Abstract
Increased cell migration is an acquired feature of metastatic cancer cells and relies on derailed signal transduction pathways. Intracellular vesicular trafficking plays a key role in cell migration due to its intricate involvement in cargo transport and membrane composition. In the last decade, endocytosis has been implicated in cell migration and found to be responsible for the internalization of membrane receptors at the plasma membrane, where integrin trafficking and fine-tuning of receptor tyrosine kinase signaling by internalization are major mechanisms. Accumulating evidence has suggested a link between endosome dynamics, cell migration, and invasion, in which small GTPases of the Rab family have central roles. We have recently determined that Rab5 activation is a crucial event in promoting focal adhesion disassembly, which is concomitant with the migration and invasion of metastatic cancer cells. The mechanisms underlying this novel role for Rab5 are currently unclear, and their elucidation will provide insight into the role of Rab5 function in cancer cell metastasis.
Cancer cell metastasis is a complex pathological process that generally compromises patient prognosis.Citation1 It is responsible for most deaths due to cancer and is therefore a central issue for public health.Citation2 Metastasis involves the migration and invasion of tumor cells from the primary tumor to adjacent tissues and then distant organs through the circulatory system, establishing secondary tumors that lead to systemic failure.Citation3 The invasive and migratory abilities of tumor cells have been extensively studied in order to develop therapies that prevent cancer cell spread and metastasis.Citation4 In this respect, cell migration has been characterized as a multi-step process that involves cell polarization, the dynamic remodeling of the cytoskeleton, and membrane protrusion driven by coordinated activation of the small GTPases Rac1, RhoA, and Cdc42, as well as the regulated turnover of cell adhesions with the extracellular matrix (ECM).Citation5 Turnover of cell-ECM adhesions is crucial for cell migration, because it is required for cell detachment from the matrix and for the dynamic formation and disassembly of anchoring structures that permit cell movement.Citation6 These anchoring structures are the focal adhesions (FAs), which are composed of integrins and a wide variety of adaptor proteins that form adhesion plaques at the cytosolic side of the plasma membrane.Citation7,Citation8
FAs are continuously remodeled in migrating cells, as they are disassembled in response to pro-migratory stimuli, leading to integrin internalization to form part of an intracellular endosomal pool. It has been proposed that most endosomal integrins are spatially restricted and recycled, thus allowing the formation of new adhesions at the leading edge. Here, the role of the endocytic machinery in cell migration has been recently investigated, and components of the clathrin-mediated endocytosis have been shown to be required for FA turnover and cell migration.Citation9,Citation10 This evidence suggests that FA disassembly proceeds through endocytosis, involving a continuous flux of integrins through endosomal compartments. Integrins are internalized into vesicles at disassembling FAs, trafficked, and sorted to intracellular endosomal compartments,Citation9-Citation11 from which they are recycled back to the plasma membrane during migration (for a review, see ref. Citation12). Alternatively, ligand-bound integrins can also be targeted to the late endosome/lysosome pathway of degradation, in order to facilitate the formation of new adhesion sites by unligated integrins.Citation13 Because FA disassembly is highly coordinated, endocytosis of integrins is expected to be spatio-temporally regulated. Therefore, further studies of the regulation of integrin traffic and endosomal dynamics are required.
