Abstract
Development of the nervous system requires efficient extension and guidance of axons and dendrites culminating in synapse formation. Axonal growth and navigation during embryogenesis are controlled by extracellular cues. Many of the same extracellular signals also regulate axonal branching. The emergence of collateral branches from the axon augments the complexity of nervous system innervation and provides an additional mechanism for target selection. Rho-family GTPases play an important role in regulating intracellular cytoskeletal and signaling pathways that facilitate axonal morphological changes. RhoA/G and Rac1 GTPase functions are complex and they can induce or inhibit branch formation, depending on neuronal type, cell context or signaling mechanisms. Evidence of a role of Cdc42 in axon branching is mostly lacking. In contrast, Rac3 has thus far been implicated in the regulation of axon branching. Future analysis of the upstream regulators and downstream effectors mediating the effects of Rho-family GTPase will provide insights into the cellular processes effected, and shed light on the sometimes opposing roles of these GTPases in the regulation of axon branching.
Introduction
The function of the nervous system depends on the establishment and maintenance of synaptic circuitry. Each neuron generates a single axon, but makes synaptic contacts with many target neurons, often in different parts of the nervous system. The formation of axon branches from the main axon shaft is fundamental to the ability of neurons to establish such complex patterns of connectivity during development. In addition to their significant role in the developing nervous system, axon branches also emerge from intact and damaged axons in response to external injury or neurodegeneration resulting in remodeling and adaptive plasticity of the adult nervous system.Citation1,Citation2
Axon branches can arise from phenomenologically distinct methods (reviewed in 3). As the growth cone is navigating, it can split in half generating two separate growth cones independently leading two axonal branches, termed bifurcation of the axon. In contrast, axonal collateral branches (also referred to as interstitial branches or axon sprouting) emerge from protrusive filopodia and lamellipodia initiated along the shaft of the axon. In one form of this mechanism, the protrusive activity is reflective of sites along the axon's path at which the growth cone stalled, leaving behind a domain of protrusive activity, followed by resumption of its advance. In a related and more common form of collateral branching, protrusive activity is generated de novo, in the form of filopodia or lamellipodia, from the otherwise generally quiescent axon shaft.
The cytoskeletal mechanisms of axonal branching are orchestrated by Rho GTPases. RhoA, Cdc42 and Rac are the most studied mammalian GTPases. Cdc42 activity has not been implicated in branch formation, although Cdc42 is involved in axon formation and growth cone filopodia formation.Citation4 The role of RhoA in formation of axonal branches is complicated and not universal among neurons. In response to RhoA signaling, the actin cytoskeleton is rearranged to support growth cone collapse and axon retraction.Citation5 In some cases, RhoA has been shown to inhibit the emergence of protrusive activity from the axon, and thus negatively regulate axon branching.Citation6-Citation8 In contrast, other studies revealed that RhoA activity promotes axon branching.Citation9,Citation10 Similarly, Rac1 GTPase facilitates and prevents axonal branching in different systems, whereas Rac3 and RhoG have a positive effect in axonal branch regulation. This review will focus on the role of Rho-family GTPases in mechanisms of axon collateral branching by primary neurons (summarized in ).
Table 1. Summary of reported roles of RhoA-Family GTPases in axon branching
Overview of the Cytoskeletal Mechanism of Axon Collateral Branching
The actin and microtubule cytoskeleton is critical for collateral branching (). The first step in the formation of a collateral branch involves the actin filament dependent initiation of axonal filopodia, and in some cases lamellipodia. As formation of axonal filopodia is the most common first step in branch emergence (reviewed in ref. Citation3), this review will largely focus on this issue. Unlike growth cones, the shaft of axons contains relatively low levels of actin filaments and protrusive activity.Citation11 However, the axon is still capable of generating filopodia and extracellular signals that promote collateral branching drive the formation of axonal filopodia. Direct evidence for the requirement of filopodia in collateral branching has been provided by filopodia elimination studies. Specific depletion of Enabled (xENA)/xVASP (vasodilator-stimulated phosphoprotein) proteins in Xenopus retinal axons in vivo results in severe impairments of terminal axon branching,Citation12 without major effects on axon extension and path finding.
Figure 1. Collateral axon branching and axonal actin patch precursors to the emergence of axonal filopodia. (A) Schamatic of the steps involved in axon collateral branching. (i) The axon forms a filopodium. (ii) The filopodium becomes invaded by axonal microtubules. (iii) The filopodium develops polarity and matures into a branch. (B) Example of axonal actin patches and filopodial emergence along a cultured chicken embryonic sensory axon expressing eYFP-β-actin. White arrowheads (a,b) show the presence of prexisiting patches which dissipate during the time-lapse sequence. At 6 s a new patch forms (yellow arrowhead) and by 12 s a filopodium emergences from the patch (red arrowhead). Between 6–12 s the actin patch that gives rise to the filopodium elaborates as reflected by the increase in fluorescence. The filopodium and patch have retarcted and disspipated, respectively, by 24 s. (C) Diagram of the current understanding of the role of Rho-GTPases in the regulation of axonal actin patches and filopodia emergence along embryonic sensory axons. The phases of actin patch development are shown as a function of time with assigned roles for Rho-GTPases as positive or negative regulators. For the roles of additional proteins in this mechanism, see reference Citation14.
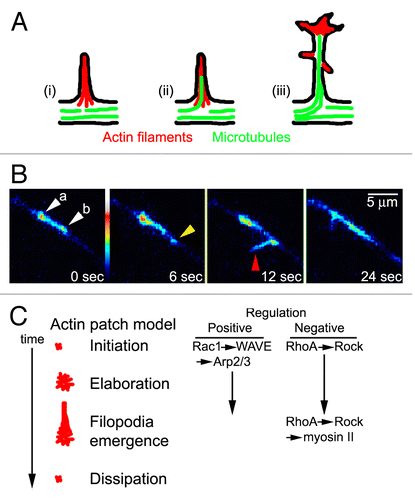
Although axons generate multiple filopodia during the process of branching, only a subset of these filopodia mature into collateral branches. The maturation of filopodia into branches requires the invasion of the filopodium by axonal microtubules, which must then be stabilized in situ in order to allow the transformation of the filopodium into an axon branch (). Microtubules can be targeted into axonal filopodia both through the dynamic instability of microtubule tips, or the transport of microtubules into the filopodia in a cell and/or context dependent manner.Citation3 The targeting of microtubules into filopodia is considered to allow for the directed transport of organelles and proteins into the filopodium, culminating in the transformation of the filopodium into a bonafide axon branch capable of continued extension.
