol 2003; 162:113 - 24; http://dx.doi.org/10.1083/jcb.200301006; PMID: 12847086
- Shteyn E, Pigati L, Fölsch H. Arf6 regulates AP-1B-dependent sorting in polarized epithelial cells. J Cell Biol 2011; 194:873 - 87; http://dx.doi.org/10.1083/jcb.201106010; PMID: 21911479
- Raiborg C, Bache KG, Mehlum A, Stang E, Stenmark H. Hrs recruits clathrin to early endosomes. EMBO J 2001; 20:5008 - 21; http://dx.doi.org/10.1093/emboj/20.17.5008; PMID: 11532964
- Raiborg C, Wesche J, Malerød L, Stenmark H. Flat clathrin coats on endosomes mediate degradative protein sorting by scaffolding Hrs in dynamic microdomains. J Cell Sci 2006; 119:2414 - 24; http://dx.doi.org/10.1242/jcs.02978; PMID: 16720641
- Shi A, Sun L, Banerjee R, Tobin M, Zhang Y, Grant BD. Regulation of endosomal clathrin and retromer-mediated endosome to Golgi retrograde transport by the J-domain protein RME-8. EMBO J 2009; 28:3290 - 302; http://dx.doi.org/10.1038/emboj.2009.272; PMID: 19763082
- Peter BJ, Kent HM, Mills IG, Vallis Y, Butler PJ, Evans PR, et al. BAR domains as sensors of membrane curvature: the amphiphysin BAR structure. Science 2004; 303:495 - 9; http://dx.doi.org/10.1126/science.1092586; PMID: 14645856
- Gallop JL, Jao CC, Kent HM, Butler PJ, Evans PR, Langen R, et al. Mechanism of endophilin N-BAR domain-mediated membrane curvature. EMBO J 2006; 25:2898 - 910; http://dx.doi.org/10.1038/sj.emboj.7601174; PMID: 16763559
- Shinozaki-Narikawa N, Kodama T, Shibasaki Y. Cooperation of phosphoinositides and BAR domain proteins in endosomal tubulation. Traffic 2006; 7:1539 - 50; http://dx.doi.org/10.1111/j.1600-0854.2006.00480.x; PMID: 17010122
- Hattula K, Furuhjelm J, Arffman A, Peränen JA. A Rab8-specific GDP/GTP exchange factor is involved in actin remodeling and polarized membrane transport. Mol Biol Cell 2002; 13:3268 - 80; http://dx.doi.org/10.1091/mbc.E02-03-0143; PMID: 12221131
- Ang AL, Fölsch H, Koivisto UM, Pypaert M, Mellman I. The Rab8 GTPase selectively regulates AP-1B-dependent basolateral transport in polarized Madin-Darby canine kidney cells. J Cell Biol 2003; 163:339 - 50; http://dx.doi.org/10.1083/jcb.200307046; PMID: 14581456
- Ang AL, Taguchi T, Francis S, Fölsch H, Murrells LJ, Pypaert M, et al. Recycling endosomes can serve as intermediates during transport from the Golgi to the plasma membrane of MDCK cells. J Cell Biol 2004; 167:531 - 43; http://dx.doi.org/10.1083/jcb.200408165; PMID: 15534004
- Schuck S, Gerl MJ, Ang A, Manninen A, Keller P, Mellman I, et al. Rab10 is involved in basolateral transport in polarized Madin-Darby canine kidney cells. Traffic 2007; 8:47 - 60; http://dx.doi.org/10.1111/j.1600-0854.2006.00506.x; PMID: 17132146
- Sharma M, Giridharan SS, Rahajeng J, Naslavsky N, Caplan S. MICAL-L1 links EHD1 to tubular recycling endosomes and regulates receptor recycling. Mol Biol Cell 2009; 20:5181 - 94; http://dx.doi.org/10.1091/mbc.E09-06-0535; PMID: 19864458
- Rahajeng J, Giridharan SS, Cai B, Naslavsky N, Caplan S. MICAL-L1 is a tubular endosomal membrane hub that connects Rab35 and Arf6 with Rab8a. Traffic 2012; 13:82 - 93; http://dx.doi.org/10.1111/j.1600-0854.2011.01294.x; PMID: 21951725
- Egami Y, Fukuda M, Araki N. Rab35 regulates phagosome formation through recruitment of ACAP2 in macrophages during FcγR-mediated phagocytosis. J Cell Sci 2011; 124:3557 - 67; http://dx.doi.org/10.1242/jcs.083881; PMID: 22045739
- Chesneau L, Dambournet D, Machicoane M, Kouranti I, Fukuda M, Goud B, et al. An ARF6/Rab35 GTPase cascade for endocytic recycling and successful cytokinesis. Curr Biol 2012; 22:147 - 53; http://dx.doi.org/10.1016/j.cub.2011.11.058; PMID: 22226746