Abstract
Epithelial cells differentiate and polarize to build complete epithelial organs during development. The study of epithelial morphogenesis is instrumental to the understanding of disease processes where epithelial polarity is disrupted. Recently, we demonstrated that matrix-induced cell confinement controls the acquisition of three-dimensional epithelial polarity, by modulating the initiation of the apical membrane to form a central lumen (J Cell Biol 2012; 198:1011-1026). Cell confinement can be achieved by use of micropatterned culture chips that allow precise micrometric-scale control of the cell adhesion surface and its composition. Using micropattern chips, we demonstrated that polarizing epithelial cells require high confinement conditions to properly position the centrosome and the trafficking machinery toward the cell-cell contacts and to initiate lumen morphogenesis. Low confinement induces LKB1 and RhoA-mediated cell contractility, which inhibits this mechanism for lumen formation. Deactivation of Myosin-II-mediated contractility rescued normal lumen initiation in low confinement conditions. Our results indicate that a mechanotransduction pathway coordinates nuclear and centrosome positioning to initiate epithelial morphogenesis. Here we discuss the potential candidates that control this process, specifically the polarized activation of Rho and Rab-family GTPases, and also a group of recently characterized nuclear transcription factors.
Introduction
Epithelial organs are involved in the majority of the vital functions of higher animals. The epithelial tissue constitutes the outermost layer of an epithelial organ and acts as a functional barrier that surrounds a central lumen, an extracellular fluid-filled space connected to the outside world. The single functional unit of an epithelial organ is the polarized epithelial cell, which requires the establishment and maintenance of distinct apical and basolateral plasma membrane domains, separated by the apical junction complexes that connect each cell to its neighbors.Citation1 Pioneering studies in the field of cell-to-cell interactions demonstrated that the establishment of cell-cell contacts constitutes the spatial cue that initiates the process of cell polarization in epithelial differentiation.Citation2,Citation3 However, an in-depth understanding of the involvement of cell-to-matrix contacts in epithelial morphogenesis had remained elusive until the introduction of three-dimensional (3D) matrix cell cultures.Citation4 These 3D-culture methods, which use extracts of collagen-I, laminin and other extracellular matrix (ECM) gels, constitute a more physiological approach to mimic the internal organ conditions of epithelial cells and allow the unraveling of multiple molecular pathways involved in epithelial cell polarity and morphogenesis. However, only a few studies to date have performed a separate analysis of the individual properties of the ECM and their contribution to epithelial cell polarization.Citation5 Recent work using micropatterned devices, from our laboratory and others, has begun to shed light on the function of cell-to-matrix interactions. Our results suggest that cells translate not only biochemical signals, but also biophysical extracellular conditions, into signaling pathways which are required for epithelial morphogenesis.Citation6
Cell confinement control of epithelial lumen formation
The effect of mechanical stress on morphogenesis has been extensively analyzed in developmental biology.Citation7 Early works using in vitro models established that different types of cells, including epithelial tissues, could modify their behavior depending on the mechanical properties of the underlying matrix via a complex mechanism requiring the cytoskeletal contractility machinery. More recent in vivo data from C. elegans embryos has also shown that forces generated by contraction of the whole animal can also be translated to the epidermis through hemidesmosome-like structures and conserved Rac1 signaling, and these forces are required for normal epithelial morphogenesis.Citation8 To underpin the mechanical basis of morphogenesis, numerous tools have been developed, including the use of cell chips and micropatterns to control the adhesive properties of the cell environment.Citation9 Using these tools a recent study has revealed that ciliogenesis, a hallmark of differentiated epithelia, is controlled by the ability of the cell to sense spatial confinement through changes in actin-mediated contractility.Citation10 In this work, Pitaval et al. analyzed ciliogenesis using a variety of micropatterned adhesive surfaces to modify cell confinement (). Their results showed that cells on low confinement did not polarize or initiate ciliogenesis correctly and also formed fewer shorter ventral cilia. In contrast, cells on high confinement (using a smaller micropattern size per cell) formed typical longer apical primary cilia. Furthermore, cells on low confinement presented a very pronounced contractile phenotype, with mature focal adhesions and abundant stress fiber formation. Consistently, inhibition of myosin-II-mediated contractility with blebbistatin, or depolymerization of actin with cytochalasin D, was sufficient to prevent stress fiber formation under low confinement, and restored primary cilia formation in the apical part of the cell. These results indicate that cell contractility and primary cilia formation are mutually exclusive processes in cellular physiology. Furthermore, they introduce the possibility that other processes required for epithelial morphogenesis could be modulated by contractility and thus, more easily studied using micropatterns.