Mechanism of the Formation of Axonal Filopodia
Filopodia are thin, finger-like extensions mainly composed of a bundle of parallel actin filaments and actin associated proteins. The orientation of actin filaments in filopodia is polarized. The rapidly polymerizing barbed ends of filaments are oriented toward the filopodial tip, generating force that pushes the membrane forward.Citation13 A fine balance between actin polymerization at filopodial tips, and the centripetal retrograde flow of the filaments away from the tip, determines filopodia dynamics. In order for filopodia to form, actin filaments must be nucleated, elongated and bundled into parallel fibers. Despite the apparent simplicity of filopodial structures, consideration of the literature indicates cell/context specificity regarding the specific mechanism involved in the formation of filopodia.Citation14
The mechanism underlying the formation of axonal filopodia has been most extensively investigated in embryonic sensory axons, and these axons will be the focus of this discussion. As noted previously, the axon shaft behind the advancing growth cone exhibits low levels of actin filaments and minimal protrusive activity. The localized formation of filopodia is due to the submembranous formation of actin filament based precursor structures termed actin patches (; reviewed in ref. Citation14). Actin patches are detected along axons in vitro and in vivo.Citation15,Citation16 As revealed by platinum replica electron microscopy, actin patches consist of micron sized domains of filaments with a general organization similar to that observed in lamellipodial structures.Citation15 Live imaging of GFP/YFP-actin dynamics along axons has determined that actin patches are dynamic and transient. Patches appear along the axon, initially grow in size and intensity, and eventually dissipate. In vitro and in vivo, the mean duration of actin patches is in the order of 20–40 s. While axons make many patches per unit time, only a subset of these patches gives rise to filopodia before dissipating. However, most filopodia are noted to arise from actin patches. Interestingly, actin patches have also been described in the most proximal segment of the axon, termed the axon initial segment, where they have a role in capturing transport cargo that is not intended for delivery into the axon.Citation17 Actin patches in the distal axon may thus similarly serve to locally trap molecules or organelles relevant to branching.
Along distal sensory axons, the sites of actin filament patch formation are determined by localized microdomains of phosphoinositide 3-kinase (PI3K) activity, and the branching inducing signal nerve growth factor promotes the colocalization of sites of actin patch formation with stalled axonal mitochondria.Citation21 Furthermore, the location of formation of axonal filopodia, and ultimately branches, is determined by the presence of actively respirating mitochondria.Citation89 The rate of formation of axonal actin patches also exhibits a distal (higher) to proximal (lower) gradient along axons,Citation15 which is reminiscent of the distribution of mitochondria in distal axons.Citation21 In conjunction with the demonstration that mitochondria stalling along axons is required for the formation of axon branches and correlates with site for branch formation,Citation29,Citation89 these considerations indicate that the positioning of axonal mitochondria has a fundamental role in determining sites of actin patch formation and subsequent formation of filopodia and ultimately branching.
The Arp2/3 complex is an actin filament nucleator that binds the side of an existing filament and nucleates a new filament emerging at an approximate 70° angle from the “mother” filament. Arp2/3 mediated filament nucleation is a major contributor to the formation and maintenance of lamellipodial structures. Arp2/3 subunits target to axonal actin patches, in both sensory and central nervous system neurons.Citation15,Citation18 Inhibition of the Arp2/3 complex or upstream regulators decreases the formation of axonal actin patches, filopodia and branches.Citation15,Citation18,Citation19 Collectively, these studies identify Arp2/3 as a regulator of the earliest stages of axon branching. It seems likely that additional actin nucleators (e.g., formins or cordon bleu) also contribute to the formation of actin patches, probably through the generation of mother filaments required by Arp2/3 to establish branch filament arrays. Based on the requirement for Arp2/3, the convergent elongation (CE) model for filopodia formationCitation20 appears to be most relevant to the formation of axonal filopodia. In the CE model, the barbed ends of filaments nucleated by Arp2/3 are brought together into a bundle which then determines the site of filopodia formation. Consistently, platinum replica microscopy of actin filament organization in axonal filopodia reveals filaments converging from the population within actin patches giving rise to filopodia.Citation15 The organization of actin filaments in axonal patches observed through platinum replica microscopy resembles that observed in lamellipodia (e.g., a meshwork of filaments at various orientations). Although not directly determined, this observation indicates that the filaments in patches are likely to have mixed polarity.
PI3K is a lipid kinase that generates PIP3 (phosphatidylinositol [3,4,5] triphosphate) from PIP2 (phosphatidylinositol [4,5] biphosphate) in the plasma membrane. PIP3 then recruits the Akt kinase to the plasma membrane, which in turn can inhibit GSK-3β signaling and activate other signaling pathways such as mTOR. These kinase systems have been implicated in collateral branching. In sensory neurons, NGF signaling induces filopodia and collateral branch formation through PI3K-Akt-mTOR by increasing the rate of formation of actin patches without affecting the probability that a patch will give rise to a filopodium.Citation21 Additionally, this study revealed that temporal and spatial development of actin patches correlate spatio-temporally with localized microdomains of PIP3 accumulation in the membrane. Genetic deletion of phosphate and tensin homolog on chromosome ten (PTEN), which has lipid phosphatase activity against PIP3 and thus antagonizes PI3K signaling, results in increased branching in vivo.Citation22 Similar effects on increased branching has been demonstrated by PTEN downregulation through Nedd4 (E3 ligase, part of the ubiquitin proteasome system) in Xenopus retinal ganglion cells in vivo.Citation23 Downstream of PI3K, Akt targets to the membrane through its plekstrin homology domain. The inhibition of Akt activity blocks F-actin patch and filopodia formation.Citation21 This is consistent with the increased branching that occurs in response to constitutively active Akt in sensory neurons.Citation24 Akt signaling inhibits GSK-3β, and studies in hippocampal neurons and cerebellar slices have shown that inhibition of GSK-3β activity or GSK-3β knockdown promotes axonal branching.Citation25,Citation26 Additionally, cyclic GMP-dependent protein kinase 1 (PrKG1), which also inhibits GSK-3, induces collateral branching of rat embryonic DRG sensory neurons.Citation27 Other kinases that play a role in branching involve SAD kinases activated in response to NT-3. In response to NT-3, Raf/MEK/ERK pathway induces SAD kinase activity, which promotes axon branching of cultured DRGs and branching of proprioceptive sensory neurons in terminal fields within ventral spinal cord.Citation28 The related LKB1-NUAK1 kinase pathway regulates branching of cortical axons.Citation29
Intra-Axonal Protein Synthesis in Collateral Branching
Localized protein synthesis in the developing and injured adult nervous system provides an efficient mechanism for the regulation of the axonal proteome. Multiple mRNAs coding for proteins that regulate the axonal cytoskeleton (β-actin, cofilin, GAP43, Arp2, cortactin, WAVE1, fascin, tubulin) are targeted into axons.Citation30-Citation32 mRNAs are targeted into axons through motifs located within the 3′ untranslated region (3′UTR) or the 5′UTR (reviewed by refs. Citation33, Citation34). The transport and subcellular translation of mRNAs are regulated by neurotrophins, injury, and neuronal activity,Citation34 all of which have been involved in the regulation of axon branching.
