Abstract
Mechanical forces influence nearly all aspects of biology. Cells are equipped with numerous mechanosensitive proteins that activate various signaling cascades in response to mechanical cues from their environment. Much interest lies in understanding how cells respond to external stresses. A number of studies have highlighted the coordination of mechanical and chemical signaling cascades downstream of integrins. In recent years, the study of mechanotransduction has expanded to other mechanosensitive adhesion receptors, such as platelet endothelial cell adhesion molecule-1 (PECAM-1). This commentary will highlight our current understanding of integrin and PECAM-1-mediated mechanotransduction and expand on the observation that a localized mechanical stress can elicit a global mechanosignaling response.
Introduction
Mechanotransduction, the process by which cells convert mechanical force into biochemical signaling events, is central to nearly all aspects of biology.Citation1 Mechanical forces regulate cell behavior and function during all stages of life and are central to a number of physiological and pathological processes. Therefore, a great deal of interest lies in how cells sense and respond to mechanical cues from their environment. Cells are decorated with numerous mechanosensitive proteins that work in concert to regulate the cellular response to mechanical stimuli such as stretching, shear stress, or extracellular matrix (ECM) rigidity. In addition to external forces, cells also generate their own forces (via actomyosin-based contractility) that regulate adhesion and maintenance of cell-cell junctions.Citation2,Citation3 A model of tensegrity has been proposed to explain how the cell coordinates internal and external stresses. This model defines the cytoskeleton as a series of interconnected compression-resistant structures that are surrounded by tensional elements. Organization of the cytoskeleton creates internal tension to provide cellular structure and support.Citation4 Application of force to the entire structure results in rearrangement of cytoskeletal elements without loss of tension. Furthermore, redistribution of cytoskeletal elements also allows force to be transmitted throughout the cell to other mechanosensitive proteins.
The best characterized mechanosensitive proteins to date are integrins: transmembrane proteins that couple the internal actin cytoskeleton to the ECM.. Several studies have revealed that cells respond to exogenous force on integrins by strengthening or stiffening adhesions to resist increased tensile strain.Citation5-Citation7 Applied force on integrins involves recruitment of vinculin and growth of focal adhesions in the direction of applied force.Citation8 Adaptive stiffening also requires coordination of mechanically activated signaling cascades, including activation of RhoA and its effectors, which ultimately mediate local changes in focal adhesion growth and actomyosin contractility.Citation5-Citation7 In recent years, researchers have begun to probe other mechanosensitive proteins, such as platelet endothelial cell adhesion molecule-1 (PECAM-1). PECAM-1 is expressed in only a subset of cells, including endothelial cells, where it plays an important role in transducing shear stress due to blood flow into various biochemical signals.Citation9 Mechanical probing of PECAM-1 has revealed that adaptive stiffening or strengthening in response to exogenous force is not specific to integrins, but may be a commonality shared by multiple mechanosensitive proteins. However, recent work has shown that while common sets of molecular mechanisms exist, specialized systems display unique mechanotransduction responses. This commentary will compare and contrast known mechanotransduction pathways in response to applied tension to integrins and PECAM-1.
Common Mechanotransduction Responses
A number of experimental approaches to apply tension on integrins, including optical or magnetic tweezers and electromagnetic microneedles, have shown that cells can sense the applied tension and respond by strengthening their cytoskeletal linkages to oppose the force.Citation6,Citation7,Citation10-Citation12 Similar to integrins, application of tensional forces on anti-PECAM-1-coated beads bound to endothelial cells induces a stiffening response that also depends on the actin cytoskeleton.
In addition to adaptive stiffening, several other mechanosensitive responses are also shared between PECAM-1 and integrins. Force application on either adhesion receptor results in RhoA activation through the same two GEFs, GEF-H1 and LARG.Citation12,Citation13 Interestingly, RhoA activation in response to tensional force on PECAM-1 is integrin-dependent, as inhibition of new integrin-ECM connections quenches force-induced RhoA activity.Citation13 This integrin-dependent RhoA activation might explain the shared GEFs in both systems. Importantly, RhoA-mediated signaling is required for adaptive stiffening downstream of both integrins and PECAM-1, as inhibition of RhoA, its GEFs, or its effectors abolishes the force-dependent stiffening response. Finally, tension on either integrins or PECAM-1 also results in growth of focal adhesions, which mechanically couple the internal actin cytoskeleton to the extracellular matrix.Citation8,Citation13 Indeed, the best-defined response to applied tension on integrins is reinforcement of the focal adhesions to counteract the exogenously applied force.Citation5 Similarly, force on PECAM-1 results in growth of focal adhesions. However, in contrast to the local adhesion growth observed in response to tension on integrins, PECAM-1-mediated adhesion growth is a global phenomenon. This leads us to the important differences in mechanotransduction cascades between these two cell adhesion molecules that will be discussed below.