Figure 1. Models for matrix-mediated control of cell polarity and epithelial morphogenesis. (A) Cell confinement modulates cell spreading, focal adhesion formation and F-actin stress fiber polymerization and contraction. Cells in high confinement or low stiffness spread poorly and do not form contractile fibers. In low confinement (or stiff matrices) cells extend their surface and form large actin stress fibers and focal adhesions. The orientation of the centrosome changes from the apical (dorsal) region of the cell in high confinement to the basal (ventral) region in low confinement and controls ciliogenesis. (B) Cell confinement induces formation of lumens between adjacent cells. In low confinement, cell contractility prevents centrosome orientation toward the junctions and lumen initiation. (C) The mechanism of lumen initiation activates Cdc42 and Rab8 through GEFs localized to the Golgi and the centrosome that are polarized toward the junctions in high confinement. (D) Transcription factors such as YAP/TAZ, SRF-coactivator MAL and β-catenin present changes in their nuclear localization regulated by F-actin fiber polymerization and contractility. In high confinement, G-actin levels are high and induce MAL transport to the nucleus where it induces SRF-mediated transcription of MAL target genes. High confinement also induces YAP/TAZ phosphorylation and prevents its nuclear localization. In contrast, high contractility induces actin polymerization and stress fiber formation, and prevents MAL nuclear shuttling, while it induces YAP/TAZ transport to the nucleus.
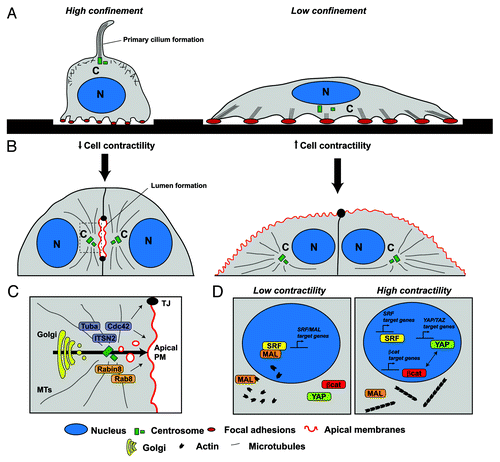
As some of the pathways involved in ciliogenesis and lumen formation appear to be common, we decided to analyze whether contractility could affect lumen initiation in the model of MDCK cyst formation.Citation11 For this purpose we took advantage of a specific feature of this model, which is the clearly visible formation of the initial lumen at the two cell stage, after the first cell division, by staining the apical marker podocalyxin/gp135. Previous work on lumen formation provided evidence that initial polarity orientation requires laminin and Rac1-GTPase signaling, and that Rac1 defects can be rescued by inhibiting Rho kinase (ROCK), thus suggesting that contractility might play a role in this process.Citation12,Citation13 We followed the same premise as Pitaval and colleagues and seeded MDCK cells on a substrate where they could adhere and stretch (collagen-I) and then modified the adhesive surface by using micropatterns of different sizes (). On low confinement, MDCK cells produced numerous stress fibers and mature focal adhesions, and cells did not form an initial lumen after the first cell division. In contrast, high confinement was sufficient to induce correct lumen initiation. Similarly to the effects previously observed in ciliogenesis, lumen initiation was rescued on low confinement by myosin-II inhibition. Furthermore, forcing contractility, by overexpression of constitutively active myosin-II regulatory chain, showed that stress fiber formation was sufficient to prevent correct centrosomal positioning and lumen formation in high confinement. Thus, cell-cell junctions and increased confinement produced by cell-confluency were not sufficient to form the initial lumen in conditions that promote high cellular contractility. Also, we observed that RhoA and ROCK activity regulate contractility in the 3D-MDCK model, and an inhibition of Rho activity consistently rescued lumen formation, thus resembling other mechanosensing pathways described during embryonic endothelial tubulogenesis.Citation14 Moreover, we found that liver kinase B1 (LKB1) activity was required for maintaining RhoA activation in this model, suggesting that this oncosuppressor is activated in highly contractile cells and functions to prevent apical membrane reorganization.
