Abstract
Microtubule (MT) organization and dynamics downstream of external cues is crucial for maintaining cellular architecture and the generation of cell asymmetries. In interphase cells RhoA, Rac, and Cdc42, conspicuous members of the family of small Rho GTPases, have major roles in modulating MT stability, and hence polarized cell behaviors. However, MTs are not mere targets of Rho GTPases, but also serve as signaling platforms coupling MT dynamics to Rho GTPase activation in a variety of cellular conditions. In this article, we review some of the key studies describing the reciprocal relationship between small Rho-GTPases and MTs during migration and polarization.
Introduction
It is now well established that members of the Ras superfamily of small GTPases along with their upstream regulators and downstream effectors function as fundamental signaling stations controlling a wide array of morphogenetic events.Citation1,Citation2 The initial functional characterization of the prototypical members of this superfamily, namely RhoA, Rac, and Cdc42 established that the actin cytoskeleton was one of its main targets.Citation1 Later studies extended these observations revealing that small Rho GTPases in concert with guanine nucleotide exchange factors (GEFs), GTPase activating proteins (GAPs), guanine dissociation inhibitors (GDIs), and factors that control their lifespan (i.e., ubiquitin E3-ligase), serve as “spatiotemporal signaling modulesCitation3 that also regulate MT organization, dynamics, plus-end capture and the cross talk with the actin cytoskeleton. Equally, MTs have emerged as key regulators of Rho GTPase functioning.Citation4-Citation6
Microtubule organization and dynamics in short
MTs are non-covalent cytoskeletal polymers involved in virtually every aspect of cell biology, including mitosis, cell motility, adhesion, intracellular transport and polarity. The main component of MTs is the tubulin polymer, composed of α- and β- heterodimer subunits assembled into 13 linear protofilaments that form a 20 nm hollow tube; in addition, a wide variety of associated proteins decorate and/or interact with the MT lattice or tips.Citation6 MTs typically undergo cycles of rapid growth and disassembly, a phenomenon known as dynamic instability, which has been observed in vitro and in vivo.Citation7,Citation8 This property allows MTs to probe the intracellular environment for interacting partners (e.g., search, capture, and stabilization at the cell cortex), to rapidly reorganize in response to environmental cues, and to spatially and temporally differentiate to generate cell polarization.Citation5,Citation6 While most MTs are very dynamic, with a half-life ranging between 5–10 min (dynamic MTs), a minor population exhibits a more stable behavior, lasting for up to 20 h (stable MTs).Citation7 In non-polarized cells these two types of MTs arrays coexist intertwined in the same cytoplasm in which stable ones (long-lived polymer) are formed and maintained next to the very dynamic population (short-lived polymer). In many cell types, including neurons, cell motility, and/or polarization have been mechanistically linked with the selective stabilization (temporal differentiation) and spatial segregation (spatial differentiation) of a subset of MTs.Citation5,Citation6,Citation9
Stable MTs accumulate post-translational modifications (PTM) of α-tubulin, such as detyrosination and acetylation.Citation10-Citation12 Detyrosination removes the gene-encoded C-terminal tyrosine resulting in tubulin monomers ending with glutamine; the resulting detyrosinated tubulin, also termed Glu-tubulin, can be further converted into Δ2-tubulin by the irreversible removal of the penultimate glutamate.Citation12 Early studies showed that acetylation of stable MT (no acetylation has been demonstrated on dynamic MT) occurs on lysine 40 of α-tubulin, which faces the MT lumen;Citation13-Citation15 the biological significance of this finding has remained intriguing since most MT interactions occur on the outer surface of the polymer. Recent studies have identified novel acetylation sites on tubulin some of which are located on the outer MT lattice.Citation16 Neither detyrosination nor acetylation are the cause of MT stability, but appear to function as moieties that protect MTs from depolymerizing agents, such as members of the kinesin 13 superfamily, or as tags and/or markers allowing binding of MT-based molecular motors, such as Kif5, or preventing the one of certain types of plus-end microtubule-associates proteins or +TIPS.Citation12,Citation17
From Rho-GTPases to microtubules
Microtubule stabilization by Rho A-mDia in migrating fibroblasts
Perhaps one of the more striking examples of the role of Rho-GTPases in controlling MT organization and dynamics is cell migration.Citation18 Using wounded monolayers of serum-starved NIH-3T3 fibroblasts, Gregg Gundersen and colleaguesCitation18-Citation21 identified, several years ago, a RhoA signaling pathway that induces the formation of a stable Glu-MT subset involved in cell migration. These and related studies established that in different types of migrating cells the radially oriented array of MTs reorganizes and polarizes in response to an external cue by the spatio-temporal transformation of a group of dynamic MTs into a stable one that contains Glu- or acetylated α-tubulin. It also became clear that migration requires the formation of this stabilized subset of MTs.Citation20,Citation21 These observations fit very well with the selective stabilization model proposed by Kirschner and Mitchison in the late 80s to explain the generation of cell asymmetry; in this proposal external signals would locally activate cortical factors to stabilize dynamic MTs.Citation9 One such cue is lysophosphatidic acid (LPA), a cytokine known for its ability to stimulate RhoA activity and stress fiber formation.Citation22 Addition of LPA in wounded monolayers of starved fibroblasts results in a rapid (within 30–60 min) and robust activation of RhoA that precedes the appearance of the stable Glu-MT array that polarizes toward the leading edge;Citation23 inhibition of RhoA activity with both C3 botulinum toxin or dominant negative RhoA prevents the formation of stable Glu-MTs and cell migration.Citation23,Citation24
LPA receptors are coupled to heterotrimeric G proteins (Gi, Gq, Gα12/13 α-subunits) and elicit multiple cellular responses.Citation25-Citation27 In scratch-wound directed cell migration of mouse embryonic fibroblasts (MEFs), the G protein–coupled receptors (GPCR) Gα12/13 are essential and sufficient for LPA-induced RhoA activation, Glu-MT formation, and cell progression.Citation28 Activated Gα13 tightly binds p115 RhoGEF and stimulates its capacity to catalyze nucleotide exchange on RhoA,Citation29 while activated Gα12 inhibits Gα13-induced stimulation. Thus, p115 RhoGEF can directly link heterotrimeric G protein α subunits to the regulation of RhoA activity upstream of MT stabilizationCitation29; on the other hand, MTs may have a role in targeting p115 RhoGEF to specific subcellular locations.Citation30 Leukemia-associated RhoGEF (LARG) has also been shown to promote Gα12/13 dependent RhoA activation in vivo.Citation31
The question of how activated RhoA induces polarized MT stabilization and subsequent cell migration is complex and far from being solved. One straightforward possibility is the local activation of a MT stabilization pathway within the cell's leading edge, where RhoA activity has been detected.Citation32,Citation33 Several lines of evidence suggest that LPA induces polarized stable MT formation in wound edge migrating fibroblasts through the mammalian homolog of Drosophila diaphanous (mDia), a formin that is a RhoA effector; in this model MTs that neither growth nor shrink, have a half-life of more than 1 h and contain Glu-tubulin are the result of capture and plus end stabilization or capping ().Citation4
Figure 1. Drawing representing the MT-RhoA-mDia signaling pathway at the leading edge of migrating fibroblasts. Molecules are drawn schematically, roughly preserving relative molecular sizes and when possible, known crystallographic and/or functional spatial conformations. (A) Dynamic MTs: In the absence of LPA, most MTs in wound-facing fibroblasts are highly dynamic showing high rate of α/β heterodimer exchange on + tips. The MTs contain + tips proteins, like EB1 and APC having a comet-like appearance, being highly enriched at the most distal part of the MTs and tapering down within few micrometers. In the absence of LPA signaling, RhoA is inactive (GDP-RhoA), mDia (also inactive) doesn’t bind MTs; there are no focal adhesions and hence FAK remains inactivated. (B) Stable and/or Capped Microtubule: Upon LPA stimulation, focal adhesions start assembling in the leading edge of wound-facing fibroblasts. Typically, focal adhesion formation involves integrin receptor activation and accumulation leading to further enrichment of several other proteins involved in the regulation of the actin network, providing mechanical and signaling means for F-actin binding. Of particular importance during FA formation is the activation of FAK, which, among other effects, promotes the accumulation of GM1 on the leading edge plasma membrane (big dark-yellow arrow). GM1-enriched membranes provide sites for the insertion of RhoA, and activation by LPA receptor signaling. GTP-RhoA activation releases mDia auto-inhibitory conformation leading to its binding to MT + tips; mDia also binds EB1 and APC forming a trimeric protein complex which functions as a MT plus cap. This cap prevents heterodimer exchange stabilizing MT at the leading edge.