The relationship between endocytosis and migration was demonstrated by interfering with components of the clathrin-dependent endocytosis, such as clathrin, dynamin, and endocytic adaptors, including AP2, ARH, and Dab2, which led to decreased rates of FA disassembly, dephosphorylation of focal adhesion kinase (FAK), and cell migration induced by microtubules.Citation9,Citation10 Despite the fact that components of the clathrin machinery were implicated in FA disassembly, other relevant regulators of endosome dynamics have not been evaluated, such as Rab GTPases, molecular switches that cycle between active and inactive conformations, and are involved in cell migration (for a review, see ref. Citation14). Among the regulators of endocytosis and migration, a particular Rab protein, Rab5, the master regulator of early endosome dynamics, has been shown to be essential in cell invasion and metastasis.Citation15,Citation16
Our recent findings using wound healing and spreading assays have shown that Rab5-GTP loading increases at the periphery of metastatic cancer cells,Citation11 suggesting that the engagement of integrins promotes Rab5 activation, leading to augmented internalization and endosome fusion events that facilitate integrin trafficking and recycling to new adhesion sites. Rab5 downregulation by shRNA targeting leads to a significant reduction in FA disassembly, cell migration and spreading, whereas reconstitution of functional Rab5 but not the dominant negative mutant (S34N, high affinity for GDP, locked in the inactive state) recovered the migratory phenotype. Thus, Rab5 GTP-loading is an important switch that turns on the endosomal machinery, providing the flux of integrins required for directional cell migration. Intriguingly, Rab5 was found to be associated in a physical complex with FA components, such as Paxillin, Vinculin, FAK, and β1, and this interaction was further increased when synchronizing FAs with the microtubule-disrupting drug nocodazole. The association of Rab5 and FA components was also increased during migration, paralleling the kinetics of Rab5 activation. These observations suggest an interaction between GTP-loaded Rab5 and FAs, which was supported by FA fractionation experiments, in which we observed an enrichment of constitutively active Rab5 (Q79L, GTPase-dead, locked in the active conformation) compared with the inactive mutant and wild-type version. This hypothesis is also supported by previous reports showing higher levels of Rab5/Q79L in β1 integrin immunoprecipitates and by co-localization with β1 integrin.Citation16 β1 integrins are known to accumulate in Rab5/Q79L enlarged endosomes following microtubule-induced FA disassemblyCitation10. Accordingly, we observed increased peripheral staining of the biosensor for GTP-loaded Rab5, mCherry-R5BD, but not mCherry alone in cells spreading over fibronectin, the main ligand for α5β1, which can account for the continuous remodeling of the adhesion front while the cell is re-adhering to the matrix.Citation11
The association between Rab5-stained endosomes and FAs was supported by two alternative approaches: synchronizing FAs with nocodazole and studying FA dynamics in live-cells.Citation11 Additionally, re-distribution of the biosensor for mCherry-R5BD could be observed in cells stimulated to migrate to a wounded area.Citation11 GTP-loaded Rab5 was predominantly found in the perinuclear region but was also found to a lesser extent in close proximity to FAs (), supporting the notion that active Rab5 is recruited to FA sites to promote their internalization during cell migration.
Figure 1. MDA-MB-231 cells were grown at confluence on glass-coverslips and transfected with the mCherry-R5BD construct described in Mendoza et al.Citation11 Post-transfection (24 h), confluent monolayers were wounded and allowed to migrate for 30 min. Samples were fixed and stained for Rab5 (monoclonal antibody, green) and paxillin (polyclonal antibody, blue). mCherry-R5BD is shown in red. Samples were analyzed by confocal microscopy. A representative deconvoluted image is shown. Arrows indicate co-localization at focal adhesions; arrowheads indicate co-localization at intracellular compartments. Bar represents 10 μm.