Taken together, these results indicate that both ciliogenesis and lumen initiation occur in conditions of low contractility and also that stress fiber formation inhibits some important molecular mechanisms required for proper epithelial morphogenesis. This data also suggested that not only the presence of laminin (and thus activation of Rac1) but also high confinement (and thus inactivation of RhoA) is required for proper initial epithelial polarization. Interestingly, we found that laminin prevents proper spreading and contractility of MDCK cells, suggesting that laminin could be sufficient for both activation of Rac1 and inactivation of RhoA. MDCK cells express laminin-binding receptors, such as α6-β1 integrin, which might be responsible for this observed effect. Indeed, β1 blocking antibodies prevent proper laminin organization and result in loss of polarity.Citation12 Recent progress has shown that laminin is organized in epithelial organoids through collective rotation of cells, and triggering this movement is essential for epithelial morphogenesis.Citation15 Furthermore, laminin-mediated integrin activation controls microtubule orientation through integrin-linked kinase (ILK), which might be responsible for preventing or controlling basal actin contractility and 3D cell spreading.Citation16
After polarity is orientated, lumen formation requires vesicular trafficking and de novo assembly of tight junctions at the site where the new apical membrane is going to be formed.Citation17 We believe that centrosome positioning and microtubule organization play a decisive role in this process (). Indeed, the vesicular trafficking required for lumen formation occurs through a complex molecular mechanism orchestrated by the small Rho GTPase Cdc42 and the Rab-family GTPases, Rab11, Rab8 and Rab27.Citation18‑Citation20 The specific guanine nucleotide exchange factors (GEFs) for Rab8 and Cdc42, Rabin8 and Intersectin-2, respectively, bind to the centrosome, suggesting that centrosome orientation might position the site of de novo apical plasma membrane formation. Interestingly, primary ciliogenesis shares, with lumen formation, a common polarized trafficking machinery.Citation21-Citation23 In summary, we propose that laminin signaling and cell confinement mediate the orientation of the nucleo-centrosomal polarity axis, which results in a polarized activation of Cdc42 and specific RabGTPases. These RabGTPases and Cdc42, in turn, recruit the necessary effectors involved in the generation of polarized membrane trafficking to form the lumen and the primary cilium.
Data gathered in the last decade have provided important evidence regarding the molecular mechanisms associated with lumen formation. Additionally, modifications in gene expression are also known to occur and to be required during epithelial morphogenesis. As an example, we have described recently a set of 16 regulators of lumen formation, including two synaptotagmin-like proteins that belong to a family of Rab-effectors, which are induced very early (a few hours) after seeding cells on laminin-rich ECMs, and are required for lumen formation.Citation18 Despite this recent progress, it is still currently unknown which signaling pathways and nuclear transcription factors are involved in translating ECM signaling to gene expression and how they function to induce lumen formation. In this final part of the review we will suggest some possible mechanotransduction pathways that have been studied in other models of epithelial differentiation, which might shed some light in this direction.
Mechanosensory control of cell differentiation
During the last decade several studies have shown that cells can sense ECM mechanical properties through the activation of specific cytoskeletal machinery. Activation of cytoskeletal signaling, in turn, modulates nuclear transcriptional programs leading to differentiation to a variety of cell types.Citation24 These mechanotransduction pathways are constituted by matrix-binding molecules, such as integrins, focal adhesion forming complexes and actin fibers and bundles, as well as a series of nuclear membrane protein complexes, such as the LINC complexes, which are capable of translating the cytoskeletal signal into the nucleus.Citation25 Additionally, several studies have characterized a series of transcription factors, including β-catenin, serum response factor (SRF), Yes-activated protein (YAP), NFκB, etc., which are transported into or out of the nucleus depending on the levels of mechanical cell stress ().Citation26
The role of YAP in cellular mechanotransduction was uncovered through analysis of differential gene expression in cells cultured on stiff vs. soft matrices.Citation27,Citation28 YAP behavior was also analyzed in cells using micropatterns of different size, resulting in the observation that dephosphorylated YAP accumulates in the nucleus in low confinement conditions where it activates genes required for cell proliferation.Citation28 On soft substrates, or low confinement, YAP is phosphorylated and excluded from the nucleus. Both, actin polymerization and myosin-II inhibitors induce YAP phosphorylation and resultant exclusion from the nucleus, suggesting that contractility and stress fiber formation are involved in YAP regulation.