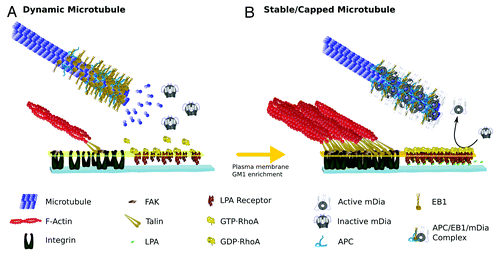
Formins are a family of multidomain proteins first described as potent actin regulators capable of modulating a wide variety of cellular processes such as cytokinesis, cell polarization and morphogenesis.Citation34,Citation35 Diaphanous-related formins (DRF) are a subfamily bearing a GTP-binding domain (GBD) near the N-terminal region and a Diaphanous auto regulatory domain (DAD) near the C-terminal side of a formin homology domain (FH2) that facilitates intramolecular binding. The binding of Rho-GTP to GBD is thought to activate DRF by relieving an auto inhibitory interaction between an adjacent N-terminal inhibitory domain formed by armadillo repeats and DAD on the C-terminal region; RhoA uses its “switches” regionsCitation1,Citation2 for interacting with the two formin domains.Citation36 In addition to their known role in regulating actin-dependent processes DRF are also involved in regulating the MT cytoskeleton. In wound-edge migrating fibroblasts constitutively active mDia or activation of endogenous mDia with the mDia auto-inhibitory domain stimulates the formation of stable MTs.Citation37 Besides, mDia co-localizes with stable MTs when overexpressed and associates with MTs in vitro. These observations establish that mDia is sufficient to generate and orient stable MTs, and that DRF could be part of a conserved pathway that regulates the dynamics of MTs + ends.Citation37 A more recent study has provided evidence suggesting that actin capping protein induces release of mDia from actin filaments near the leading edge allowing binding to MTs and subsequent stabilization.Citation38
Focal adhesions (FA) may serve as signaling platforms directing the RhoA-mDia pathway to stabilize MTs. It has been proposed that integrins and focal adhesion kinase (FAK) act as “adhesion checkpoints,” coordinating both spatially and temporally the formation of stable Glu-MTs. FAK activation localizes ganglioside GM1 to specialized domains in the cell surface, perhaps in the form of lipid rafts, harboring regulatory proteins and enabling RhoA to stimulate mDia ().Citation39
End-binding protein 1 (EB1) and adenomatous polyposis coli (APC), which target MT ends, directly bind to each other and to mDia. The three proteins are enriched on cortical MT + ends as detected by TIRF microscopy, where presumably active RhoA would accumulate.Citation20 Together these data suggest that upon LPA-GPCR activation near the cell cortex, an event facilitated by integrin/FAK signaling, RhoA activates mDia, which further binds EB1 and APC forming a cortical complex that captures and stabilizes dynamic MTs. The capture event would give enough time for tubulin modifying enzymes to detyrosinate and acetylate individual MTs, leading to further specialization ().
MT stabilization by RhoA/mDia in other biological systems
Evidence from different systems suggests that the signaling pathway involving RhoA, mDia, and + TIPS leading to MT stabilization is conserved across diverse cell types and cellular contexts. For example, in budding yeast the axis of cell division is determined by signaling molecules that control bud site selection; it has been proposed that this spot is established by interactions between cytoplasmic MTs and asymmetrically localized cortical proteins during cell cycle progression.Citation40 MTs captured at bud sites exhibit controlled shrinkage but do not persist for hours like those in mammalian cells; this pathway is regulated by Rho GTPases, such as RhoA and Cdc42 and the formin Bni1, the yeast ortholog of mDia. Budding yeast Kar9 serves as a functional homolog of APC and Bim1 as the one of EB1. The two yeast proteins have been directly implicated as direct Bni1 effectors in capturing and controlling MT dynamics in the nascent yeast bud.Citation40-Citation42
RhoA-dependent MT stabilization has also been observed in the epithelial to mesenchymal transition during gastrulation.Citation43 Basal membrane (BM) breakdown is the first recognizable step of this process and is controlled by loss of basally localized RhoA activity leading to loss of stable MTs. Reduction of RhoA activity in normal epithelium leads to BM breakdown while failure of RhoA downregulation during epithelial-mesenchymal transition to BM retention, a phenomenon partly due to RhoA-regulated MT stabilization.Citation44
Kaposi sarcoma (KS)-associated herpes virus, which is implicated in the pathogenesis of KS and other lymphoproliferative disorders, infects a variety of target cells both in vivo and in vitro.Citation45 Early during infection, the virus induces the activation of the RhoA/mDia2 pathway promoting the stabilization and subsequent acetylation of MTs. Using stable MTs the virus promotes the trafficking of viral capsids toward the cell nucleus and the establishment of infection. Capsids proteins colocalize along MT tracks supporting the need of an intact MT array for capsid trafficking. The inactivation of Rho by Clostridium difficile toxin B significantly reduces MT acetylation and delivery of viral DNA to the nucleus while constitutive active RhoA increases viral infection.Citation45 In T cells, the reorientation of the MT organizing center (MTOC) to the immune synapse (IS) during antigen presentation also involves Diaphanous 1 (DIA1, the human ortholog of mDia) and Formin-like-1 (FMNL1); besides, inverted formin 2 (INF2) mediates formation of an array of stable Glu-MTs that is necessary for MTOC reorientation to the IS.Citation46,Citation47
Finally, in developing cerebellar granule cells the RhoA/mDia pathway promotes axonal elongation in response to stromal cell–derived factor (SDF)-1α. The authors suggested that mDia-promoted axonal elongation could be related to the regulation of actin dynamics.Citation48 It will be of interest to test whether or not mDia also regulates axonal MT stability, a key event for initiating and maintaining neuronal polarity.Citation2,Citation6
Cdc42 and microtubule reorganization during cell migration
Several lines of evidence favor the view that Cdc42 also plays a major role in regulating MT organization and dynamics during cell migration. For example, it was initially established that Cdc42 regulates changes in centrosome orientation in T cells.Citation49 Then, using monolayers of primary astrocytes or fibroblasts in a scratch-induced migration assay, it was demonstrated that Cd42 is required for both polarized membrane protrusion at the leading edge and microtubule organizing center-Golgi apparatus (MTOC-GA) orientation toward the direction of migration ().Citation50,Citation51
Figure 2. Drawings representing proteins involved in regulating nuclear movement and MTOC-GA orientation during cell migration. Molecules are drawn schematically, roughly preserving relative molecular sizes and, when possible, known crystallographic and/or functional spatial conformations. (A) Non-polarized MTOC-nuclear axis: In wound-facing fibroblasts and in the absence of LPA, MTs extending near the plasma membrane are dynamic and are neither stabilized nor anchored; these MTs also have dynein/dynactin complexes, most likely involved in carrying membranous organelles (organelles not shown). Under this condition RhoA is inactive and not bound to the plasma membrane. The signaling and scaffold proteins Par3 and Par6 are also inactive and membrane unbound. F-Actin, myosin-dependent rearward flow is very low with most myosin II in an inhibited (auto-inhibition) conformation. (B) Polarized MTOC-nuclear axis: Upon LPA stimulation, Cdc42 is activated (GTP·Cdc42) and accumulates at the leading edge. GTP·Cdc42 activates MRCK (not shown), which in turns releases the auto-inhibitory conformation of myosin II. Activated myosin II (phosphorylated) binds to the F-actin network promoting rearward actin movement, which is coupled with the rearward movement of transversal actin cables. These actin cables are coupled to the nucleus by transmembrane actin-associated nuclear (TAN) lines98 (not shown) thereby moving the nucleus in the opposite direction of migration. GTP·Cdc42 also stimulates the assembly of the Par3/Par6/PKC (PKC not shown) polarity complex, which becomes activated and accumulates at the leading edge plasma membrane. The localization of Par3 is still a matter of debate.71,99 One study reported that Par 3 is enriched at the leading edge of migrating keratinocytes,71 while other that it associates with dynein at cell-cell contacts of migrating fibroblasts.99 Regardless, it is likely that the polarity complex provides sites for binding to the dynein and/or dynactin complex. Upon binding to the cell cortex the dynein and/or dynactin generates an opposing force on MTs, which in turn oppose the rearward nuclear movement. Membrane-cortical factors, dynein and/or dynactin, and MTs generate a force that serves to maintain the MTOC at the cell centroid, while the nucleus is moved rearward by actin cables, polarizing the nucleus-MTOC-GA in the direction of migration.