PI3K signaling is a major determinant of axon branching and can regulate translation through the mammalian target of rapamycin (mTOR) pathway. A role for protein synthesis in collateral sprouting in vivo is suggested by the finding that intra-peritoneal administration of rapamycin, an inhibitor of mTOR, results in a 50% decrease in sprouting of mouse dentate gyrus inter-neurons in induced status epilepticus.Citation35 In cultured chicken sensory axons in vitro, NGF-PI3K signaling promotes collateral branch formation dependent on intra-axonal protein synthesis of Arp2 subunit of the Arp2/3 complex, WAVE1, and the complex stabilizer cortactin.Citation32 In this study, inhibition of protein synthesis and PI3K-mTOR signaling in axons severed from their cell bodies impaired collateral branching induced by acute treatment with nerve growth factor. Additional evidence on the role of localized protein synthesis and collateral formation is provided by studies of the mRNA binding/localizing protein FMRP and its gene fmr1. Knockout of fmr1 in mice is associated with extensive collateral formation from the granule cell axons in the hippocampus.Citation36 Loss of FMRP function and associated defects in axonal arborization are also observed in the Drosophila model of fragile X syndrome.Citation37 Motor neurons demonstrate increased branching in the absence of dFmr1, and decreased branching when dFMRP is overexpressed. These findings suggest that local protein synthesis is disrupted in fragile X syndrome. Injection of morpholino antisense oligonucleotides in zebrafish embryos to inhibit translation of target mRNA and knockdown of the survival motor neuron (SMN) protein significantly increases motor neuron branching.Citation38 SMN is involved in the formation of ribonucleoprotein complexes and its mutation/deletion results in spinal muscular atrophy.Citation39 Additionally, a possible role for axonal protein synthesis in branching is also suggested by the accumulation of β-actin and actin depolymerizing factor mRNAs at the base of branches in vitro.Citation40,Citation41 Translation of axonal β-actin mRNA in cultured rat neurons and in vivo in sensory neurons of chicken spinal cord results in increased branching.Citation42 Furthermore, the mRNA binding proteins Vg1RBP and Hermes regulate axon branching in developing retinal ganglion cell axons.Citation43,Citation44
Microtubule Invasion of Filopodia and Branch Maturation
The second critical step of collateral branch formation requires the involvement of the microtubule cytoskeleton. Axonal microtubules are organized in bundles along the axon with the majority of their plus-end oriented toward the growth cone. The mechanisms that regulate the interaction of the actin and microtubule cytoskeleton in axonal branch formation are not well understood. Early steps of collateral branching involve the unbundling and splaying apart of microtubule bundles.Citation3,Citation45 In cortical neuronal cultures, sites of collateral branches demarcated by growth cone pausing display disruption in microtubule bundling, exploration of axonal filopodia by microtubules, breakdown of bundles, and invasion of the nascent axonal branch by microtubules.Citation46 Localization of Septin-7 protein to the base of filopodia along the axon correlates with microtubule splaying apart and microtubule exploration of axonal filopodia in chicken sensory neurons.Citation47 The stabilization and continued extension of nascent branches correlates with microtubule invasion.Citation48 Invasion of axonal filopodia by microtubules requires microtubule dynamic instability and/or the transport of small microtubules.Citation3 Fragmented microtubules have been detected at axonal branching sites of hippocampal neurons.Citation49 Microtubule associated proteins that function in microtubule depolymerization and severing have also been shown to play a role in axon collateral branching. For example, kinesin superfamily protein 2A (KIF2A), implicated in microtubule depolymerization, functions downstream of phosphatidyl 4-phosphate 5-kinase α to negatively regulate the length and the number of collateral branches longer that 50 μm in hippocampal neurons.Citation50 Microtubule severing at sites of branching is accomplished by microtubule severing proteins, katanin and spastin. The expression of these proteins are enhanced in hippocampal cultures treated with basic fibroblast growth factor (bFGF) which result in increased levels of short microtubules and promotion of axon branching.Citation51 The effect of katanin in branch enhancement is enabled by tau phosphorylation and subsequent dissociation from microtubules in response to bFGF.
Emerging Roles of Rho GTPases in Axon Branching
The most commonly studied Rho-GTPases that regulate the actin cytoskeleton in the mammalian system are RhoA, Rac1 and Cdc42. Investigation of Rho GTPase function in vivo by utilizing global animal knockout has been limited due to the embryonic lethality of RhoA, Rac1, and Cdc42 mutant mice.Citation52 Rho GTPases are monomeric G proteins that are switched on when bound to GTP, a process facilitated by guanine exchange factors (GEFs), and switched off when bound to GDP, a process promoted by GTPase-activating proteins (GAPs).Citation4 When bound to GTP, membrane-bound Rho GTPases initiate singaling cascades that reorganize the cytoskeleton. They are important for axonal growth, axonal morphology, pathfinding, and neuronal plasticity. Their functional implications are complex and display specificity toward neuronal cell type, context or developmental stage. The current understanding of the role of Rho-GTPases in the regulation of axonal actin patches and filopodia along embryonic sensory neurons is summarized in .
Overall, the current literature does not implicate Cdc42 function as a major regulator in axon branch formation. Consistent with a general lack of reported roles for Cdc42 in branching, relative to the growth cone the axon exhibits lower levels of Cdc42 activation,Citation53 and dominant negative Cdc42 has no effect on formation of axonal filopodia but it decreases the number of growth cone filopodia in cultured sensory neurons.Citation32 However, a role for Cdc42 in axon branching was suggested in a study investigating branching that coincides with the retraction of the axon tip following contact with repellent signals.Citation5 This form of branching may thus be different from the branching elicited by branch inducing signals such as neurotrophins. However, Cdc42 function has been associated with axonogenesis. Expression of constitutively active Cdc42 (V12) or hyperactivated Cdc42 (L28) in rat hippocampal neurons result in inhibition of neurite extension and formation of multiple axons, respectively.Citation54 This study revealed that Rap1B GTPase functions upstream of Cdc42, whereas PI3K functions upstream of both GTPases to determine axonal initiation. Additionally, Cdc42 signals through its downstream effectors Par6/aPKC.Citation4 Alternatively, Cdc42-mediated actin reorganization and filopodia formation can involve the activation of Wiskott-Aldrich-syndrome protein (WASP) and neuronal WASP (N-WASP).Citation55 WASP and N-Wasp are activators of the Arp2/3 complex which, as previously discussed, contributes to the formation of filopodia and lamellipodia. The subcellular functions of Cdc42 in the formation of filopodia remain poorly understood. The lack of a role for Cdc42 in axon branching may reflect the utilization of Arp2/3 activators of the WAVE family by branching signals, or a predominance of WAVE proteins along the axon shaft relative to the growth cone. Moreover, even along the same axons (e.g., sensory axons) Cdc42 can contribute to filopodia formation at the growth cone, but not along the axon shaft.Citation32
The RhoA-Rock pathway has been associated with negative regulation of the axonal actin cytoskeleton. In chicken sensory neurons, RhoA-Rock signaling negatively regulates actin patch elaboration without affecting the rate of actin patch initiation.Citation6 Thus, patches form at normal rates but grow larger than in baseline conditions. These effects appear to be independent of myosin II contractility, a major effector downstream of Rock. However, through Myosin II activation RhoA-Rock suppresses axonal protrusive activity by negatively regulating the emergence of filopodia from actin patches. Consistently, inhibition of myosin II activity in this model system promotes axon branching (G. Gallo unpublished results). Semaphorin-3A is an inhibitor of axon elongation and branching. Semaphorin-3A signaling inhibits the formation of axonal actin patches through Rock.Citation56 RhoA signaling also negatively regulates axon branch formation in Drosophila mushroom body neurons. Inactivating p190RhoGAP, activating RhoA or its effector Drok/Rock all results in the retraction of branches into the main axon shaft.Citation7 These findings are generally consistent with the roles of RhoA-Rock-myosin II in mediating axon retraction in response to repellent signals.Citation56 RhoA also negatively regulates axon branching of Purkinje cells in the deep cerebellar nuclei.Citation8 In this study, cell specific ablation of FAK increased axon terminals in vivo. In vitro experiments revealed that FAK recruits and activates p190RhoGEF, which in turn activates RhoA in order to inhibit axon branching.
