Up Scaling Production of Myristoylated Arf6, pp. 3–8
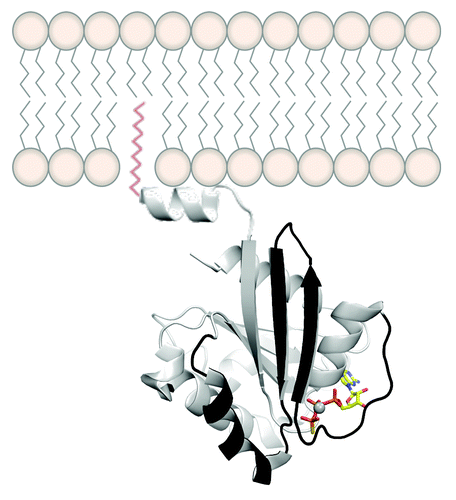
One way to study the molecular mechanisms that the Arf family of GTPases uses to regulate intracellular traffic and organelle structure requires in vitro biochemical assays. However, it is important to use a recombinant Arf protein that is in its cellular, myristoylated form, a form that is difficult to purify. In this research paper, Padovani et al. have found an efficient way to produce highly pure N-myristoylated Arf6 on a large scale, thus paving a way to produce other myristoylated Arfs in the future ().
Rac1b Off the Hook, pp.9–15
Although Rac1b is often expressed in human cancer cells, what effects it may be exerting are unclear. In this research paper, Mori et al. investigate these effects by constructing a constitutively active Rac1V12 gene that is inducible upon application of the plant protein auxin. Using MDCK cysts as their model for a polarized epithelial structure, the team was able to determine that Rac1b may be involved in the progression of cancer, but not necessarily be able to drive cell proliferation.
The Making of Lysosome-Related Organelles with Rab32 and Rab38, pp. 16–22
Some cell types produce two similar organelles, lysosomes and lysosome-related organelles (LROs). But, what mechanism decides which organelle a certain cargo will be sent to? Bultema and Di Pietro discuss their recent research on a specific type of LRO, the melanosome, focusing on Rab32 and Rab38 and how these proteins are involved in the production of LROs and the trafficking of cargo to melanosomes.
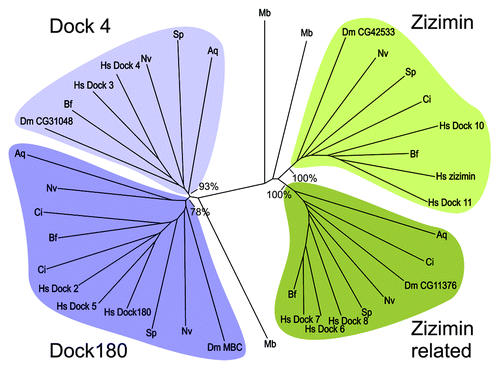
Cell Function According to Zizimin and Dock, pp.22–7
The Rho family of small GTPases plays an important role in many different cellular processes, such as migration, phagocytosis and proliferation. The switching of these GTPases from an active to an inactive state is controlled by the RhoGEF Zizimins, proteins from the Dock superfamily. In this commentary, Pakes et al. discuss the structure of Zizimin from an evolutionary standpoint and also compare them to Dock proteins. Understanding how these proteins function may provide insight into diseases stemming from mutations in Dock/Zizimin proteins ().
Small Yeast GTPase Control of a Key Survival Response, pp.28–33
Vesicular trafficking is vital for eukaryotes as it is involved in many processes: signaling, proliferation and development, to name just a few. Studying yeast, Tsvetanova et al. have discovered another important task for protein trafficking, the regulation of an important survival process, the unfolded protein response (UPR). In this commentary, Tsvetanova speculates on how the small yeast GTPase Ypt family regulates this novel secretory pathway and controls endoplasmic reticulum homeostasis.
Plexin Guides Neurons Via Ras and Rap, pp. 34–41
Axon extension and guidance is dependent on the regulation of cytoskeletal dynamics and adhesion. Small GTPases play critical roles in this regulatory process with the ability to be able to be switched on and off by extracellular guidance cues working through various guanine nucleotide exchange factors (GEFs) and GTPase activating proteins (GAPs). In this commentary, Yang and Terman delve into the role of plexin and its GAP activity that affects small GTPases activity via the Ras/Rap family.
RhoG Builds Neuronal Processes, pp. 42–6
RhoG is central to the regulation of cytoskeletal organization that occurs under many circumstances; therefore, it is not surprising that quite a bit is known about this small GTPase and how it is able to exert its effects. RhoG’s involvement in neuronal differentiation, however, has not yet been deduced. Schumacher and Franke comment on their recent research and discuss what effects RhoG has on axonal and dendritic differentiation along with interactions with Rac1, Cdc42 and microRNA miR-124.
RASA3 Regulates Blood Cell Production, pp.47–50
Mouse models have greatly contributed to our understanding of how red blood cells are produced and have also provided us with insight into the etiology of some red blood cell disorders. In this commentary, Peters, Paw and Blanc review their recent findings of a missense mutation found in the Rasa3 gene—encoding for a Ras GTPase activating protein—in a severe combined anemia and thrombocytopenia (scat) mouse model. The authors also highlight the many functions of RASA3 that surround red blood cell and platelet production.
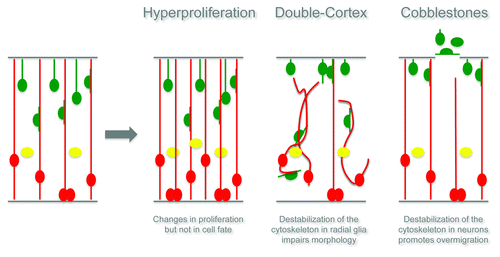
A Structured Brain Requires RhoA, pp.51–6
Rho GTPases are critical for cytoskeletal remodeling, directed cell migration and cell polarity in a variety of tissues, but what about in neuronal tissue? In this commentary, Silvia Cappello reviews his lab’s findings on the effects of the small GTPase RhoA on neuronal migration. Using a mouse model, Cappello’s group determined that the deletion of RhoA causes serious cortical malformations during development. These findings provide definitive role for Rho GTPases in the development of neuronal tissue ().
Targeting Ras Nanoclusters, pp.57–60
Ras/MAPK signaling relies greatly on the spatiotemporal dynamics of membrane bound Ras, or Ras nanoclusters. Cho and Hancock review how inhibiting BRaf can alter nanoclustering of different kinds of Ras and how this can affect Ras signal transmission. The authors speculate that targeting the dynamics of Ras nanoclusters may provide a beneficial therapeutic intervention.