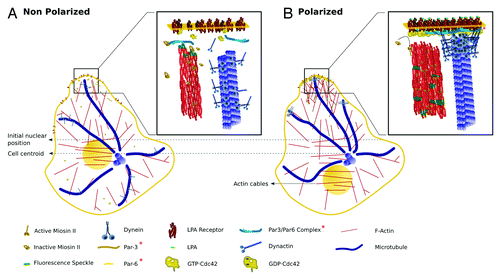
In migrating astrocytes this phenomenon involves Par6, a scaffold protein that is a direct target of Cdc42 and PKCζ.Citation52 It was shown that both proteins directly interact regulating glycogen synthase kinase-3β (GSK-3β) to promote MTOC reorientation and control the site of protrusive activity.Citation53 In migrating fibroblasts Cdc42-GSK-3β pathway only regulates MTOC positioning; protrusive activity is controlled by the Cdc42-dependent activation of P21-activated protein kinase (PAK), which controls the localization of Rac activity through recruitment of β-Pix, a GEF for Cdc42 and Rac, to the leading edge.Citation54 Cdc42-dependent phosphorylation of GSK-3β occurs specifically at the leading edge of migrating cells, and induces the interaction of APC protein with MT + ends.Citation53 In fibroblasts, MTOC cell-centroid reorientation and/or maintenance requires dynein pulling on capped MT; the presence of APC, mDia, and EB1 at the end of MT contributes to their capping and stabilization.Citation20,Citation55 The same signaling pathway has been implicated in axon formation, a phenomenon involving polarized protrusive activity, as well as remodeling of the growth cone actin and MT cytoskeleton.Citation56-Citation60 Centrosome movement during astrocyte migration also requires dynein, Cdc42, and APC,Citation61,Citation62 but does not involve MT plus end capture. Thus, it has recently been shown that a Cdc42 signaling pathway engaging the polarity protein disk large 1 (Dlg1) and the scaffolding protein GKAP, recruits dynein to leading edge MT.Citation61 In astrocytes, MTs bend in close proximity to the plasma membrane (approximately 3 µm) in a dynein-dependent manner; thus, dynein could provide a cortical anchor allowing minus end MT-based motor activity to pull the centrosome in the direction of migration.Citation62
Live imaging of fibroblasts has also revealed that at the onset of migration the nucleus moves rearward while the MTOC remains stationary.Citation63 Rearward nuclear movement is coupled to retrograde actin-myosin flow and is regulated by Cdc42 and its effector, Myotonic related Cdc42-binding kinase (MRCK).Citation63 Dynein is not involved in nuclear movement, but is essential to maintain the MTOC at the cell centroid. Together, these results reveal that there are at least two Cdc42 pathways involved in cell migration that regulate either nuclear movement or MTOC positioning and/or reorientation ().
Neutrophil chemotaxis is a form of directed migration that involves persistent polarization with a leading pseudopod, responsible for directional locomotion and the uropod, on the opposite site, mostly involved in cell-to-cell interaction and in a variety of leukocyte activities including activation, apoptosis, and immune interactions.Citation64 Several lines of evidence suggest that uropod formation involves microfilaments, ezrin-radixin-moesin (ERM) proteins, and integrin signaling, as well as rearrangement of the MTOC and activation of Cdc42. A recent study has also provided evidence suggesting that Cdc42 controls neutrophil polarity (pseudopod and/or uropod bipolar organization) by regulating WASP at the uropod.Citation65 WASP regulates the relocalization of CD11b integrin into a detergent resistant membrane domain, which in turn recruits EB1 to capture and stabilize MTs at the uropod.Citation65
Rac-1/PAK and the regulation of MT dynamics during cell migration
In randomly migrating PtK1 cells (a marsupial kidney epithelial cell line), Rac1 and the effector PAK1 regulate the dynamics of a subset of growing MTs.Citation66 In these cells a group of MTs, that resembles “pioneer” MTs,Citation67 grow well into leading edge protrusions, showing markedly less catastrophes events and spending more time in a growing phase than the rest of the MT array. The study by Wittmann et al. (ref. Citation66) revealed that nearly all MTs in constitutively active Rac1-expressing cells exhibit a kinetic behavior similar to pioneer MTs, while most MTs in dominant-negative Rac1expressing cells behave as central MTs, in which catastrophe frequency and time spent in pause are high.
In various cancer cells and in PtK1 cells stathmin/Op18, a MT-destabilizing factor, becomes phosphorylated in response to treatment with epidermal growth factor (EGF) in a Rac1-PAK1-dependent manner.Citation67,Citation68 PAK1 can directly phosphorylate Op18 at serine 16Citation67 inhibiting its catastrophe promoting activity in vitro, while inhibition of PAKs downstream of constitutive active Rac increases MT-destabilizing activity of Op18 in PtK1 cells, indicating that Op18 activity, and hence MT dynamics, can be regulated by PAKs in vivo.Citation68 Interestingly, this pathway operates in other cell types. Assembly and stabilization of growth cone dynamic MTs are key events during axon formation,Citation5 where Rac1 also plays a crucial role.Citation2 In primary cultured neurons DOCK7, a Rac GEF, selectively localizes to the tip of growing axons; DOCK7 and Rac activation lead to phosphorylation and inactivation of stathmin/Op18 in the nascent axon and this event is important for axon formation.Citation69
It is likely that other factors downstream of Rac and/or PAK are involved in the regulation of either leading edge or growth cone MT dynamics, because PAK activity is necessary but not sufficient for Rac1-mediated promotion of MT growth.Citation66 It has been proposed that Rac1 and PAK-mediated inactivation of stathmin/Op18 initiates MT growth by locally lowering the catastrophe frequency, which could then be sustained with further MT stabilization by +TIPs, such as CLIP-170, EB1, or APC.Citation68
Rac may also utilize different upstream regulators to control MT dynamics. For example, in wound-edge migrating primary mouse astrocytes, the Rac1 GEF Tiam1 is required for outgrowth of protrusions, wound closure and proper organization of the MT cytoskeleton.Citation70 Tiam1-deficient astrocytes contain reduced pools of Glu-tubulin, with stable MT restricted to an area close to the nucleus and failing to spread toward the leading edge, typical of migrating cells.Citation70 These results are in line with previous observations showing that Tiam1 can regulate migration and MT stability in single migrating keratinocytesCitation71 and that its downregulation results in reduced levels of acetylated tubulin.Citation72
CLIP-associated proteins (CLASPs) are a group of +TIPs, which stabilize specific subsets of MT in response to signaling cues.Citation71 CLASPs relocalize to distal segments of MTs at the leading edge of motile fibroblasts, an event mediated by PI3-kinase and GSK-3β and regulated by Cdc42-Rac signaling.Citation71-Citation74 Interestingly, MEFs lacking Tiam1 display alterations in migration and MT organization similar to those described in CLIP-associating protein 2 (CLASP2)-deficient ones.Citation69 CLASP2, a member of this family, has been implicated in MT stabilization during migration presumably by capture and crosslinking of MTs to the cell cortex. In these cells, CLASP2 is required for the formation of a stable polarized MT array independent of CLIP-170 and APC-EB1 interactions.Citation75,Citation76 In migrating PtK1 epithelial cells, although CLASPs associate with MT + ends in the cell body, they dynamically decorate the entire MT lattice in the leading lamella; interestingly, it was found that CLASP2 binding to the MT lattice is promoted by Rac.Citation77 Thus, by activating Rac1, Tiam1 could be an upstream regulator of CLASP2-mediated MT stabilization in migrating cells.