In contrast, RhoA activity has also been implicated in the promotion of axon branching. Inhibition of RhoA activity diminishes axonal branching in hippocampal neurons of embryonic mice, whereas expression of constitutively active RhoA has no effect of branching.Citation9 Activation and inhibition of RhoA in organotypic cortical slice cultures resulted in increased and decreased axon branching.Citation10 RhoA activity in these organotypic cortical slices is promoted by neural activity as demonstrated by inhibition of sodium channels and glutamate receptors. The role of RhoA in these central nervous system neurons may thus be related to their activity patterns and activity dependent mechanisms. A possible candidate downstream effector of RhoA that enables actin polymerization is the mammalian diaphanous formin protein 1 (mDia1). mDia1 contains a Rho-binding domain (RBD), which upon binding to activated Rho (A, B, or C) proteins is released from auto-inhibition.Citation55,Citation57 The differences in the role of RhoA in branching between studies may reflect differences between cell types, culturing environment (or in vitro relative to in situ), or as suggested by the study by Ohnami et al.Citation10 the electrophysiological state of the neurons. Indeed, it will be of interest to determine further how neuronal activity regulates or orchestrates the function of GTPases.
Rac GTPases have been shown to have roles in the regulation of axon branching. Rac1 negatively regulates axon branching in the Drosophila giant fiber system.Citation58 Overexpression of dominant-negative Rac1 (N17) in the giant fibers is accompanied with enhanced axon branching. Purkinje cells of transgenic mice expressing the human constitutively active Rac1 (V12) display decreased axon terminals in the deep cerebellar nuclei.Citation59 In contrast to these roles of Rac1 in the suppression of branching, Rac1 has been shown to positively regulate axon branching in other neuronal systems. The activities of Rac GTPases (Rac1, Rac2, and related Mig-2-like (Mtl)) are critical for axon branching of mushroom body neurons in Drosophila.Citation60 Loss-of-function mutations of endogenous dRac genes revealed that compared with axon growth and guidance, axon branching displays a higher dependence on Rac GTPase activity, in which Rac1 function is more critical than Rac2. Moreover, the frequency of axon branching defects correlates with progressive loss-of-function induced by removal of wild-type copies of Rac genes. The axon branching defects seem to be independent of other observed defects in guidance and axon growth. Rac2/3 double mutations in C. elegans revealed abnormal and premature axon branching, indicative of the role or Rac GTPases in controlling axonal branching.Citation61 Expression of neuron-specific Rac also resulted in increased branching of axons. Additionally, this study revealed that UNC-115, an actin-binding protein, mediates the effects of Rac2 in regulating axonal morphology and pathfinding. Furthermore, the regulation of Rac1 GTPase activity by the Vav2 GEF positively regulates branch formation of Xenopus retinal ganglion neurons.Citation62 In spinal neurons, expression of Vav2 or constitutively active Rac1 promotes axonal branching on laminin substratum. Loss-of-function of Vav2 in neurons cultured on L1 substratum results in significant branch inhibition, despite the ability of L1 to promote branching. Similarly, Vav2 activity regulates branching of commissural interneurons as they approach the ventral spinal cord midline. In chicken sensory neuronal cultures, the activity of Rac1 is promoted by NGF-PI3K signaling and it induces formation of axonal actin patches and filopodia, which are precursors of collateral branch formation.Citation32 This effect of Rac1 is likely performed by activation of the downstream effector, WAVE. WAVE induces filopodia and axon collateral branching by promoting actin filament branching and polymerization through the Arp2/3 complex.Citation32,Citation63
Other GTPases implicated in axon branch formation are Rac3 and RhoG. Rac3 was first identified in chicken as cRac1B. It specifically expresses in the nervous system and it is developmentally regulated, with the highest expression correlating developmentally with axon branching.Citation64,Citation65 Overexpression of cRac1B/Rac3 in cultured retinal cells promotes neurite branching.Citation65 Overexpression of the cRac1A/Rac1 in retinal cells had no effect on neurite morphology. Kalirin-GEF1 activates Rho GTPases and it has specificity for RhoG activation in postnatal sympathetic neurons.Citation66 Expression of Kalirin-GEF1 enhances multiple axon-like processes from the soma and branching, a phenotype consistent with expression of activated RhoG. In contrast to its role in postnatal sympathetic neurons, RhoG decreases the number of branches along the axons of embryonic hippocampal neurons.Citation74 In this cell system, ELMO-Dock180-Rac1 downstream of RhoG act to suppress the number of axon branches. However, the Rac and Cdc42 GTPases guanine exchange factor (GEF) αPIX induces axonal branching in embryonic hippocampal neurons.Citation67 Overexpression or depletion of αPIX or the associated protein GIT2 induce and impair axonal branching, respectively. Furthermore, inhibition of Rac1 and Cdc42 activity using cell permeable peptides in cultured retinal ganglion cells blocks the formation of axon collaterals in axons undergoing retraction following contact with repellent guidance signals.Citation5 The differences in the role of RhoG and downstream GTPases in branching between these studies may reflect a variety of differences (e.g., cell type, developmental stage, culturing substratum), including the possibility that different upstream activating mechanisms (e.g., the GEFs Dock180 and αPIX) which may drive a concerted response involving multiple effectors.
Ras family GTPases are also implicated in axonal morphology regulation by signaling through PI3K and the actin filament binding protein afadin (AF-6).Citation68 This study revealed that R-Ras induces axon branching in cortical neurons by translocating afadin to the cell membrane and by inhibiting GSK-3β through PI3K/Akt signaling pathway. Ras GTPases directly activates PI3K by interacting with the PI3K-p110 subunit.Citation69 Other GTPases functioning downstream of PI3K, such as Rac1–3, Cdc42, and RhoG are also capable of activating PI3K indirectly and inducing a positive feedback loop of PI3K activation. Whether positive feedback loops between GTPases and PI3K signaling contribute to axon branching remains to be elucidated.
Extracellular Signals and the Regulation of Axon Branches: Involvement of RhoA-Family GTPases
In vivo the formation and maintenance of axon branches in under bi-directional control by extracellular signals, cell-to-cell communication and neuronal activity patterns. While beyond the scope of this review, it is worth noting that these extrinsic regulatory signals have been described to act through RhoA-family GTPases. Neurotrophins are major regulators of axon branching in a variety of neural systems, and neurotrophin binding to Trk receptors regulates RhoA GTPases.Citation75 Similarly, netrins also regulate axon branching and signal through RhoA GTPases.Citation76-Citation80 Interestingly, RhoA also acts to suppress the targeting of netrin receptors to the membrane,Citation81 revealing a multifunctional role for GTPases in this signaling system. Semaphorins also regulate axon branching, usually inhibiting branching (but see refs. Citation82, Citation92), and can activate RhoA GTPases.Citation83 Chondroitin sulfate proteoglycans (CSPGs) suppress axon branching in vivo and in vitro and also use RhoA-family GTPases to signal.Citation84-Citation87 Finally, synaptic activity can also regulate RhoA-family GTPases,Citation88 emphasizing the notion that in electrically active neuronal cultures or in vivo circuits activity may further assist in the determination of GTPase activity or function. As noted by the multiple discrepancies in the literature regarding the roles of RhoA-family GTPases in axon branching, it will be important to further determine how the functions of GTPase signaling are determined in the context of additional signaling events induced by ligand binding to specific receptor systems and in different neuronal populations and contexts.