Cell spreading and contractility also regulate the SRF cofactor MAL through binding and detection of globular actin (G-actin).Citation29,Citation30 In high confinement conditions, SRF activates specific targets in the nucleus and induces epidermal stem cell differentiation. In low confinement (high-stress) conditions, filamentous actin (F-actin) is polymerized actively in the cytoplasm to maintain cell shape and movement, and this prevents epidermal stem cell differentiation. Thus, the G-actin/F-actin ratio controls SRF binding to MAL and promotes the activation of different MAL-dependent and independent SRF-target genes. Even though progress has been made, so far it remains unclear exactly how these and other transcription factors modulate epithelial cell morphogenesis. Recent data have shown that SRF is required for polarized oriented cell division and morphogenesis in the epidermis.Citation31 In addition, a subset of genes that are induced and required during lumen morphogenesis and ciliogenesis (such as ITSN2, Slp2-a, Ninein or Cep164) contain consensus promoter sequences for SRF binding. In this regard, it will be of crucial importance to understand the processes by which mechanosensory-mediated gene expression controls known molecular pathways of epithelial morphogenesis. It will also be necessary to understand the role of these mechanisms in a broader array of epithelial cell types and animal models.
Conclusions: Mechanotransduction as a main driver of cell differentiation and polarity
Mechanotransduction pathways are beginning to be understood, both at the initial molecular events controlling cell stretching, nuclear movement and centrosome positioning, and also later nuclear signaling cascades directing gene expression and cell differentiation. Mechanotransduction pathway analysis is progressively stepping into the limelight of important human disease conditions, such as cancer and polycystic kidney disease, where epithelial cell division and polarity are affected.Citation32-Citation34 In this sense we expect that new and exciting findings will continue to fill the gaps in our present knowledge. First, additional efforts should be directed toward uncovering novel transcription factors and mechanisms involved in the nuclear translation of external mechanical cues. Second, it will be indispensable to understand better how subtle changes in contractility activate these transcription factors, to relay the signal to the nucleus. Additionally, increasing evidence suggests that β-catenin, YAP/TAZ and many other transcription factors can also be regulated by recruitment to mature cell–cell junctions, which also sense mechanotransduction by different means.Citation35 Thus, the equilibrium between cell confinement and cell junction maturation will most likely modulate a variety of transcription factors to shift the balance of differentiation and proliferation in polarizing epithelial cells. These efforts will be crucial for the design of next-generation pharmacological approaches which attempt to reestablish cell differentiation in the treatment of epithelial diseases such as cancer, as well as in the development of more robust strategies for stem cell culture and targeted differentiation.
Acknowledgments
We thank Carmen M. Ruiz-Jarabo for comments on the manuscript and members of the Martin-Belmonte lab for discussions. This work was supported by grants from the Human Frontiers Science Program (HFSP-CDA 00011/2009), MICINN (BFU2011-22622) and CONSOLIDER (CSD2009-00016) to F.M-B. A.E.R-F. is a recipient of a JAE fellowship, from CSIC. An institutional Grant from Fundación Ramón Areces to CBMSO is also acknowledged.
References
- St Johnston D, Sanson B. Epithelial polarity and morphogenesis. Curr Opin Cell Biol 2011; 23:540 - 6; http://dx.doi.org/10.1016/j.ceb.2011.07.005; PMID: 21807488