From microtubules to Rho GTPases
Pioneer work by C Waterman-Storer and T Salmon in the late 90s established that MT growth at leading-edge lamellipodia locally activates Rac1 to drive actin polymerization and lamellipodial protrusion required for cell migration.Citation78-Citation80 It was also shown that MT depolymerization in fibroblasts resulted in an increase in the level of GTP-bound RhoA, a phenomenon paralleled by formation of contractile actin bundles and focal adhesions. These studies gave rise to the idea that MTs play a major role in regulating the crosstalk between Rho-GTPases and microfilaments to control polarized actin assembly dynamics during cell migration.Citation79,Citation80 In addition, these observations implied that growing MTs might carry signals that promote Rac-mediated protrusive activity while shrinking ones factors that stimulate Rho activity and actin contractility. Subsequent studies identified several GEFs, such as GEF-H1, p190RhoGEF, Tiam1, and STEF (Tiam2) that link MTs with Rac and/or Rho and the actin cytoskeleton.Citation81-Citation84
The Lfc-Tctex-1 connection and RhoA
GEF-H1, or its mouse homolog Lfc, is negatively regulated by MT bindingCitation81 and is crucial for coupling MT dynamics to Rho A activation in different biological situations, including cell migrationCitation82,Citation83 and neuronal polarization.Citation84-Citation86 Lfc mutants unable to bind MTs have increased GEF activity and stress fiber formation, while Lfc downregulation in non-neuronal cells attenuates LPA-mediated actin reorganization, raising the possibility that it may be one critical GEF mediating MT-microfilament crosstalk.Citation81
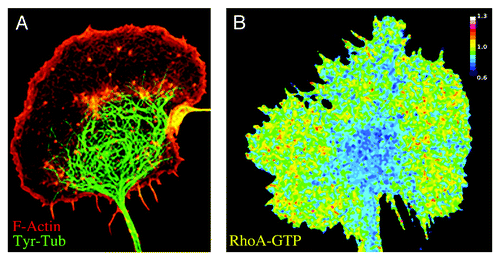
Lfc participates in several aspects of neuronal development, including neurogenesis,Citation85 axon outgrowth,Citation84 and spine formation.Citation86 In primary cultured neurons, confocal and TIRF microscopy revealed that Lfc associates with MTs in the growth cone central region (C domain) where Rho activity is low (); it is also present at the growth cone P domain,Citation84 where few MTs are present and RhoA activity is elevated (). It has been proposed that a Lfc-RhoA-Rho-Kinase (RhoK) signaling pathway exerts an inhibitory control on axon formation by targeting Tiam1 and disrupting a self-activating module that includes STEF (Tiam2), the Par polarity complex, Cdc42, and Rac that drives growth cone MT/actin remodeling required for axon formation; thus, local inactivation of Lfc-RhoA could be a signal required to trigger axon formation.Citation6,Citation84
Figure 3. Distribution of MTs, F-actin, and RhoA-activity in growth cones of developing neurons. (A) Double immunofluorescence micrograph showing an axonal growth cone of a cultured dorsal root ganglion cell (DRG) stained with phalloidin (red; F-actin) and a monoclonal antibody against (mAb) against tyrosinated tubulin (green; Tyr-tubulin). Dynamic MTs containing Tyr-tubulin predominate in the central growth cone region (C domain); the peripheral lamellipodial veil contains very few MTs. (B) FRET map image showing Rho-A activity in a growth cone of a DRG neuron. FRET measurements were performed using a unimolecular RhoA biosensor3,33 and a radiometric method. RhoA activity is low in the central MT-enriched growth cone domain and high in the actin-rich peripheral lamellipodial veil.
Recent studies have identified candidate molecules responsible for anchoring Lfc to the MT array and for regulating its activity. Tctex-1 is a dynein light chain that serves as an adaptor protein linking rhodopsin-bearing vesicles to the dynein motor complex for transport;Citation87 interestingly, Tctex-1 has dynein-independent functions, including promotion of Rac-mediated actin dynamics required for axon outgrowth.Citation88 Two recent studiesCitation84,Citation89 have demonstrated that Tctex directly interacts with Lfc, both in vitro and in vivo, inhibiting its GEF activity.
The Tiam1-MAP1B connection and Rac1
Reorganization of the growth cone cytoskeleton involving lamellipodial protrusion and expansion, shortening of actin ribs, increased actin dynamics, Cdc42-Rac1 signaling, paralleled by MT growth, invasion, and capture within the P-domain are events essential for axon formation.Citation90,Citation91 Tiam1-mediated activation of Rac1 is required for axon outgrowth and the rapid elongation of the newly formed axon;Citation91,Citation92 besides, Par6-Par-3 mediated Cdc42-induced Rac activation involves Tiam1/Tiam2.Citation92,Citation93 In cultured neurons, Tiam1 localizes to the growth cone of the nascent axon where it associates with dynamic MT and the subcortical cytoskeleton;Citation91 biochemical studies have confirmed these observation revealing that Tiam1 purifies with brain-derived MT.Citation91
MAP1B is a microtubule-associated protein preferentially expressed in developing neurons that is enriched at the tip of growing axons and required for their development.Citation94,Citation95 MAP1B-deficient neurons fail to properly elaborate an axon, displaying neurites with small growth cones containing fewer dynamic MT than their wild type counterparts.Citation94 It was also demonstrated that MAP1B deficiency decreases Rac1/Cdc42 while increasing that of RhoA, and that ectopic expression of Tiam1 rescues the MAP1B KO phenotype of cultured neurons.Citation96 A more recent study showed that Tiam1 interacts with MAP1B light chain 1 (LC1) through its pleckstrin homology (PH) domain; thus, MAP1B can be envisioned as a “flag” that targets Tiam1 to growth cone dynamic MTs to locally activate Rac1.Citation97 Together, with the Lfc-Tctex1 interaction, these observations illustrate the key role that MTs and MT-interacting components have in regulating Rho-GTPase signaling during axon formation, and its underlying events, namely neurite outgrowth (e.g., protrusion), growth cone advance (motility) and breaking of cell symmetry (polarization).
Conclusion and Future Directions
During the past decade great advances have been made in our understanding of the reciprocal relationship between MTs and member of the Ras family of small GTPases. Both protein systems are master regulators of key biological events including cell division, motility, and polarization. It is now evident that MTs, and not only actin filaments, are main cytoskeletal targets of RhoA, Rac, and Cdc42; more importantly, this regulation is central for controlling the dynamic organization of MTs, and hence most of its biological functions. Besides, due to its dynamicity and the presence of + TIPs, MTs can reach the cell cortex and interact with small Rho GTPases. This is of great functional importance since MTs also serve as platforms harboring factors, such as GEFs, capable of promoting the activity of at least RhoA and Rac. This reciprocal relationship generates positive and negative feedback loops, which have proved to be essential for events like cell migration and neuronal polarization.