Concluding Statement
The mechanisms of axon collateral branching are only partially understood. RhoA-family GTPases are key regulators of cytoskeletal reorganization, but specific roles for these GTPases in axon branching largely remain to be determined. The literature indicates complex roles for these GTPases in a neuronal cell type/context dependent manner. A major goal of future investigations will be to determine the upstream regulators and downstream effectors that determine the multi-functional nature of these GTPases. As an example, although the Rac1 GTPase is canonically considered to drive the elaboration of protrusive structures, it is also required for the loss of protrusive activity by growth cones in response to extracellular repellent guidance molecules. In this context, the function of Rac1 appears to undergo a switch from regulating protrusive activity to promoting endocytosis in response to repellent guidance molecules.Citation70 These types of observations underscore the importance of understanding the cellular roles of these GTPases in a context dependent manner. Ultimately, a unifying model for axon branching, beyond the basic sequence of cytoskeletal events (), is not supported by the literature. Indeed, even the same kinases contribute to branching depending on the cell type they are expressed in in vivo, and also likely depending on which extracellular signals are regulating branching in that neuronal population.Citation29,Citation89,Citation90 Given that Rho-GTPases have been ascribed a variety of cellular functions, it will be necessary to determine their specific contributions to cytoskeletal dynamics, subcellular organization, organelle function, and membrane traffic, all components of axon branching.Citation3,Citation29,Citation91 Through regulation of the cytoskeleton, Rho-GTPases may also control aspects of somatic protein synthesis.Citation71 It will thus be of interest to determine if they may have such roles in axons in the context of axonal protein synthesis dependent axon branching. Furthermore, although microtubules have a fundamental role in axon branching, and some Rho-GTPases have been shown to regulate the microtubule cytoskeleton,Citation72,Citation73 studies of these GTPases in the regulation of axonal microtubules during branching are lacking.
Finally, the majority of studies perform morphometric analysis and report on the number or length of axon branches, sometimes at single experimental time points. Differences in these morphometric variables can arise through the separate regulation of different aspects of the mechanism of branching. For example, an increase in the number of axon branches can arise from increased initiation of branches, and/or increased retention of nascent branches that would otherwise be retracted.Citation29,Citation89 Thus, it is important to consider the dynamics of the branching process in the determination of the roles of specific molecules, and ultimately which aspects of the basic cytoskeletal events underlying branching are under regulation by GTPases. Future live imaging analysis of branch formation, and the underlying cytoskeletal dynamics, may shed light on some of the observed discrepancies between different studies.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
This work was supported by NIH grant NS078030 (G.G.).
References
- Onifer SM, Smith GM, Fouad K. Plasticity after spinal cord injury: relevance to recovery and approaches to facilitate it. Neurotherapeutics 2011; 8:283 - 93; http://dx.doi.org/10.1007/s13311-011-0034-4; PMID: 21384221
- Harel NY, Strittmatter SM. Can regenerating axons recapitulate developmental guidance during recovery from spinal cord injury?. Nat Rev Neurosci 2006; 7:603 - 16; http://dx.doi.org/10.1038/nrn1957; PMID: 16858389
- Gallo G. The cytoskeletal and signaling mechanisms of axon collateral branching. Dev Neurobiol 2011; 71:201 - 20; http://dx.doi.org/10.1002/dneu.20852; PMID: 21308993
- Hall A, Lalli G. Rho and Ras GTPases in axon growth, guidance, and branching. Cold Spring Harb Perspect Biol 2010; 2:a001818; http://dx.doi.org/10.1101/cshperspect.a001818; PMID: 20182621
- Thies E, Davenport RW. Independent roles of Rho-GTPases in growth cone and axonal behavior. J Neurobiol 2003; 54:358 - 69; http://dx.doi.org/10.1002/neu.10135; PMID: 12500311
- Loudon RP, Silver LD, Yee HF Jr., Gallo G. RhoA-kinase and myosin II are required for the maintenance of growth cone polarity and guidance by nerve growth factor. J Neurobiol 2006; 66:847 - 67; http://dx.doi.org/10.1002/neu.20258; PMID: 16673385
- Billuart P, Winter CG, Maresh A, Zhao X, Luo L. Regulating axon branch stability: the role of p190 RhoGAP in repressing a retraction signaling pathway. Cell 2001; 107:195 - 207; http://dx.doi.org/10.1016/S0092-8674(01)00522-0; PMID: 11672527
- Rico B, Beggs HE, Schahin-Reed D, Kimes N, Schmidt A, Reichardt LF. Control of axonal branching and synapse formation by focal adhesion kinase. Nat Neurosci 2004; 7:1059 - 69; http://dx.doi.org/10.1038/nn1317; PMID: 15378065
- Ahnert-Hilger G, Höltje M, Grosse G, Pickert G, Mucke C, Nixdorf-Bergweiler B, Boquet P, Hofmann F, Just I. Differential effects of Rho GTPases on axonal and dendritic development in hippocampal neurones. J Neurochem 2004; 90:9 - 18; http://dx.doi.org/10.1111/j.1471-4159.2004.02475.x; PMID: 15198662
- Ohnami S, Endo M, Hirai S, Uesaka N, Hatanaka Y, Yamashita T, Yamamoto N. Role of RhoA in activity-dependent cortical axon branching. J Neurosci 2008; 28:9117 - 21; http://dx.doi.org/10.1523/JNEUROSCI.1731-08.2008; PMID: 18784292
- Letourneau PC. Actin in axons: stable scaffolds and dynamic filaments. Results Probl Cell Differ 2009; 48:65 - 90; http://dx.doi.org/10.1007/400_2009_15; PMID: 19582412
- Dwivedy A, Gertler FB, Miller J, Holt CE, Lebrand C. Ena/VASP function in retinal axons is required for terminal arborization but not pathway navigation. Development 2007; 134:2137 - 46; http://dx.doi.org/10.1242/dev.002345; PMID: 17507414
- Mattila PK, Lappalainen P. Filopodia: molecular architecture and cellular functions. Nat Rev Mol Cell Biol 2008; 9:446 - 54; http://dx.doi.org/10.1038/nrm2406; PMID: 18464790