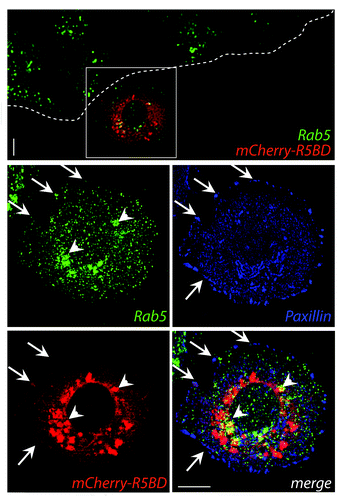
However, it is unclear whether Rab5 promotes FA turnover via specific interactions with FAs or by promoting fusion events of vesicles containing FA complexes with endosomes. A previous study showed that knocking-down Rab5 significantly decreases the fraction of internalized integrins,Citation16 indicating a role for this GTPase in delivering integrins from the plasma membrane to vesicular compartments. Using live cell imaging, we tracked the endosomal dynamics during FA disassembly and observed co-localization of GFP-Rab5 positive endosomes with FA complexes in extremely narrow timeframes. This action preceded their disassembly, suggesting that this interaction triggers the break-down of adhesion plaques and the departure of their components to endosomes.Citation11
An interesting observation from this study was the dependence of FA disassembly from Rab5 function. Although the contribution of the endosome fusion events in FA disassembly is not yet clear, the endocytosis of FAs appears to be a reasonable mechanism. In this manner, Rab5 activation promotes integrin internalization and boosts endosome fusion events, which is followed by an enhanced flow of trans-membrane proteins from clathrin-coated vesicles to early endosomes, an endocytic route previously described for integrins.Citation10 This action mobilizes the internalized pools of integrins to advanced stages of vesicular traffic and contributes to their recycling, leading to sustained tumor cell migration. In this context, the relevance of different components of the recycling machinery has been extensively studied during the last decade. Both Rab4-dependent short loop and Rab11-dependent long loop of recycling are known to control not only the dynamics, but also the function of integrins that differ in substrate specificity and function (for a review, see refs. Citation12 and Citation14). Moreover, recycling Rabs cooperate with Rab5 in additional mechanisms involved in cell migration, such as Rac signaling and localization during collective tumor cell migration,Citation17 and the trafficking of cell adhesion molecules during neuronal migration.Citation18
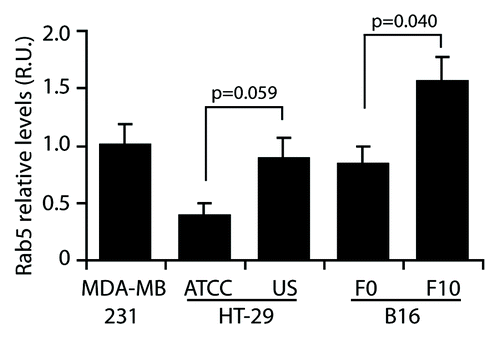
It is tempting to speculate a role for Rab5 in tumor progression, because of its migration promoting function, which provides tumor cells with the capability to detach from their matrix and colonize adjacent tissues. In line with this ability, augmented Rab5 levels have been observed in tumors associated with poor-prognosis compared with the adjacent non-transformed tissue,Citation15 suggesting that Rab5 upregulation represents an oncogenic characteristic. Nevertheless, in normal adult cells, Rab5 is known to be differentially regulated, which appears to be dependent on the cellular context. If Rab5 is an adhesion remodeling promoter, a relationship between the expression and/or activation of Rab5, and the dynamics of cellular adhesions might be expected. Recent evidence supports this view, since Rab5 activity was shown to be necessary for the dynamics of long-term integrin adhesion complexes in the fly myotendinous junctions.Citation19 Based on this model, it is tempting to speculate that at early stages of development, higher levels of active Rab5 accelerate the turnover of adhesions, leading to continuous tissue remodeling, whereas at late stages of development, the active fraction of Rab5 decreases, leading to low adhesion turnover and the stabilization of adhesion complexes. However, more studies are needed to confirm whether this is a general phenomenon, because it is well known that several differentiated cells types depict highly dynamic and organized endocytosis and recycling rates. Most importantly, the influence of these trafficking routes on the dynamics of cell adhesion complexes remains to be evaluated. Regardless, these data contribute to understand the re-acquired migratory capabilities of tumor cells, which show increased turnover of FAs through upregulation of Rab5. In this respect, we measured the levels of Rab5 in different cancer cell lines and observed interesting differences (). In cells with low metastatic potential, Rab5 protein levels were lower than in their highly metastatic counterparts. Specifically, colon carcinoma cells HT29 (ATCC) have lower levels of Rab5 than the highly metastasic HT29(US) cells (previously characterized in ref. Citation20). The same situation was observed in a mouse melanoma model, in which B16-F0 cells expressed lower overall Rab5 protein levels than its derived, highly-metastatic sub-cell line B16-F10. Despite the fact that these cell models derive from different species, tissues and cellular contexts, there is a clear correlation between their metastatic potential and Rab5 protein levels, and these data provide insights about the relevance of deregulated Rab5 expression in cancer cell migration and metastasis.