One of the major challenges for future studies will be to correlate different MT behavior (growth, shrinkage, bundling, capture, etc.) with specific patterns of Rho-GTPase activation and/or signaling in time and space. The visualization of multiple Rho-GTPase activation patterns with next generation biosensorsCitation3 and multiplexing techniquesCitation33 in combination with live imaging of MT dynamics will certainly help characterizing the spatio-temporal circuits and programs involving MTs and small GTPases and how they relate with morphogenetic events.
| Abbreviations: | ||
| MT | = | Microtubule |
| GEF | = | guanine nucleotide exchange factor |
| GAP | = | GTPase activating protein |
| GDI | = | Guanine Dissociation Inhibitor |
| PTM | = | Post-Translational modifications |
| MEF | = | Mouse embryonic fibroblasts |
| LPA | = | Lysophosphatidic acid |
| GPCR | = | G protein–coupled receptors |
| LARG | = | Leukemia-associated RhoGEF |
| DRF | = | Diaphanous-related formin |
| GBD | = | GTP-binding domain |
| DAD | = | Diaphanous autoregulatory domain |
| FH | = | Formin homology |
| FAK | = | Focal adhesion kinase |
| EB1 | = | End-binding protein 1 |
| APC | = | Adenomatous polyposis coli |
| BM | = | Basement membrane |
| KS | = | Kaposi's sarcoma |
| SDF | = | stromal cell–derived factor |
| MTOC | = | Microtubule organizing center |
| GA | = | Golgi apparatus |
| aPKC | = | Atypical protein kinase C |
| GSK-3β | = | glycogen synthase kinase-3beta |
| PAK | = | p21-activated kinase |
| MRCK | = | Myotonic related Cdc42-binding kinase |
| ERM | = | Ezrin, radixin, moesin |
| EGF | = | Epidermal growth factor |
| CLASPs | = | CLIP-associating proteins |
| TIRFM | = | Total internal reflection fluorescence microscopy |
| MAP | = | Microtubule-associated protein |
| PH | = | Pleckstrin homology |
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
Research at the Cáceres’s laboratory is supported by grants from ANPCyT (MINCyT, Argentina) and Agencia Cordoba Ciencia. A.C. is an established scientist from CONICET. J.W. and G.Q. are fellows from CONICET. M.P.M. is supported by FONDECYT grant 1110382 and Millenium Nucleus in Regenerative Biology (MINREB), RC120003 (Iniciativa Científica Milenio, Ministerio de Economia, Fomento y Turismo).
References
- Etienne-Manneville S, Hall A. Rho GTPases in cell biology. Nature 2002; 420:629 - 35; http://dx.doi.org/10.1038/nature01148; PMID: 12478284
- González-Billault C, Muñoz-Llancao P, Henriquez DR, Wojnacki J, Conde C, Cáceres A. The role of small GTPases in neuronal morphogenesis and polarity. Cytoskeleton (Hoboken) 2012; 69:464 - 85; http://dx.doi.org/10.1002/cm.21034; PMID: 22605667
- Pertz O. Spatio-temporal Rho GTPase signaling - where are we now?. J Cell Sci 2010; 123:1841 - 50; http://dx.doi.org/10.1242/jcs.064345; PMID: 20484664
- Etienne-Manneville S. Microtubules in cell migration. Annu Rev Cell Dev Biol 2013; 29:471 - 99; http://dx.doi.org/10.1146/annurev-cellbio-101011-155711; PMID: 23875648
- Li R, Gundersen GG. Beyond polymer polarity: how the cytoskeleton builds a polarized cell. Nat Rev Mol Cell Biol 2008; 9:860 - 73; http://dx.doi.org/10.1038/nrm2522; PMID: 18946475
- Conde C, Cáceres A. Microtubule assembly, organization and dynamics in axons and dendrites. Nat Rev Neurosci 2009; 10:319 - 32; http://dx.doi.org/10.1038/nrn2631; PMID: 19377501
- Burbank KS, Mitchison TJ. Microtubule dynamic instability. Curr Biol 2006; 16:R516 - 7; http://dx.doi.org/10.1016/j.cub.2006.06.044; PMID: 16860721
- Schulze E, Kirschner M. Microtubule dynamics in interphase cells. J Cell Biol 1986; 102:1020 - 31; http://dx.doi.org/10.1083/jcb.102.3.1020; PMID: 3512576
- Kirschner M, Mitchison T. Beyond self-assembly: from microtubules to morphogenesis. Cell 1986; 45:329 - 42; http://dx.doi.org/10.1016/0092-8674(86)90318-1; PMID: 3516413
- Verhey KJ, Gaertig J. The tubulin code. Cell Cycle 2007; 6:2152 - 60; http://dx.doi.org/10.4161/cc.6.17.4633; PMID: 17786050
- Janke C, Kneussel M. Tubulin post-translational modifications: encoding functions on the neuronal microtubule cytoskeleton. Trends Neurosci 2010; 33:362 - 72; http://dx.doi.org/10.1016/j.tins.2010.05.001; PMID: 20541813
- Janke C, Bulinski JC. Post-translational regulation of the microtubule cytoskeleton: mechanisms and functions. Nat Rev Mol Cell Biol 2011; 12:773 - 86; http://dx.doi.org/10.1038/nrm3227; PMID: 22086369
- Palazzo A, Ackerman B, Gundersen GG. Cell biology: Tubulin acetylation and cell motility. Nature 2003; 421:230; http://dx.doi.org/10.1038/421230a; PMID: 12529632
- LeDizet M, Piperno G. Identification of an acetylation site of Chlamydomonas alpha-tubulin. Proc Natl Acad Sci U S A 1987; 84:5720 - 4; http://dx.doi.org/10.1073/pnas.84.16.5720; PMID: 2441392
- Nogales E, Wolf SG, Downing KH. Structure of the alpha beta tubulin dimer by electron crystallography. Nature 1998; 391:199 - 203; http://dx.doi.org/10.1038/34465; PMID: 9428769
- Choudhary C, Kumar C, Gnad F, Nielsen ML, Rehman M, Walther TC, Olsen JV, Mann M. Lysine acetylation targets protein complexes and co-regulates major cellular functions. Science 2009; 325:834 - 40; http://dx.doi.org/10.1126/science.1175371; PMID: 19608861
- Steinmetz MO, Akhmanova A. Capturing protein tails by CAP-Gly domains. Trends Biochem Sci 2008; 33:535 - 45; http://dx.doi.org/10.1016/j.tibs.2008.08.006; PMID: 18835717
- Gundersen GG, Bulinski JC. Selective stabilization of microtubules oriented toward the direction of cell migration. Proc Natl Acad Sci U S A 1988; 85:5946 - 50; http://dx.doi.org/10.1073/pnas.85.16.5946; PMID: 3413068
- Gundersen GG, Khawaja S, Bulinski JC. Generation of a stable, posttranslationally modified microtubule array is an early event in myogenic differentiation. J Cell Biol 1989; 109:2275 - 88; http://dx.doi.org/10.1083/jcb.109.5.2275; PMID: 2681230
- Wen Y, Eng CH, Schmoranzer J, Cabrera-Poch N, Morris EJ, Chen M, Wallar BJ, Alberts AS, Gundersen GG. EB1 and APC bind to mDia to stabilize microtubules downstream of Rho and promote cell migration. Nat Cell Biol 2004; 6:820 - 30; http://dx.doi.org/10.1038/ncb1160; PMID: 15311282