- Gallo G. Mechanisms underlying the initiation and dynamics of neuronal filopodia: from neurite formation to synaptogenesis. Int Rev Cell Mol Biol 2013; 301:95 - 156; http://dx.doi.org/10.1016/B978-0-12-407704-1.00003-8; PMID: 23317818
- Spillane M, Ketschek A, Jones SL, Korobova F, Marsick B, Lanier L, Svitkina T, Gallo G. The actin nucleating Arp2/3 complex contributes to the formation of axonal filopodia and branches through the regulation of actin patch precursors to filopodia. Dev Neurobiol 2011; 71:747 - 58; http://dx.doi.org/10.1002/dneu.20907; PMID: 21557512
- Andersen EF, Asuri NS, Halloran MC. In vivo imaging of cell behaviors and F-actin reveals LIM-HD transcription factor regulation of peripheral versus central sensory axon development. Neural Dev 2011; 6:27; http://dx.doi.org/10.1186/1749-8104-6-27; PMID: 21619654
- Watanabe K, Al-Bassam S, Miyazaki Y, Wandless TJ, Webster P, Arnold DB. Networks of polarized actin filaments in the axon initial segment provide a mechanism for sorting axonal and dendritic proteins. Cell Rep 2012; 2:1546 - 53; http://dx.doi.org/10.1016/j.celrep.2012.11.015; PMID: 23246006
- Mingorance-Le Meur A, O’Connor TP. Neurite consolidation is an active process requiring constant repression of protrusive activity. EMBO J 2009; 28:248 - 60; http://dx.doi.org/10.1038/emboj.2008.265; PMID: 19096364
- Strasser GA, Rahim NA, VanderWaal KE, Gertler FB, Lanier LM. Arp2/3 is a negative regulator of growth cone translocation. Neuron 2004; 43:81 - 94; http://dx.doi.org/10.1016/j.neuron.2004.05.015; PMID: 15233919
- Svitkina TM, Bulanova EA, Chaga OY, Vignjevic DM, Kojima S, Vasiliev JM, Borisy GG. Mechanism of filopodia initiation by reorganization of a dendritic network. J Cell Biol 2003; 160:409 - 21; http://dx.doi.org/10.1083/jcb.200210174; PMID: 12566431
- Ketschek A, Gallo G. Nerve growth factor induces axonal filopodia through localized microdomains of phosphoinositide 3-kinase activity that drive the formation of cytoskeletal precursors to filopodia. J Neurosci 2010; 30:12185 - 97; http://dx.doi.org/10.1523/JNEUROSCI.1740-10.2010; PMID: 20826681
- Kwon C-H, Luikart BW, Powell CM, Zhou J, Matheny SA, Zhang W, Li Y, Baker SJ, Parada LF. Pten regulates neuronal arborization and social interaction in mice. Neuron 2006; 50:377 - 88; http://dx.doi.org/10.1016/j.neuron.2006.03.023; PMID: 16675393
- Drinjakovic J, Jung H, Campbell DS, Strochlic L, Dwivedy A, Holt CE. E3 ligase Nedd4 promotes axon branching by downregulating PTEN. Neuron 2010; 65:341 - 57; http://dx.doi.org/10.1016/j.neuron.2010.01.017; PMID: 20159448
- Grider MH, Park D, Spencer DM, Shine HD. Lipid raft-targeted Akt promotes axonal branching and growth cone expansion via mTOR and Rac1, respectively. J Neurosci Res 2009; 87:3033 - 42; http://dx.doi.org/10.1002/jnr.22140; PMID: 19530170
- Kim WY, Zhou FQ, Zhou J, Yokota Y, Wang YM, Yoshimura T, Kaibuchi K, Woodgett JR, Anton ES, Snider WD. Essential roles for GSK-3s and GSK-3-primed substrates in neurotrophin-induced and hippocampal axon growth. Neuron 2006; 52:981 - 96; http://dx.doi.org/10.1016/j.neuron.2006.10.031; PMID: 17178402
- Bilimoria PM, de la Torre-Ubieta L, Ikeuchi Y, Becker EB, Reiner O, Bonni A. A JIP3-regulated GSK3β/DCX signaling pathway restricts axon branching. J Neurosci 2010; 30:16766 - 76; http://dx.doi.org/10.1523/JNEUROSCI.1362-10.2010; PMID: 21159948
- Zhao Z, Wang Z, Gu Y, Feil R, Hofmann F, Ma L. Regulate axon branching by the cyclic GMP pathway via inhibition of glycogen synthase kinase 3 in dorsal root ganglion sensory neurons. J Neurosci 2009; 29:1350 - 60; http://dx.doi.org/10.1523/JNEUROSCI.3770-08.2009; PMID: 19193882
- Lilley BN, Pan YA, Sanes JR. SAD kinases sculpt axonal arbors of sensory neurons through long- and short-term responses to neurotrophin signals. Neuron 2013; 79:39 - 53; http://dx.doi.org/10.1016/j.neuron.2013.05.017; PMID: 23790753
- Courchet J, Lewis TL Jr., Lee S, Courchet V, Liou DY, Aizawa S, Polleux F. Terminal axon branching is regulated by the LKB1-NUAK1 kinase pathway via presynaptic mitochondrial capture. Cell 2013; 153:1510 - 25; http://dx.doi.org/10.1016/j.cell.2013.05.021; PMID: 23791179
- Willis DE, van Niekerk EA, Sasaki Y, Mesngon M, Merianda TT, Williams GG, Kendall M, Smith DS, Bassell GJ, Twiss JL. Extracellular stimuli specifically regulate localized levels of individual neuronal mRNAs. J Cell Biol 2007; 178:965 - 80; http://dx.doi.org/10.1083/jcb.200703209; PMID: 17785519
- Zivraj KH, Tung YCL, Piper M, Gumy L, Fawcett JW, Yeo GSH, Holt CE. Subcellular profiling reveals distinct and developmentally regulated repertoire of growth cone mRNAs. J Neurosci 2010; 30:15464 - 78; http://dx.doi.org/10.1523/JNEUROSCI.1800-10.2010; PMID: 21084603
- Spillane M, Ketschek A, Donnelly CJ, Pacheco A, Twiss JL, Gallo G. Nerve growth factor-induced formation of axonal filopodia and collateral branches involves the intra-axonal synthesis of regulators of the actin-nucleating Arp2/3 complex. J Neurosci 2012; 32:17671 - 89; http://dx.doi.org/10.1523/JNEUROSCI.1079-12.2012; PMID: 23223289
- Andreassi C, Riccio A. To localize or not to localize: mRNA fate is in 3’UTR ends. Trends Cell Biol 2009; 19:465 - 74; http://dx.doi.org/10.1016/j.tcb.2009.06.001; PMID: 19716303
- Yoo S, van Niekerk EA, Merianda TT, Twiss JL. Dynamics of axonal mRNA transport and implications for peripheral nerve regeneration. Exp Neurol 2010; 223:19 - 27; http://dx.doi.org/10.1016/j.expneurol.2009.08.011; PMID: 19699200
- Buckmaster PS, Wen X. Rapamycin suppresses axon sprouting by somatostatin interneurons in a mouse model of temporal lobe epilepsy. Epilepsia 2011; 52:2057 - 64; http://dx.doi.org/10.1111/j.1528-1167.2011.03253.x; PMID: 21883182
- Qiu L-F, Lu T-J, Hu X-L, Yi Y-H, Liao W-P, Xiong Z-Q. Limbic epileptogenesis in a mouse model of fragile X syndrome. Cereb Cortex 2009; 19:1504 - 14; http://dx.doi.org/10.1093/cercor/bhn163; PMID: 18832330
- Bhogal B, Jongens TA. Fragile X syndrome and model organisms: identifying potential routes of therapeutic intervention. Dis Model Mech 2010; 3:693 - 700; http://dx.doi.org/10.1242/dmm.002006; PMID: 20682752
- McWhorter ML, Monani UR, Burghes AHM, Beattie CE. Knockdown of the survival motor neuron (Smn) protein in zebrafish causes defects in motor axon outgrowth and pathfinding. J Cell Biol 2003; 162:919 - 31; http://dx.doi.org/10.1083/jcb.200303168; PMID: 12952942