Figure 2. Rab5 expression in cancer cell lines. Whole cell lysates were obtained from MDA-MB-231 human breast cancer cells, human colon adenocarcinoma cell lines with low (HT-29/ATCC) and high (HT-29/US) metastatic potential and mouse melanoma cell lines with low (B16-F0) and high (B16-F10) metastatic potential. Total Rab5 levels were analyzed by western blotting, and relative levels were normalized by actin. Data were expressed as relative units with respect to Rab5 levels in MDA-MB-231 cells and represent the mean of three independent experiments (mean ± s.e.m.). As indicated, data were compared using unpaired t tests with the GraphPad Prism 5 software.
Many questions remain unanswered regarding how Rab5 promotes FA disassembly, including the identity of upstream signaling pathways leading to Rab5 activation, the degree of integrity of the adhesion complexes in the internalized fraction and the nature of the cascade that accelerates endosomal trafficking in pro-migratory situations. In this respect, the equilibrium GEF/GAP that accounts for the overall activation of Rab5 is unclear, and recently, a very interesting study identified a Rab5-GAP that is implicated in FA disassembly.Citation21 RN-Tre, a Rab5-GAP, localizes to FAs and delays their disassembly upon growth factor stimulation in a GAP-dependent manner, highlighting the role of GTP-loaded Rab5 in FA disassembly as a cell migration switch.
In summary, these and previous results indicate that Rab5 activity is an important mechanism for the adhesion remodeling machinery involved in cell migration, and endocytosis appears to be the mechanism by which FAs disassemble; however, further studies are needed to elucidate the upstream regulation in different contexts.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
This work was supported by FONDECYT Initiation #11100287, CONICYT #79090021 “Insertion of Young Postdoctoral Researchers in the Academy” and CONICYT fellowships (to J.D. and P.S.).
References
- Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer 2010; 127:2893 - 917; http://dx.doi.org/10.1002/ijc.25516; PMID: 21351269
- Talmadge JE, Fidler IJ. AACR centennial series: the biology of cancer metastasis: historical perspective. Cancer Res 2010; 70:5649 - 69; http://dx.doi.org/10.1158/0008-5472.CAN-10-1040; PMID: 20610625
- Gupta GP, Massagué J. Cancer metastasis: building a framework. Cell 2006; 127:679 - 95; http://dx.doi.org/10.1016/j.cell.2006.11.001; PMID: 17110329
- Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell 2011; 144:646 - 74; http://dx.doi.org/10.1016/j.cell.2011.02.013; PMID: 21376230
- Ridley AJ, Schwartz MA, Burridge K, Firtel RA, Ginsberg MH, Borisy G, Parsons JT, Horwitz AR. Cell migration: integrating signals from front to back. Science 2003; 302:1704 - 9; http://dx.doi.org/10.1126/science.1092053; PMID: 14657486
- Burridge K, Fath K, Kelly T, Nuckolls G, Turner C. Focal adhesions: transmembrane junctions between the extracellular matrix and the cytoskeleton. Annu Rev Cell Biol 1988; 4:487 - 525; http://dx.doi.org/10.1146/annurev.cb.04.110188.002415; PMID: 3058164
- Burridge K, Chrzanowska-Wodnicka M. Focal adhesions, contractility, and signaling. Annu Rev Cell Dev Biol 1996; 12:463 - 518; http://dx.doi.org/10.1146/annurev.cellbio.12.1.463; PMID: 8970735