- Gundersen GG, Wen Y, Eng CH, Schmoranzer J, Cabrera-Poch N, Morris EJ, Chen M, Gomes ER. Regulation of microtubules by Rho GTPases in migrating cells. Novartis Found Symp 2005; 269:116 - 26, discussion 116-26, 223-30; http://dx.doi.org/10.1002/047001766X.ch10; PMID: 16358406
- Ridley AJ, Hall A. The small GTP-binding protein rho regulates the assembly of focal adhesions and actin stress fibers in response to growth factors. Cell 1992; 70:389 - 99; http://dx.doi.org/10.1016/0092-8674(92)90163-7; PMID: 1643657
- Gundersen GG, Kim I, Chapin CJ. Induction of stable microtubules in 3T3 fibroblasts by TGF-beta and serum. J Cell Sci 1994; 107:645 - 59; PMID: 8006078
- Cook TA, Nagasaki T, Gundersen GG. Rho guanosine triphosphatase mediates the selective stabilization of microtubules induced by lysophosphatidic acid. J Cell Biol 1998; 141:175 - 85; http://dx.doi.org/10.1083/jcb.141.1.175; PMID: 9531557
- Choi JW, Herr DR, Noguchi K, Yung YC, Lee CW, Mutoh T, Lin ME, Teo ST, Park KE, Mosley AN, et al. LPA receptors: subtypes and biological actions. Annu Rev Pharmacol Toxicol 2010; 50:157 - 86; http://dx.doi.org/10.1146/annurev.pharmtox.010909.105753; PMID: 20055701
- Xiang SY, Dusaban SS, Brown JH. Lysophospholipid receptor activation of RhoA and lipid signaling pathways. Biochim Biophys Acta 2013; 1831:213 - 22; http://dx.doi.org/10.1016/j.bbalip.2012.09.004; PMID: 22986288
- Lin ME, Herr DR, Chun J. Lysophosphatidic acid (LPA) receptors: signaling properties and disease relevance. Prostaglandins Other Lipid Mediat 2010; 91:130 - 8; http://dx.doi.org/10.1016/j.prostaglandins.2009.02.002; PMID: 20331961
- Goulimari P, Kitzing TM, Knieling H, Brandt DT, Offermanns S, Grosse R. Galpha12/13 is essential for directed cell migration and localized Rho-Dia1 function. J Biol Chem 2005; 280:42242 - 51; http://dx.doi.org/10.1074/jbc.M508690200; PMID: 16251183
- Hart MJ, Jiang X, Kozasa T, Roscoe W, Singer WD, Gilman AG, Sternweis PC, Bollag G. Direct stimulation of the guanine nucleotide exchange activity of p115 RhoGEF by Galpha13. Science 1998; 280:2112 - 4; http://dx.doi.org/10.1126/science.280.5372.2112; PMID: 9641916
- Slattum G, McGee KM, Rosenblatt J. P115 RhoGEF and microtubules decide the direction apoptotic cells extrude from an epithelium. J Cell Biol 2009; 186:693 - 702; http://dx.doi.org/10.1083/jcb.200903079; PMID: 19720875
- Fukuhara S, Chikumi H, Gutkind JS. Leukemia-associated Rho guanine nucleotide exchange factor (LARG) links heterotrimeric G proteins of the G(12) family to Rho. FEBS Lett 2000; 485:183 - 8; http://dx.doi.org/10.1016/S0014-5793(00)02224-9; PMID: 11094164
- Pertz O, Hodgson L, Klemke RL, Hahn KM. Spatiotemporal dynamics of RhoA activity in migrating cells. Nature 2006; 440:1069 - 72; http://dx.doi.org/10.1038/nature04665; PMID: 16547516
- Machacek M, Hodgson L, Welch C, Elliott H, Pertz O, Nalbant P, Abell A, Johnson GL, Hahn KM, Danuser G. Coordination of Rho GTPase activities during cell protrusion. Nature 2009; 461:99 - 103; http://dx.doi.org/10.1038/nature08242; PMID: 19693013
- Young KG, Copeland JW. Formins in cell signaling. Biochim Biophys Acta 2010; 1803:183 - 90; http://dx.doi.org/10.1016/j.bbamcr.2008.09.017; PMID: 18977250
- Breitsprecher D, Goode BL. Formins at a glance. J Cell Sci 2013; 126:1 - 7; http://dx.doi.org/10.1242/jcs.107250; PMID: 23516326
- Wallar BJ, Alberts AS. The formins: active scaffolds that remodel the cytoskeleton. Trends Cell Biol 2003; 13:435 - 46; http://dx.doi.org/10.1016/S0962-8924(03)00153-3; PMID: 12888296
- Palazzo AF, Cook TA, Alberts AS, Gundersen GG. mDia mediates Rho-regulated formation and orientation of stable microtubules. Nat Cell Biol 2001; 3:723 - 9; http://dx.doi.org/10.1038/35087035; PMID: 11483957
- Bartolini F, Ramalingam N, Gundersen GG. Actin-capping protein promotes microtubule stability by antagonizing the actin activity of mDia1. Mol Biol Cell 2012; 23:4032 - 40; http://dx.doi.org/10.1091/mbc.E12-05-0338; PMID: 22918941
- Palazzo AF, Eng CH, Schlaepfer DD, Marcantonio EE, Gundersen GG. Localized stabilization of microtubules by integrin- and FAK-facilitated Rho signaling. Science 2004; 303:836 - 9; http://dx.doi.org/10.1126/science.1091325; PMID: 14764879
- Kohno H, Tanaka K, Mino A, Umikawa M, Imamura H, Fujiwara T, Fujita Y, Hotta K, Qadota H, Watanabe T, et al. Bni1p implicated in cytoskeletal control is a putative target of Rho1p small GTP binding protein in Saccharomyces cerevisiae. EMBO J 1996; 15:6060 - 8; PMID: 8947028
- Lee L, Klee SK, Evangelista M, Boone C, Pellman D. Control of mitotic spindle position by the Saccharomyces cerevisiae formin Bni1p. J Cell Biol 1999; 144:947 - 61; http://dx.doi.org/10.1083/jcb.144.5.947; PMID: 10085293
- Gundersen GG, Gomes ER, Wen Y. Cortical control of microtubule stability and polarization. Curr Opin Cell Biol 2004; 16:106 - 12; http://dx.doi.org/10.1016/j.ceb.2003.11.010; PMID: 15037313
- Nakaya Y, Sheng G. Epithelial to mesenchymal transition during gastrulation: an embryological view. Dev Growth Differ 2008; 50:755 - 66; http://dx.doi.org/10.1111/j.1440-169X.2008.01070.x; PMID: 19046163
- Nakaya Y, Sukowati EW, Wu Y, Sheng G. RhoA and microtubule dynamics control cell-basement membrane interaction in EMT during gastrulation. Nat Cell Biol 2008; 10:765 - 75; http://dx.doi.org/10.1038/ncb1739; PMID: 18552836
- Naranatt PP, Krishnan HH, Smith MS, Chandran B. Kaposi’s sarcoma-associated herpesvirus modulates microtubule dynamics via RhoA-GTP-diaphanous 2 signaling and utilizes the dynein motors to deliver its DNA to the nucleus. J Virol 2005; 79:1191 - 206; http://dx.doi.org/10.1128/JVI.79.2.1191-1206.2005; PMID: 15613346
- Gomez TS, Kumar K, Medeiros RB, Shimizu Y, Leibson PJ, Billadeau DD. Formins regulate the actin-related protein 2/3 complex-independent polarization of the centrosome to the immunological synapse. Immunity 2007; 26:177 - 90; http://dx.doi.org/10.1016/j.immuni.2007.01.008; PMID: 17306570
- Andrés-Delgado L, Antón OM, Bartolini F, Ruiz-Sáenz A, Correas I, Gundersen GG, Alonso MA. INF2 promotes the formation of detyrosinated microtubules necessary for centrosome reorientation in T cells. J Cell Biol 2012; 198:1025 - 37; http://dx.doi.org/10.1083/jcb.201202137; PMID: 22986496