- Wang W, van Niekerk E, Willis DE, Twiss JL. RNA transport and localized protein synthesis in neurological disorders and neural repair. Dev Neurobiol 2007; 67:1166 - 82; http://dx.doi.org/10.1002/dneu.20511; PMID: 17514714
- Lee S-K, Hollenbeck PJ. Organization and translation of mRNA in sympathetic axons. J Cell Sci 2003; 116:4467 - 78; http://dx.doi.org/10.1242/jcs.00745; PMID: 13130093
- Olink-Coux M, Hollenbeck PJ. Localization and active transport of mRNA in axons of sympathetic neurons in culture. J Neurosci 1996; 16:1346 - 58; PMID: 8778286
- Donnelly CJ, Park M, Spillane M, Yoo S, Pacheco A, Gomes C, Vuppalanchi D, McDonald M, Kim HH, Merianda TT, et al. Axonally synthesized β-actin and GAP-43 proteins support distinct modes of axonal growth. J Neurosci 2013; 33:3311 - 22; http://dx.doi.org/10.1523/JNEUROSCI.1722-12.2013; PMID: 23426659
- Hörnberg H, Wollerton-van Horck F, Maurus D, Zwart M, Svoboda H, Harris WA, Holt CE. RNA-binding protein Hermes/RBPMS inversely affects synapse density and axon arbor formation in retinal ganglion cells in vivo. J Neurosci 2013; 33:10384 - 95; http://dx.doi.org/10.1523/JNEUROSCI.5858-12.2013; PMID: 23785151
- Kalous A, Stake JI, Yisraeli JK, Holt CE. RNA-binding protein Vg1RBP regulates terminal arbor formation but not long-range axon navigation in the developing visual system. Dev Neurobiol 2014; 74:303 - 18; http://dx.doi.org/10.1002/dneu.22110; PMID: 23853158
- Kalil K, Szebenyi G, Dent EW. Common mechanisms underlying growth cone guidance and axon branching. J Neurobiol 2000; 44:145 - 58; http://dx.doi.org/10.1002/1097-4695(200008)44:2<145::AID-NEU5>3.0.CO;2-X; PMID: 10934318
- Dent EW, Callaway JL, Szebenyi G, Baas PW, Kalil K. Reorganization and movement of microtubules in axonal growth cones and developing interstitial branches. J Neurosci 1999; 19:8894 - 908; PMID: 10516309
- Hu J, Bai X, Bowen JR, Dolat L, Korobova F, Yu W, Baas PW, Svitkina T, Gallo G, Spiliotis ET. Septin-driven coordination of actin and microtubule remodeling regulates the collateral branching of axons. Curr Biol 2012; 22:1109 - 15; http://dx.doi.org/10.1016/j.cub.2012.04.019; PMID: 22608511
- Gallo G, Letourneau PC. Different contributions of microtubule dynamics and transport to the growth of axons and collateral sprouts. J Neurosci 1999; 19:3860 - 73; PMID: 10234018
- Yu W, Ahmad FJ, Baas PW. Microtubule fragmentation and partitioning in the axon during collateral branch formation. J Neurosci 1994; 14:5872 - 84; PMID: 7931550
- Noda Y, Niwa S, Homma N, Fukuda H, Imajo-Ohmi S, Hirokawa N. Phosphatidylinositol 4-phosphate 5-kinase alpha (PIPKα) regulates neuronal microtubule depolymerase kinesin, KIF2A and suppresses elongation of axon branches. Proc Natl Acad Sci U S A 2012; 109:1725 - 30; http://dx.doi.org/10.1073/pnas.1107808109; PMID: 22307638
- Qiang L, Yu W, Liu M, Solowska JM, Baas PW. Basic fibroblast growth factor elicits formation of interstitial axonal branches via enhanced severing of microtubules. Mol Biol Cell 2010; 21:334 - 44; http://dx.doi.org/10.1091/mbc.E09-09-0834; PMID: 19940015
- Heasman SJ, Ridley AJ. Mammalian Rho GTPases: new insights into their functions from in vivo studies. Nat Rev Mol Cell Biol 2008; 9:690 - 701; http://dx.doi.org/10.1038/nrm2476; PMID: 18719708
- Myers JP, Robles E, Ducharme-Smith A, Gomez TM. Focal adhesion kinase modulates Cdc42 activity downstream of positive and negative axon guidance cues. J Cell Sci 2012; 125:2918 - 29; http://dx.doi.org/10.1242/jcs.100107; PMID: 22393238
- Schwamborn JC, Püschel AW. The sequential activity of the GTPases Rap1B and Cdc42 determines neuronal polarity. Nat Neurosci 2004; 7:923 - 9; http://dx.doi.org/10.1038/nn1295; PMID: 15286792
- Govek E-E, Newey SE, Van Aelst L. The role of the Rho GTPases in neuronal development. Genes Dev 2005; 19:1 - 49; http://dx.doi.org/10.1101/gad.1256405; PMID: 15630019
- Gallo G. RhoA-kinase coordinates F-actin organization and myosin II activity during semaphorin-3A-induced axon retraction. J Cell Sci 2006; 119:3413 - 23; http://dx.doi.org/10.1242/jcs.03084; PMID: 16899819
- Sit S-T, Manser E. Rho GTPases and their role in organizing the actin cytoskeleton. J Cell Sci 2011; 124:679 - 83; http://dx.doi.org/10.1242/jcs.064964; PMID: 21321325
- Allen MJ, Shan X, Murphey RK. A role for Drosophila Drac1 in neurite outgrowth and synaptogenesis in the giant fiber system. Mol Cell Neurosci 2000; 16:754 - 65; http://dx.doi.org/10.1006/mcne.2000.0903; PMID: 11124895
- Luo L, Hensch TK, Ackerman L, Barbel S, Jan LY, Jan YN. Differential effects of the Rac GTPase on Purkinje cell axons and dendritic trunks and spines. Nature 1996; 379:837 - 40; http://dx.doi.org/10.1038/379837a0; PMID: 8587609
- Ng J, Nardine T, Harms M, Tzu J, Goldstein A, Sun Y, Dietzl G, Dickson BJ, Luo L. Rac GTPases control axon growth, guidance and branching. Nature 2002; 416:442 - 7; http://dx.doi.org/10.1038/416442a; PMID: 11919635
- Struckhoff EC, Lundquist EA. The actin-binding protein UNC-115 is an effector of Rac signaling during axon pathfinding in C. elegans.. Development 2003; 130:693 - 704; http://dx.doi.org/10.1242/dev.00300; PMID: 12506000
- Moon MS, Gomez TM. Balanced Vav2 GEF activity regulates neurite outgrowth and branching in vitro and in vivo.. Mol Cell Neurosci 2010; 44:118 - 28; http://dx.doi.org/10.1016/j.mcn.2010.03.001; PMID: 20298788
- Derivery E, Gautreau A. Generation of branched actin networks: assembly and regulation of the N-WASP and WAVE molecular machines. Bioessays 2010; 32:119 - 31; http://dx.doi.org/10.1002/bies.200900123; PMID: 20091750
- de Curtis I. Functions of Rac GTPases during neuronal development. Dev Neurosci 2008; 30:47 - 58; http://dx.doi.org/10.1159/000109851; PMID: 18075254
- Albertinazzi C, Gilardelli D, Paris S, Longhi R, de Curtis I. Overexpression of a neural-specific rho family GTPase, cRac1B, selectively induces enhanced neuritogenesis and neurite branching in primary neurons. J Cell Biol 1998; 142:815 - 25; http://dx.doi.org/10.1083/jcb.142.3.815; PMID: 9700168
- May V, Schiller MR, Eipper BA, Mains RE. Kalirin Dbl-homology guanine nucleotide exchange factor 1 domain initiates new axon outgrowths via RhoG-mediated mechanisms. J Neurosci 2002; 22:6980 - 90; PMID: 12177196