- Petit V, Thiery JP. Focal adhesions: structure and dynamics. Biol Cell 2000; 92:477 - 94; http://dx.doi.org/10.1016/S0248-4900(00)01101-1; PMID: 11229600
- Chao WT, Kunz J. Focal adhesion disassembly requires clathrin-dependent endocytosis of integrins. FEBS Lett 2009; 583:1337 - 43; http://dx.doi.org/10.1016/j.febslet.2009.03.037; PMID: 19306879
- Ezratty EJ, Bertaux C, Marcantonio EE, Gundersen GG. Clathrin mediates integrin endocytosis for focal adhesion disassembly in migrating cells. J Cell Biol 2009; 187:733 - 47; http://dx.doi.org/10.1083/jcb.200904054; PMID: 19951918
- Mendoza P, Ortiz R, Díaz J, Quest AF, Leyton L, Stupack D, Torres VA. Rab5 activation promotes focal adhesion disassembly, migration and invasiveness in tumor cells. J Cell Sci 2013; 126:3835 - 47; http://dx.doi.org/10.1242/jcs.119727; PMID: 23813952
- Jones MC, Caswell PT, Norman JC. Endocytic recycling pathways: emerging regulators of cell migration. Curr Opin Cell Biol 2006; 18:549 - 57; http://dx.doi.org/10.1016/j.ceb.2006.08.003; PMID: 16904305
- Lobert VH, Brech A, Pedersen NM, Wesche J, Oppelt A, Malerød L, Stenmark H. Ubiquitination of alpha 5 beta 1 integrin controls fibroblast migration through lysosomal degradation of fibronectin-integrin complexes. Dev Cell 2010; 19:148 - 59; http://dx.doi.org/10.1016/j.devcel.2010.06.010; PMID: 20643357
- Caswell PT, Norman JC. Integrin trafficking and the control of cell migration. Traffic 2006; 7:14 - 21; http://dx.doi.org/10.1111/j.1600-0854.2005.00362.x; PMID: 16445683
- Liu SS, Chen XM, Zheng HX, Shi SL, Li Y. Knockdown of Rab5a expression decreases cancer cell motility and invasion through integrin-mediated signaling pathway. J Biomed Sci 2011; 18:58; http://dx.doi.org/10.1186/1423-0127-18-58; PMID: 21849022
- Torres VA, Mielgo A, Barbero S, Hsiao R, Wilkins JA, Stupack DG. Rab5 mediates caspase-8-promoted cell motility and metastasis. Mol Biol Cell 2010; 21:369 - 76; http://dx.doi.org/10.1091/mbc.E09-09-0769; PMID: 19923319
- Ramel D, Wang X, Laflamme C, Montell DJ, Emery G. Rab11 regulates cell-cell communication during collective cell movements. Nat Cell Biol 2013; 15:317 - 24; http://dx.doi.org/10.1038/ncb2681; PMID: 23376974
- Kawauchi T, Sekine K, Shikanai M, Chihama K, Tomita K, Kubo K, Nakajima K, Nabeshima Y, Hoshino M. Rab GTPases-dependent endocytic pathways regulate neuronal migration and maturation through N-cadherin trafficking. Neuron 2010; 67:588 - 602; http://dx.doi.org/10.1016/j.neuron.2010.07.007; PMID: 20797536
- Yuan L, Fairchild MJ, Perkins AD, Tanentzapf G. Analysis of integrin turnover in fly myotendinous junctions. J Cell Sci 2010; 123:939 - 46; http://dx.doi.org/10.1242/jcs.063040; PMID: 20179102
- Torres VA, Tapia JC, Rodriguez DA, Lladser A, Arredondo C, Leyton L, Quest AF. E-cadherin is required for caveolin-1-mediated down-regulation of the inhibitor of apoptosis protein survivin via reduced beta-catenin-Tcf/Lef-dependent transcription. Mol Cell Biol 2007; 27:7703 - 17; http://dx.doi.org/10.1128/MCB.01991-06; PMID: 17785436
- Palamidessi A, Frittoli E, Ducano N, Offenhauser N, Sigismund S, Kajiho H, Parazzoli D, Oldani A, Gobbi M, Serini G, et al. The GTPase-activating protein RN-tre controls focal adhesion turnover and cell migration. Curr Biol 2013; 23:2355 - 64; http://dx.doi.org/10.1016/j.cub.2013.09.060; PMID: 24239119