Differences in Mechanotransduction Pathways
Several differences exist when comparing the cellular response to force application on integrins vs. PECAM-1. Closer examination of some of the shared responses, such as adaptive stiffening or RhoA activation, also reveal some notable differences in the cellular responses downstream of each receptor. While mechanical stimulation of either adhesion receptor results in adaptive stiffening, the time course of the response varies between the two proteins. Tension applied to fibronectin (FN)-binding integrins results in an immediate stiffening response, as differences in cellular stiffness can be detected immediately following force application. Conversely, while force application on PECAM-1 also elicits adaptive stiffening, the response is delayed and is detectable on a minute, rather than second timescale. This same trend is also observed when taking a closer look at the time course of RhoA activation. Force application on integrins results in an increase in RhoA activity in as little as 1 min. In contrast, force-induced RhoA activation downstream of PECAM-1 does not increase until 5 min of force. Therefore, it is possible that the delay in the PECAM-1-mediated adaptive stiffening response is a reflection of delayed RhoA activation. Differences in the time course of these events may be attributed to differences in mechanisms of signal transduction. Mechanosensitive signaling pathways may be chemically or mechanically propagated throughout the cell, via diffusion of small chemical messengers or mechanically transmitted through cytoskeletal filaments, respectively. Indeed, mechanical signals can be propagated at a remarkable speed of 30m/s, whereas biochemical signals diffuse at a mere rate of 2μm/s.Citation14 Given integrins’ intimate association with the cytoskeleton, it is possible that integrin-mediated mechanotransduction relies more heavily on mechanical transmission of the signaling cascade, while PECAM-1-mediated mechanotransduction is more chemically-dependent in nature.
Perhaps the most profound difference between integrin and PECAM-1-mediated mechanosignaling involves the spatial organization of the response. Several studies have revealed that integrin-mediated adaptive stiffening response is mediated, in part, by localized recruitment of focal adhesion proteins (such as β1 and β3 integrins, talin, and vinculin), which results in a local growth of adhesions at the site of force application.Citation8,Citation15 Similarly, force application on integrins induces localized recruitment of LARG and GEF-H1 to the adhesion complex. Furthermore, mRNA and ribosomes also specifically localize to focal adhesions that form under adherent FN-coated microbeads.Citation16 Surprisingly, our work has revealed that tension on PECAM-1 results in a remarkable cell-wide, or global, mechanotransduction response, as evidenced by a cell-wide increase in focal adhesion growth. This result is especially intriguing, as it is the first documented evidence of a global mechanotransduction event elicited by a localized force. While studies from Ning Wang’s group have reported Src and Rac activation at remote sites away from stress application with RGD-coated beads,Citation14,Citation17,Citation18 neither signal displayed cell-wide distribution. Nevertheless, it has been shown that global, cell-wide changes in cytoskeletal structure and mechanics can regulate mechanotransduction, such that the mechanotransduction response is governed by global mechanical cues, including isometric tension (pre-stress) within the cytoskeleton.Citation18,Citation19
Globalization of Mechanotrasduction
How does a localized force on PECAM-1 elicit a global response? Phosphoinositide 3-kinase (PI3K) is rapidly activated following application of tension on PECAM-1. Activation of PI3K results in the production of freely diffusible phosphoinositides (such as PI(3,4,5)P3) that act as second messengers to propagate signaling cascades throughout the cell. Experimental evidence suggests that: 1) activation of PI3K, and 2) diffusion of the second messenger PI(3,4,5)P3 is required for the global response downstream of PECAM-1. Localized tension on PECAM-1 not only elicits global focal adhesion formation, but also results in global integrin and RhoA activation (). Interestingly, inhibition of PI3K activation, or sequestration of the PI(3,4,5)P3 messenger, abolishes the global