- Totaro A, Tavano S, Filosa G, Gärtner A, Pennucci R, Santambrogio P, Bachi A, Dotti CG, de Curtis I. Biochemical and functional characterisation of αPIX, a specific regulator of axonal and dendritic branching in hippocampal neurons. Biol Cell 2012; 104:533 - 52; http://dx.doi.org/10.1111/boc.201100060; PMID: 22554054
- Iwasawa N, Negishi M, Oinuma I. R-Ras controls axon branching through afadin in cortical neurons. Mol Biol Cell 2012; 23:2793 - 804; http://dx.doi.org/10.1091/mbc.E12-02-0103; PMID: 22593211
- Yang HW, Shin M-G, Lee S, Kim J-R, Park WS, Cho K-H, Meyer T, Heo WD. Cooperative activation of PI3K by Ras and Rho family small GTPases. Mol Cell 2012; 47:281 - 90; http://dx.doi.org/10.1016/j.molcel.2012.05.007; PMID: 22683270
- Jurney WM, Gallo G, Letourneau PC, McLoon SC. Rac1-mediated endocytosis during ephrin-A2- and semaphorin 3A-induced growth cone collapse. J Neurosci 2002; 22:6019 - 28; PMID: 12122063
- Gross SR, Kinzy TG. Improper organization of the actin cytoskeleton affects protein synthesis at initiation. Mol Cell Biol 2007; 27:1974 - 89; http://dx.doi.org/10.1128/MCB.00832-06; PMID: 17178834
- Ridley AJ. Rho GTPases and cell migration. J Cell Sci 2001; 114:2713 - 22; PMID: 11683406
- Etienne-Manneville S, Hall A. Rho GTPases in cell biology. Nature 2002; 420:629 - 35; http://dx.doi.org/10.1038/nature01148; PMID: 12478284
- Franke K, Otto W, Johannes S, Baumgart J, Nitsch R, Schumacher S. miR-124-regulated RhoG reduces neuronal process complexity via ELMO/Dock180/Rac1 and Cdc42 signalling. EMBO J 2012; 31:2908 - 21; http://dx.doi.org/10.1038/emboj.2012.130; PMID: 22588079
- Li X, Gao X, Liu G, Xiong W, Wu J, Rao Y. Netrin signal transduction and the guanine nucleotide exchange factor DOCK180 in attractive signaling. Nat Neurosci 2008; 11:28 - 35; http://dx.doi.org/10.1038/nn2022; PMID: 18066058
- Picard M, Petrie RJ, Antoine-Bertrand J, Saint-Cyr-Proulx E, Villemure JF, Lamarche-Vane N. Spatial and temporal activation of the small GTPases RhoA and Rac1 by the netrin-1 receptor UNC5a during neurite outgrowth. Cell Signal 2009; 21:1961 - 73; http://dx.doi.org/10.1016/j.cellsig.2009.09.004; PMID: 19755150
- Murray A, Naeem A, Barnes SH, Drescher U, Guthrie S. Slit and Netrin-1 guide cranial motor axon pathfinding via Rho-kinase, myosin light chain kinase and myosin II. Neural Dev 2010; 5:16; PMID: 20569485
- Antoine-Bertrand J, Ghogha A, Luangrath V, Bedford FK, Lamarche-Vane N. The activation of ezrin-radixin-moesin proteins is regulated by netrin-1 through Src kinase and RhoA/Rho kinase activities and mediates netrin-1-induced axon outgrowth. Mol Biol Cell 2011; 22:3734 - 46; http://dx.doi.org/10.1091/mbc.E10-11-0917; PMID: 21849478
- Demarco RS, Struckhoff EC, Lundquist EA. The Rac GTP exchange factor TIAM-1 acts with CDC-42 and the guidance receptor UNC-40/DCC in neuronal protrusion and axon guidance. PLoS Genet 2012; 8:e1002665; http://dx.doi.org/10.1371/journal.pgen.1002665; PMID: 22570618
- DeGeer J, Boudeau J, Schmidt S, Bedford F, Lamarche-Vane N, Debant A. Tyrosine phosphorylation of the Rho guanine nucleotide exchange factor Trio regulates netrin-1/DCC-mediated cortical axon outgrowth. Mol Cell Biol 2013; 33:739 - 51; http://dx.doi.org/10.1128/MCB.01264-12; PMID: 23230270
- Moore SW, Correia JP, Lai Wing Sun K, Pool M, Fournier AE, Kennedy TE. Rho inhibition recruits DCC to the neuronal plasma membrane and enhances axon chemoattraction to netrin 1. Development 2008; 135:2855 - 64; http://dx.doi.org/10.1242/dev.024133; PMID: 18653556
- Fukunishi A, Maruyama T, Zhao H, Tiwari M, Kang S, Kumanogoh A, Yamamoto N. The action of Semaphorin7A on thalamocortical axon branching. J Neurochem 2011; 118:1008 - 15; http://dx.doi.org/10.1111/j.1471-4159.2011.07390.x; PMID: 21781117
- Püschel AW. GTPases in semaphorin signaling. Adv Exp Med Biol 2007; 600:12 - 23; http://dx.doi.org/10.1007/978-0-387-70956-7_2; PMID: 17607943
- Gopalakrishnan SM, Teusch N, Imhof C, Bakker MH, Schurdak M, Burns DJ, Warrior U. Role of Rho kinase pathway in chondroitin sulfate proteoglycan-mediated inhibition of neurite outgrowth in PC12 cells. J Neurosci Res 2008; 86:2214 - 26; http://dx.doi.org/10.1002/jnr.21671; PMID: 18438921
- Jain A, Brady-Kalnay SM, Bellamkonda RV. Modulation of Rho GTPase activity alleviates chondroitin sulfate proteoglycan-dependent inhibition of neurite extension. J Neurosci Res 2004; 77:299 - 307; http://dx.doi.org/10.1002/jnr.20161; PMID: 15211597
- Monnier PP, Sierra A, Schwab JM, Henke-Fahle S, Mueller BK. The Rho/ROCK pathway mediates neurite growth-inhibitory activity associated with the chondroitin sulfate proteoglycans of the CNS glial scar. Mol Cell Neurosci 2003; 22:319 - 30; http://dx.doi.org/10.1016/S1044-7431(02)00035-0; PMID: 12691734
- Fisher D, Xing B, Dill J, Li H, Hoang HH, Zhao Z, Yang XL, Bachoo R, Cannon S, Longo FM, et al. Leukocyte common antigen-related phosphatase is a functional receptor for chondroitin sulfate proteoglycan axon growth inhibitors. J Neurosci 2011; 31:14051 - 66; http://dx.doi.org/10.1523/JNEUROSCI.1737-11.2011; PMID: 21976490
- Tolias KF, Duman JG, Um K. Control of synapse development and plasticity by Rho GTPase regulatory proteins. Prog Neurobiol 2011; 94:133 - 48; http://dx.doi.org/10.1016/j.pneurobio.2011.04.011; PMID: 21530608
- Spillane M, Ketschek A, Merianda TT, Twiss JL, Gallo G. Mitochondria coordinate sites of axon branching through localized intra-axonal protein synthesis. Cell Rep 2013; 5:1564 - 75; http://dx.doi.org/10.1016/j.celrep.2013.11.022; PMID: 24332852
- Lilley BN, Pan YA, Sanes JR. SAD kinases sculpt axonal arbors of sensory neurons through long- and short-term responses to neurotrophin signals. Neuron 2013; 79:39 - 53; http://dx.doi.org/10.1016/j.neuron.2013.05.017; PMID: 23790753
- Greif KF, Asabere N, Lutz GJ, Gallo G. Synaptotagmin-1 promotes the formation of axonal filopodia and branches along the developing axons of forebrain neurons. Dev Neurobiol 2013; 73:27 - 44; http://dx.doi.org/10.1002/dneu.22033; PMID: 22589224
- Cioni JM, Telley L, Saywell V, Cadilhac C, Jourdan C, Huber AB, Huang JZ, Jahannault-Talignani C, Ango F. SEMA3A signaling controls layer-specific interneuron branching in the cerebellum. Curr Biol 2013; 23:850 - 61; http://dx.doi.org/10.1016/j.cub.2013.04.007; PMID: 23602477