- Drubin DG, Nelson WJ. Origins of cell polarity. Cell 1996; 84:335 - 44; http://dx.doi.org/10.1016/S0092-8674(00)81278-7; PMID: 8608587
- McNeill H, Ozawa M, Kemler R, Nelson WJ. Novel function of the cell adhesion molecule uvomorulin as an inducer of cell surface polarity. Cell 1990; 62:309 - 16; http://dx.doi.org/10.1016/0092-8674(90)90368-O; PMID: 2164888
- Nelson CM, Bissell MJ. Of extracellular matrix, scaffolds, and signaling: tissue architecture regulates development, homeostasis, and cancer. Annu Rev Cell Dev Biol 2006; 22:287 - 309; http://dx.doi.org/10.1146/annurev.cellbio.22.010305.104315; PMID: 16824016
- Nelson CM, Gleghorn JP. Sculpting organs: mechanical regulation of tissue development. Annu Rev Biomed Eng 2012; 14:129 - 54; http://dx.doi.org/10.1146/annurev-bioeng-071811-150043; PMID: 22524386
- Théry M. Micropatterning as a tool to decipher cell morphogenesis and functions. J Cell Sci 2010; 123:4201 - 13; http://dx.doi.org/10.1242/jcs.075150; PMID: 21123618
- Ingber DE. The riddle of morphogenesis: a question of solution chemistry or molecular cell engineering?. Cell 1993; 75:1249 - 52; http://dx.doi.org/10.1016/0092-8674(93)90612-T; PMID: 8269508
- Zhang H, Landmann F, Zahreddine H, Rodriguez D, Koch M, Labouesse M. A tension-induced mechanotransduction pathway promotes epithelial morphogenesis. Nature 2011; 471:99 - 103; http://dx.doi.org/10.1038/nature09765; PMID: 21368832
- Huh D, Hamilton GA, Ingber DE. From 3D cell culture to organs-on-chips. Trends Cell Biol 2011; 21:745 - 54; http://dx.doi.org/10.1016/j.tcb.2011.09.005; PMID: 22033488
- Pitaval A, Tseng Q, Bornens M, Théry M. Cell shape and contractility regulate ciliogenesis in cell cycle-arrested cells. J Cell Biol 2010; 191:303 - 12; http://dx.doi.org/10.1083/jcb.201004003; PMID: 20956379
- Rodríguez-Fraticelli AE, Auzan M, Alonso MA, Bornens M, Martín-Belmonte F. Cell confinement controls centrosome positioning and lumen initiation during epithelial morphogenesis. J Cell Biol 2012; 198:1011 - 23; http://dx.doi.org/10.1083/jcb.201203075; PMID: 22965908
- Yu W, Datta A, Leroy P, O’Brien LE, Mak G, Jou TS, et al. Beta1-integrin orients epithelial polarity via Rac1 and laminin. Mol Biol Cell 2005; 16:433 - 45; http://dx.doi.org/10.1091/mbc.E04-05-0435; PMID: 15574881
- O’Brien LE, Jou TS, Pollack AL, Zhang Q, Hansen SH, Yurchenco P, et al. Rac1 orientates epithelial apical polarity through effects on basolateral laminin assembly. Nat Cell Biol 2001; 3:831 - 8; http://dx.doi.org/10.1038/ncb0901-831; PMID: 11533663
- Xu K, Sacharidou A, Fu S, Chong DC, Skaug B, Chen ZJ, et al. Blood vessel tubulogenesis requires Rasip1 regulation of GTPase signaling. Dev Cell 2011; 20:526 - 39; http://dx.doi.org/10.1016/j.devcel.2011.02.010; PMID: 21396893
- Wang H, Lacoche S, Huang L, Xue B, Muthuswamy SK. Rotational motion during three-dimensional morphogenesis of mammary epithelial acini relates to laminin matrix assembly. Proc Natl Acad Sci U S A 2013; 110:163 - 8; http://dx.doi.org/10.1073/pnas.1201141110; PMID: 23248267
- Akhtar N, Streuli CH. An integrin-ILK-microtubule network orients cell polarity and lumen formation in glandular epithelium. Nat Cell Biol 2013; 15:17 - 27; http://dx.doi.org/10.1038/ncb2646; PMID: 23263281
- Apodaca G, Gallo LI, Bryant DM. Role of membrane traffic in the generation of epithelial cell asymmetry. Nat Cell Biol 2012; 14:1235 - 43; http://dx.doi.org/10.1038/ncb2635; PMID: 23196841
- Gálvez-Santisteban M, Rodriguez-Fraticelli AE, Bryant DM, Vergarajauregui S, Yasuda T, Bañón-Rodríguez I, et al. Synaptotagmin-like proteins control the formation of a single apical membrane domain in epithelial cells. Nat Cell Biol 2012; 14:838 - 49; http://dx.doi.org/10.1038/ncb2541; PMID: 22820376