signaling response. PI3K has been implicated in integrin activation in various contexts and cell types, although the exact mechanism of activation remains elusive.Citation20 PI(3,4,5)P3 has a documented half-life 60 secCitation21 and a fairly rapid diffusion rate of 0.1–1 μm2/sec.Citation22 Given that endothelial cells are relatively thin cells (generally less that 5μm in height), it is feasible that freely diffusing PI(3,4,5)P3 would be able to globally activate integrins at cellular sites proximal and distal to the site of force application. Therefore, the current model of PECAM-1-mediated mechanotransduction suggests that PI3K is rapidly activated downstream of PECAM-1, and PI(3,4,5)P3 promotes global integrin activation (). Integrin activation is followed by new binding to the ECM and enlargement of adhesions. Cell-wide activation of integrins elicits global activation of RhoA via the GEF-H1 and LARG pathway. This is a crucial aspect of the PECAM-1-mediated response, as global activation of the GTPase facilitates global growth of focal adhesions, which contributes to the adaptive stiffening response. This model also highlights cooperation of two mechanosensors in this system: PECAM-1 and integrins. Not only does PECAM-1 lead to activation of integrins, but integrin ligation with the ECM is required for force-induced RhoA activation and adaptive stiffening.
Figure 1. Localized tension on PECAM-1 elicits global RhoA activation. Endothelial cells expressing the RhoA biosensor were incubated with anti-PECAM-1-coated beads and subjected to force with a permanent ceramic magnet for the indicated times. Cells were fixed and analyzed for FRET. Autofluorescent beads are highlighted in black dotted circles.
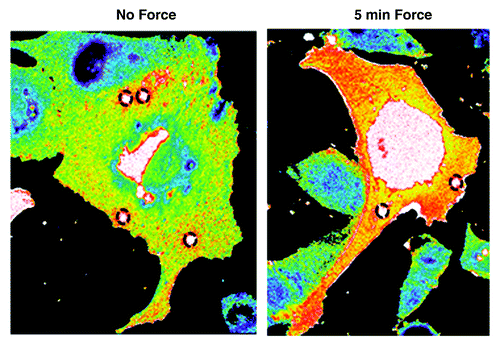
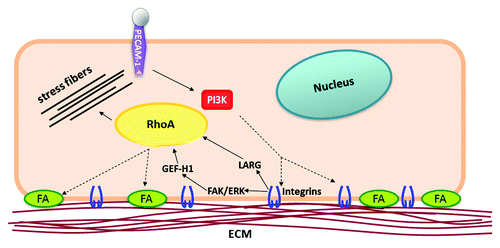
Figure 2. Model of PECAM-1-mediated mechanotransduction. Force-dependent activation of PI3K downstream of PECAM-1 promotes global activation of integrins, cell-wide activation of RhoA, and global growth of focal adhesions. Cell-wide propagation of the signaling cascade facilitates adaptive stiffening and strengthening of adhesions, which function to resist the applied force.
The requirement of PI3K activation and PI(3,4,5)P3 implies that chemical signaling is critical for the global nature of the PECAM-1-mediated response. However, it remains unclear if mechanical propagation of the signal is also required for the global response. Transmission of force through tensile cytoskeletal elements can propagate mechanosensitive signaling away from sites of applied stress. For instance, previous studies probing integrins reported rapid activation of Src and Rac at remote cytoplasmic locations away from the site of mechanical stress.Citation14,Citation17,Citation23 However, these signals were not global in nature, but rather were confined to distinct puncta that corresponded with sites of cytoskeletal deformation. It is also important to note that integrins’ physical association with the cytoskeleton has been well documented. Therefore, it is plausible that tensile cytoskeletal filaments anchored to integrins on opposing sides of the cell could facilitate transmission of a mechanical stimulus across the cell. However, PECAM-1 has not been shown to directly interact with the cytoskeleton; although indirect association via cytoplasmic interactions with β-catenin and γ-catenin have been proposed.Citation24 Furthermore, the exact of mechanism of PECAM-1-mediated mechanosensing remains unknown. Models of mechanotransduction often involve force-dependent conformational changes in proteins that permit propagation of mechanosensitive signaling cascades. For instance, α5β1 integrin undergoes a force-dependent conformation change,Citation25 which allows chemical crosslinking to fibronectin and facilitates recruitment and activation focal adhesion kinase (FAK). While it is appealing to hypothesize that PECAM-1-mediated mechanotransduction may be dependent on a conformational change, a force-dependent change in the protein structure has not yet been identified, and thus, the mechanism of mechanosensing remains elusive. Comprehensive studies investigating the structure of the PECAM-1 cytoplasmic tail may provide insights into the mechanisms of PECAM-1-mediated mechanosignaling and reveal the relative contributions of chemical signaling and mechanical transmission in the global mechanotransduction response.