- Arakawa Y, Bito H, Furuyashiki T, Tsuji T, Takemoto-Kimura S, Kimura K, Nozaki K, Hashimoto N, Narumiya S. Control of axon elongation via an SDF-1alpha/Rho/mDia pathway in cultured cerebellar granule neurons. J Cell Biol 2003; 161:381 - 91; http://dx.doi.org/10.1083/jcb.200210149; PMID: 12707308
- Stowers L, Yelon D, Berg LJ, Chant J. Regulation of the polarization of T cells toward antigen-presenting cells by Ras-related GTPase CDC42. Proc Natl Acad Sci U S A 1995; 92:5027 - 31; http://dx.doi.org/10.1073/pnas.92.11.5027; PMID: 7761442
- Etienne-Manneville S. Cdc42--the centre of polarity. J Cell Sci 2004; 117:1291 - 300; http://dx.doi.org/10.1242/jcs.01115; PMID: 15020669
- Nobes CD, Hall A. Rho GTPases control polarity, protrusion, and adhesion during cell movement. J Cell Biol 1999; 144:1235 - 44; http://dx.doi.org/10.1083/jcb.144.6.1235; PMID: 10087266
- Etienne-Manneville S, Hall A. Integrin-mediated activation of Cdc42 controls cell polarity in migrating astrocytes through PKCzeta. Cell 2001; 106:489 - 98; http://dx.doi.org/10.1016/S0092-8674(01)00471-8; PMID: 11525734
- Etienne-Manneville S, Hall A. Cdc42 regulates GSK-3beta and adenomatous polyposis coli to control cell polarity. Nature 2003; 421:753 - 6; http://dx.doi.org/10.1038/nature01423; PMID: 12610628
- Cau J, Hall A. Cdc42 controls the polarity of the actin and microtubule cytoskeletons through two distinct signal transduction pathways. J Cell Sci 2005; 118:2579 - 87; http://dx.doi.org/10.1242/jcs.02385; PMID: 15928049
- Dujardin DL, Vallee RB. Dynein at the cortex. Curr Opin Cell Biol 2002; 14:44 - 9; http://dx.doi.org/10.1016/S0955-0674(01)00292-7; PMID: 11792543
- Shi SH, Jan LY, Jan YN. Hippocampal neuronal polarity specified by spatially localized mPar3/mPar6 and PI 3-kinase activity. Cell 2003; 112:63 - 75; http://dx.doi.org/10.1016/S0092-8674(02)01249-7; PMID: 12526794
- Kim WY, Zhou FQ, Zhou J, Yokota Y, Wang YM, Yoshimura T, Kaibuchi K, Woodgett JR, Anton ES, Snider WD. Essential roles for GSK-3s and GSK-3-primed substrates in neurotrophin-induced and hippocampal axon growth. Neuron 2006; 52:981 - 96; http://dx.doi.org/10.1016/j.neuron.2006.10.031; PMID: 17178402
- Shi SH, Cheng T, Jan LY, Jan YN. APC and GSK-3beta are involved in mPar3 targeting to the nascent axon and establishment of neuronal polarity. Curr Biol 2004; 14:2025 - 32; http://dx.doi.org/10.1016/j.cub.2004.11.009; PMID: 15556865
- Zhou FQ, Zhou J, Dedhar S, Wu YH, Snider WD. NGF-induced axon growth is mediated by localized inactivation of GSK-3beta and functions of the microtubule plus end binding protein APC. Neuron 2004; 42:897 - 912; http://dx.doi.org/10.1016/j.neuron.2004.05.011; PMID: 15207235
- Dent EW, Gupton SL, Gertler FB. The growth cone cytoskeleton in axon outgrowth and guidance. Cold Spring Harb Perspect Biol 2011; 3:a001800; http://dx.doi.org/10.1101/cshperspect.a001800; PMID: 21106647
- Etienne-Manneville S, Manneville JB, Nicholls S, Ferenczi MA, Hall A. Cdc42 and Par6-PKCzeta regulate the spatially localized association of Dlg1 and APC to control cell polarization. J Cell Biol 2005; 170:895 - 901; http://dx.doi.org/10.1083/jcb.200412172; PMID: 16157700
- Manneville JB, Jehanno M, Etienne-Manneville S. Dlg1 binds GKAP to control dynein association with microtubules, centrosome positioning, and cell polarity. J Cell Biol 2010; 191:585 - 98; http://dx.doi.org/10.1083/jcb.201002151; PMID: 21041448
- Gomes ER, Jani S, Gundersen GG. Nuclear movement regulated by Cdc42, MRCK, myosin, and actin flow establishes MTOC polarization in migrating cells. Cell 2005; 121:451 - 63; http://dx.doi.org/10.1016/j.cell.2005.02.022; PMID: 15882626
- Fais S, Malorni W. Leukocyte uropod formation and membrane/cytoskeleton linkage in immune interactions. J Leukoc Biol 2003; 73:556 - 63; http://dx.doi.org/10.1189/jlb.1102568; PMID: 12714569
- Kumar S, Xu J, Perkins C, Guo F, Snapper S, Finkelman FD, Zheng Y, Filippi MD. Cdc42 regulates neutrophil migration via crosstalk between WASp, CD11b, and microtubules. Blood 2012; 120:3563 - 74; http://dx.doi.org/10.1182/blood-2012-04-426981; PMID: 22932798
- Wittmann T, Bokoch GM, Waterman-Storer CM. Regulation of leading edge microtubule and actin dynamics downstream of Rac1. J Cell Biol 2003; 161:845 - 51; http://dx.doi.org/10.1083/jcb.200303082; PMID: 12796474
- Daub H, Gevaert K, Vandekerckhove J, Sobel A, Hall A. Rac/Cdc42 and p65PAK regulate the microtubule-destabilizing protein stathmin through phosphorylation at serine 16. J Biol Chem 2001; 276:1677 - 80; http://dx.doi.org/10.1074/jbc.C000635200; PMID: 11058583
- Wittmann T, Bokoch GM, Waterman-Storer CM. Regulation of microtubule destabilizing activity of Op18/stathmin downstream of Rac1. J Biol Chem 2004; 279:6196 - 203; http://dx.doi.org/10.1074/jbc.M307261200; PMID: 14645234
- Watabe-Uchida M, John KA, Janas JA, Newey SE, Van Aelst L. The Rac activator DOCK7 regulates neuronal polarity through local phosphorylation of stathmin/Op18. Neuron 2006; 51:727 - 39; http://dx.doi.org/10.1016/j.neuron.2006.07.020; PMID: 16982419
- Ellenbroek SI, Iden S, Collard JG. The Rac activator Tiam1 is required for polarized protrusional outgrowth of primary astrocytes by affecting the organization of the microtubule network. Small GTPases 2012; 3:4 - 14; http://dx.doi.org/10.4161/sgtp.19379; PMID: 22710731
- Pegtel DM, Ellenbroek SI, Mertens AE, van der Kammen RA, de Rooij J, Collard JG. The Par-Tiam1 complex controls persistent migration by stabilizing microtubule-dependent front-rear polarity. Curr Biol 2007; 17:1623 - 34; http://dx.doi.org/10.1016/j.cub.2007.08.035; PMID: 17825562
- Akhmanova A, Hoogenraad CC, Drabek K, Stepanova T, Dortland B, Verkerk T, Vermeulen W, Burgering BM, De Zeeuw CI, Grosveld F, et al. Clasps are CLIP-115 and -170 associating proteins involved in the regional regulation of microtubule dynamics in motile fibroblasts. Cell 2001; 104:923 - 35; http://dx.doi.org/10.1016/S0092-8674(01)00288-4; PMID: 11290329
- Galjart N. CLIPs and CLASPs and cellular dynamics. Nat Rev Mol Cell Biol 2005; 6:487 - 98; http://dx.doi.org/10.1038/nrm1664; PMID: 15928712
- Kumar P, Wittmann T. +TIPs: SxIPping along microtubule ends. Trends Cell Biol 2012; 22:418 - 28; http://dx.doi.org/10.1016/j.tcb.2012.05.005; PMID: 22748381