- Bryant DM, Datta A, Rodríguez-Fraticelli AE, Peränen J, Martín-Belmonte F, Mostov KE. A molecular network for de novo generation of the apical surface and lumen. Nat Cell Biol 2010; 12:1035 - 45; http://dx.doi.org/10.1038/ncb2106; PMID: 20890297
- Martin-Belmonte F, Gassama A, Datta A, Yu W, Rescher U, Gerke V, et al. PTEN-mediated apical segregation of phosphoinositides controls epithelial morphogenesis through Cdc42. Cell 2007; 128:383 - 97; http://dx.doi.org/10.1016/j.cell.2006.11.051; PMID: 17254974
- Rodriguez-Fraticelli AE, Vergarajauregui S, Eastburn DJ, Datta A, Alonso MA, Mostov K, et al. The Cdc42 GEF Intersectin 2 controls mitotic spindle orientation to form the lumen during epithelial morphogenesis. J Cell Biol 2010; 189:725 - 38; http://dx.doi.org/10.1083/jcb.201002047; PMID: 20479469
- Schmidt KN, Kuhns S, Neuner A, Hub B, Zentgraf H, Pereira G. Cep164 mediates vesicular docking to the mother centriole during early steps of ciliogenesis. J Cell Biol 2012; 199:1083 - 101; http://dx.doi.org/10.1083/jcb.201202126; PMID: 23253480
- Westlake CJ, Baye LM, Nachury MV, Wright KJ, Ervin KE, Phu L, et al. Primary cilia membrane assembly is initiated by Rab11 and transport protein particle II (TRAPPII) complex-dependent trafficking of Rabin8 to the centrosome. Proc Natl Acad Sci U S A 2011; 108:2759 - 64; http://dx.doi.org/10.1073/pnas.1018823108; PMID: 21273506
- Discher DE, Mooney DJ, Zandstra PW. Growth factors, matrices, and forces combine and control stem cells. Science 2009; 324:1673 - 7; http://dx.doi.org/10.1126/science.1171643; PMID: 19556500
- Wang N, Tytell JD, Ingber DE. Mechanotransduction at a distance: mechanically coupling the extracellular matrix with the nucleus. Nat Rev Mol Cell Biol 2009; 10:75 - 82; http://dx.doi.org/10.1038/nrm2594; PMID: 19197334
- Mammoto A, Mammoto T, Ingber DE. Mechanosensitive mechanisms in transcriptional regulation. J Cell Sci 2012; 125:3061 - 73; http://dx.doi.org/10.1242/jcs.093005; PMID: 22797927
- Halder G, Dupont S, Piccolo S. Transduction of mechanical and cytoskeletal cues by YAP and TAZ. Nat Rev Mol Cell Biol 2012; 13:591 - 600; http://dx.doi.org/10.1038/nrm3416; PMID: 22895435
- Dupont S, Morsut L, Aragona M, Enzo E, Giulitti S, Cordenonsi M, et al. Role of YAP/TAZ in mechanotransduction. Nature 2011; 474:179 - 83; http://dx.doi.org/10.1038/nature10137; PMID: 21654799
- Vartiainen MK, Guettler S, Larijani B, Treisman R. Nuclear actin regulates dynamic subcellular localization and activity of the SRF cofactor MAL. Science 2007; 316:1749 - 52; http://dx.doi.org/10.1126/science.1141084; PMID: 17588931
- Miralles F, Posern G, Zaromytidou AI, Treisman R. Actin dynamics control SRF activity by regulation of its coactivator MAL. Cell 2003; 113:329 - 42; http://dx.doi.org/10.1016/S0092-8674(03)00278-2; PMID: 12732141
- Luxenburg C, Pasolli HA, Williams SE, Fuchs E. Developmental roles for Srf, cortical cytoskeleton and cell shape in epidermal spindle orientation. Nat Cell Biol 2011; 13:203 - 14; http://dx.doi.org/10.1038/ncb2163; PMID: 21336301
- DuFort CC, Paszek MJ, Weaver VM. Balancing forces: architectural control of mechanotransduction. Nat Rev Mol Cell Biol 2011; 12:308 - 19; http://dx.doi.org/10.1038/nrm3112; PMID: 21508987
- Patel A, Honoré E. Polycystins and renovascular mechanosensory transduction. Nat Rev Nephrol 2010; 6:530 - 8; http://dx.doi.org/10.1038/nrneph.2010.97; PMID: 20625375
- Butcher DT, Alliston T, Weaver VM. A tense situation: forcing tumour progression. Nat Rev Cancer 2009; 9:108 - 22; http://dx.doi.org/10.1038/nrc2544; PMID: 19165226
- Leckband DE, le Duc Q, Wang N, de Rooij J. Mechanotransduction at cadherin-mediated adhesions. Curr Opin Cell Biol 2011; 23:523 - 30; http://dx.doi.org/10.1016/j.ceb.2011.08.003; PMID: 21890337