| Abbreviations: | ||
| PECAM-1 | = | platelet endothelial cell adhesion molecule-1 |
| ECM | = | extracellular matrix |
| RGD | = | Arg-Gly-Asp |
| PI3K | = | phosphoinositide 3-kinase |
| FN | = | fibronectin |
| FAK | = | focal adhesion kinase |
Acknowledgments
This work was supported by NIH grant HL088632 (to E.T.). C.C. is an American Heart Association Predoctoral Fellow (12PRE119300000) and E.T. is an Ellison Medical Foundation New Scholar.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- Hoffman BD, Crocker JC. Cell mechanics: dissecting the physical responses of cells to force. Annu Rev Biomed Eng 2009; 11:259 - 88; http://dx.doi.org/10.1146/annurev.bioeng.10.061807.160511; PMID: 19400709
- Tan JL, Tien J, Pirone DM, Gray DS, Bhadriraju K, Chen CS. Cells lying on a bed of microneedles: an approach to isolate mechanical force. Proc Natl Acad Sci U S A 2003; 100:1484 - 9; http://dx.doi.org/10.1073/pnas.0235407100; PMID: 12552122
- Liu Z, Tan JL, Cohen DM, Yang MT, Sniadecki NJ, Ruiz SA, et al. Mechanical tugging force regulates the size of cell-cell junctions. Proc Natl Acad Sci U S A 2010; 107:9944 - 9; http://dx.doi.org/10.1073/pnas.0914547107; PMID: 20463286
- Ingber DE. Cellular tensegrity: defining new rules of biological design that govern the cytoskeleton. J Cell Sci 1993; 104:613 - 27; PMID: 8314865
- Bershadsky AD, Balaban NQ, Geiger B. Adhesion-dependent cell mechanosensitivity. Annu Rev Cell Dev Biol 2003; 19:677 - 95; http://dx.doi.org/10.1146/annurev.cellbio.19.111301.153011; PMID: 14570586
- Matthews BD, Overby DR, Mannix R, Ingber DE. Cellular adaptation to mechanical stress: role of integrins, Rho, cytoskeletal tension and mechanosensitive ion channels. J Cell Sci 2006; 119:508 - 18; http://dx.doi.org/10.1242/jcs.02760; PMID: 16443749
- Choquet D, Felsenfeld DP, Sheetz MP. Extracellular matrix rigidity causes strengthening of integrin-cytoskeleton linkages. Cell 1997; 88:39 - 48; http://dx.doi.org/10.1016/S0092-8674(00)81856-5; PMID: 9019403
- Riveline D, Zamir E, Balaban NQ, Schwarz US, Ishizaki T, Narumiya S, et al. Focal contacts as mechanosensors: externally applied local mechanical force induces growth of focal contacts by an mDia1-dependent and ROCK-independent mechanism. J Cell Biol 2001; 153:1175 - 86; http://dx.doi.org/10.1083/jcb.153.6.1175; PMID: 11402062
- Tzima E, Irani-Tehrani M, Kiosses WB, Dejana E, Schultz DA, Engelhardt B, et al. A mechanosensory complex that mediates the endothelial cell response to fluid shear stress. Nature 2005; 437:426 - 31; http://dx.doi.org/10.1038/nature03952; PMID: 16163360
- Wang N, Butler JP, Ingber DE. Mechanotransduction across the cell surface and through the cytoskeleton. Science 1993; 260:1124 - 7; http://dx.doi.org/10.1126/science.7684161; PMID: 7684161
- Matthews BD, Overby DR, Alenghat FJ, Karavitis J, Numaguchi Y, Allen PG, et al. Mechanical properties of individual focal adhesions probed with a magnetic microneedle. Biochem Biophys Res Commun 2004; 313:758 - 64; http://dx.doi.org/10.1016/j.bbrc.2003.12.005; PMID: 14697256