- Wittmann T, Waterman-Storer CM. Spatial regulation of CLASP affinity for microtubules by Rac1 and GSK3beta in migrating epithelial cells. [Erratum in: J Cell Biol 2005; 171: 393] J Cell Biol 2005; 169:929 - 39; http://dx.doi.org/10.1083/jcb.200412114; PMID: 15955847
- Drabek K, van Ham M, Stepanova T, Draegestein K, van Horssen R, Sayas CL, Akhmanova A, Ten Hagen T, Smits R, Fodde R, et al. Role of CLASP2 in microtubule stabilization and the regulation of persistent motility. Curr Biol 2006; 16:2259 - 64; http://dx.doi.org/10.1016/j.cub.2006.09.065; PMID: 17113391
- Lansbergen G, Grigoriev I, Mimori-Kiyosue Y, Ohtsuka T, Higa S, Kitajima I, Demmers J, Galjart N, Houtsmuller AB, Grosveld F, et al. CLASPs attach microtubule plus ends to the cell cortex through a complex with LL5beta. Dev Cell 2006; 11:21 - 32; http://dx.doi.org/10.1016/j.devcel.2006.05.012; PMID: 16824950
- Waterman-Storer CM, Worthylake RA, Liu BP, Burridge K, Salmon ED. Microtubule growth activates Rac1 to promote lamellipodial protrusion in fibroblasts. Nat Cell Biol 1999; 1:45 - 50; http://dx.doi.org/10.1038/9018; PMID: 10559863
- Wittmann T, Waterman-Storer CM. Cell motility: can Rho GTPases and microtubules point the way?. J Cell Sci 2001; 114:3795 - 803; PMID: 11719546
- Waterman-Storer CM, Salmon E. Positive feedback interactions between microtubule and actin dynamics during cell motility. Curr Opin Cell Biol 1999; 11:61 - 7; http://dx.doi.org/10.1016/S0955-0674(99)80008-8; PMID: 10047528
- Birkenfeld J, Nalbant P, Yoon SH, Bokoch GM. Cellular functions of GEF-H1, a microtubule-regulated Rho-GEF: is altered GEF-H1 activity a crucial determinant of disease pathogenesis?. Trends Cell Biol 2008; 18:210 - 9; http://dx.doi.org/10.1016/j.tcb.2008.02.006; PMID: 18394899
- Birukova AA, Adyshev D, Gorshkov B, Bokoch GM, Birukov KG, Verin AD. GEF-H1 is involved in agonist-induced human pulmonary endothelial barrier dysfunction. Am J Physiol Lung Cell Mol Physiol 2006; 290:L540 - 8; http://dx.doi.org/10.1152/ajplung.00259.2005; PMID: 16257999
- Krendel M, Zenke FT, Bokoch GM. Nucleotide exchange factor GEF-H1 mediates cross-talk between microtubules and the actin cytoskeleton. Nat Cell Biol 2002; 4:294 - 301; http://dx.doi.org/10.1038/ncb773; PMID: 11912491
- Conde C, Arias C, Robin M, Li A, Saito M, Chuang JZ, Nairn AC, Sung CH, Cáceres A. Evidence for the involvement of Lfc and Tctex-1 in axon formation. J Neurosci 2010; 30:6793 - 800; http://dx.doi.org/10.1523/JNEUROSCI.5420-09.2010; PMID: 20463241
- Gauthier-Fisher A, Lin DC, Greeve M, Kaplan DR, Rottapel R, Miller FD. Lfc and Tctex-1 regulate the genesis of neurons from cortical precursor cells. Nat Neurosci 2009; 12:735 - 44; http://dx.doi.org/10.1038/nn.2339; PMID: 19448628
- Ryan XP, Alldritt J, Svenningsson P, Allen PB, Wu GY, Nairn AC, Greengard P. The Rho-specific GEF Lfc interacts with neurabin and spinophilin to regulate dendritic spine morphology. Neuron 2005; 47:85 - 100; http://dx.doi.org/10.1016/j.neuron.2005.05.013; PMID: 15996550
- Tai AW, Chuang JZ, Bode C, Wolfrum U, Sung CH. Rhodopsin’s carboxy-terminal cytoplasmic tail acts as a membrane receptor for cytoplasmic dynein by binding to the dynein light chain Tctex-1. Cell 1999; 97:877 - 87; http://dx.doi.org/10.1016/S0092-8674(00)80800-4; PMID: 10399916
- Chuang JZ, Yeh TY, Bollati F, Conde C, Canavosio F, Caceres A, Sung CH. The dynein light chain Tctex-1 has a dynein-independent role in actin remodeling during neurite outgrowth. Dev Cell 2005; 9:75 - 86; http://dx.doi.org/10.1016/j.devcel.2005.04.003; PMID: 15992542
- Meiri D, Marshall CB, Greeve MA, Kim B, Balan M, Suarez F, Bakal C, Wu C, Larose J, Fine N, et al. Mechanistic insight into the microtubule and actin cytoskeleton coupling through dynein-dependent RhoGEF inhibition. Mol Cell 2012; 45:642 - 55 Erratum in. Mol Cell 2012; 45:844; http://dx.doi.org/10.1016/j.molcel.2012.01.027; PMID: 22405273
- Bradke F, Dotti CG. The role of local actin instability in axon formation. Science 1999; 283:1931 - 4; http://dx.doi.org/10.1126/science.283.5409.1931; PMID: 10082468
- Kunda P, Paglini G, Quiroga S, Kosik K, Caceres A. Evidence for the involvement of Tiam1 in axon formation. J Neurosci 2001; 21:2361 - 72; PMID: 11264310
- Nishimura T, Yamaguchi T, Kato K, Yoshizawa M, Nabeshima Y, Ohno S, Hoshino M, Kaibuchi K. PAR-6-PAR-3 mediates Cdc42-induced Rac activation through the Rac GEFs STEF/Tiam1. Nat Cell Biol 2005; 7:270 - 7; http://dx.doi.org/10.1038/ncb1227; PMID: 15723051
- Arimura N, Kaibuchi K. Neuronal polarity: from extracellular signals to intracellular mechanisms. Nat Rev Neurosci 2007; 8:194 - 205; http://dx.doi.org/10.1038/nrn2056; PMID: 17311006
- González-Billault C, Avila J, Cáceres A. Evidence for the role of MAP1B in axon formation. Mol Biol Cell 2001; 12:2087 - 98; http://dx.doi.org/10.1091/mbc.12.7.2087; PMID: 11452005
- González-Billault C, Jiménez-Mateos EM, Cáceres A, Díaz-Nido J, Wandosell F, Avila J. Microtubule-associated protein 1B function during normal development, regeneration, and pathological conditions in the nervous system. J Neurobiol 2004; 58:48 - 59; http://dx.doi.org/10.1002/neu.10283; PMID: 14598369
- Montenegro-Venegas C, Tortosa E, Rosso S, Peretti D, Bollati F, Bisbal M, Jausoro I, Avila J, Cáceres A, González-Billault C. MAP1B regulates axonal development by modulating Rho-GTPase Rac1 activity. Mol Biol Cell 2010; 21:3518 - 28; http://dx.doi.org/10.1091/mbc.E09-08-0709; PMID: 20719958
- Henríquez DR, Bodaleo FJ, Montenegro-Venegas C, González-Billault C. The light chain 1 subunit of the microtubule-associated protein 1B (MAP1B) is responsible for Tiam1 binding and Rac1 activation in neuronal cells. PLoS One 2012; 7:e53123; http://dx.doi.org/10.1371/journal.pone.0053123; PMID: 23300879
- Luxton GW, Gomes ER, Folker ES, Worman HJ, Gundersen GG. TAN lines: a novel nuclear envelope structure involved in nuclear positioning. Nucleus 2011; 2:173 - 81; http://dx.doi.org/10.4161/nucl.2.3.16243; PMID: 21818410
- Schmoranzer J, Fawcett JP, Segura M, Tan S, Vallee RB, Pawson T, Gundersen GG. Par3 and dynein associate to regulate local microtubule dynamics and centrosome orientation during migration. Curr Biol 2009; 19:1065 - 74; http://dx.doi.org/10.1016/j.cub.2009.05.065; PMID: 19540120