- Guilluy C, Swaminathan V, Garcia-Mata R, O’Brien ET, Superfine R, Burridge K. The Rho GEFs LARG and GEF-H1 regulate the mechanical response to force on integrins. Nat Cell Biol 2011; 13:722 - 7; http://dx.doi.org/10.1038/ncb2254; PMID: 21572419
- Collins C, Guilluy C, Welch C, O’Brien ET, Hahn K, Superfine R, et al. Localized tensional forces on PECAM-1 elicit a global mechanotransduction response via the integrin-RhoA pathway. Curr Biol 2012; 22:2087 - 94; http://dx.doi.org/10.1016/j.cub.2012.08.051; PMID: 23084990
- Na S, Collin O, Chowdhury F, Tay B, Ouyang M, Wang Y, et al. Rapid signal transduction in living cells is a unique feature of mechanotransduction. Proc Natl Acad Sci U S A 2008; 105:6626 - 31; http://dx.doi.org/10.1073/pnas.0711704105; PMID: 18456839
- Balaban NQ, Schwarz US, Riveline D, Goichberg P, Tzur G, Sabanay I, et al. Force and focal adhesion assembly: a close relationship studied using elastic micropatterned substrates. Nat Cell Biol 2001; 3:466 - 72; http://dx.doi.org/10.1038/35074532; PMID: 11331874
- Chicurel ME, Singer RH, Meyer CJ, Ingber DE. Integrin binding and mechanical tension induce movement of mRNA and ribosomes to focal adhesions. Nature 1998; 392:730 - 3; http://dx.doi.org/10.1038/33719; PMID: 9565036
- Poh YC, Na S, Chowdhury F, Ouyang M, Wang Y, Wang N. Rapid activation of Rac GTPase in living cells by force is independent of Src. PLoS One 2009; 4:e7886; http://dx.doi.org/10.1371/journal.pone.0007886; PMID: 19924282
- Alenghat FJ, Nauli SM, Kolb R, Zhou J, Ingber DE. Global cytoskeletal control of mechanotransduction in kidney epithelial cells. Exp Cell Res 2004; 301:23 - 30; http://dx.doi.org/10.1016/j.yexcr.2004.08.003; PMID: 15501441
- Ingber DE. Cellular mechanotransduction: putting all the pieces together again. FASEB J 2006; 20:811 - 27; http://dx.doi.org/10.1096/fj.05-5424rev; PMID: 16675838
- Hughes PE, Pfaff M. Integrin affinity modulation. Trends Cell Biol 1998; 8:359 - 64; http://dx.doi.org/10.1016/S0962-8924(98)01339-7; PMID: 9728397
- Tengholm A, Meyer T. A PI3-kinase signaling code for insulin-triggered insertion of glucose transporters into the plasma membrane. Curr Biol 2002; 12:1871 - 6; http://dx.doi.org/10.1016/S0960-9822(02)01223-X; PMID: 12419189
- Hammond GR, Sim Y, Lagnado L, Irvine RF. Reversible binding and rapid diffusion of proteins in complex with inositol lipids serves to coordinate free movement with spatial information. J Cell Biol 2009; 184:297 - 308; http://dx.doi.org/10.1083/jcb.200809073; PMID: 19153221
- Wang Y, Botvinick EL, Zhao Y, Berns MW, Usami S, Tsien RY, et al. Visualizing the mechanical activation of Src. Nature 2005; 434:1040 - 5; http://dx.doi.org/10.1038/nature03469; PMID: 15846350
- Ilan N, Mahooti S, Rimm DL, Madri JA. PECAM-1 (CD31) functions as a reservoir for and a modulator of tyrosine-phosphorylated beta-catenin. J Cell Sci 1999; 112:3005 - 14; PMID: 10462517
- Friedland JC, Lee MH, Boettiger D. Mechanically activated integrin switch controls alpha5beta1 function. Science 2009; 323:642 - 4; http://dx.doi.org/10.1126/science.1168441; PMID: 19179533