Abstract
Rho-associated coiled-coil containing kinases (ROCK) were originally identified as effectors of the RhoA small GTPase.Citation1–Citation5 They belong to the AGC family of serine/threonine kinasesCitation6 and play vital roles in facilitating actomyosin cytoskeleton contractility downstream of RhoA and RhoC activation. Since their discovery, ROCK kinases have been extensively studied, unveiling their manifold functions in processes including cell contraction, migration, apoptosis, survival, and proliferation. Two mammalian ROCK homologs have been identified, ROCK1 (also called ROCK I, ROKβ, Rho-kinase β, or p160ROCK) and ROCK2 (also known as ROCK II, ROKα, or Rho kinase), hereafter collectively referred to as ROCK. In this review, we will focus on the structure, regulation, and functions of ROCK.
Abbreviations
| AGC | = | protein kinase A, G and C family |
| CRD | = | cysteine-rich domain |
| CRIK | = | citron Rho-interacting kinase |
| DAMP | = | damage associated molecular pattern |
| DMPK | = | dystrophia myotonica protein kinase |
| ERM | = | ezrin/radixin/moesin |
| EST | = | expressed sequence tag |
| mDia | = | mammalian homologue of diaphanous |
| MEF | = | mouse embryo fibroblast |
| MLC | = | myosin II regulatory light chain |
| MLCK | = | MLC kinase |
| MRCK | = | myotonic dystrophy-related Cdc42-binding kinase |
| PH | = | pleckstrin homology |
| PP1M | = | myosin light chain phosphatase complex |
| RBD | = | Rho binding domain |
| ROCK | = | Rho-associated coiled-coil containing kinase |
| TiGer | = | Tissue-specific Gene Expression and Regulation database |
| VSMC | = | Vascular smooth muscle cell |
ROCK Structure
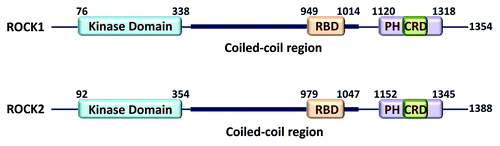
In humans, ROCK1 and ROCK2 both contain 33 exons and are located on chromosome 18 (18q11.1) and 2 (2p24) respectively. The ROCK1 open reading frame encodes 1354 amino acids, whereas ROCK2 encodes 1388 amino acids. ROCK2 also has a reported splice variant, preferentially expressed in skeletal muscle, which results in the inclusion of 57 additional amino acids.Citation7 The two ROCK homologs shares 64% identity in their primary amino acid sequences, with the highest homology (92%) within the kinase domains and the coiled-coil domains being the most diverse (55%).Citation5 The kinase domains of ROCK are closely related to many homologous domains in this family, including dystrophia myotonica protein kinase (DMPK), myotonic dystrophy kinase related-Cdc42 related kinases (MRCK) α and β, and citron Rho-interacting kinase (CRIK). To date, the crystal structures of the kinase domains from ROCK1,Citation8 ROCK2,Citation9 MRCKβ,Citation10 and DMPKCitation11 have been determined, which has highlighted the high degree of tertiary as well as primary similarity. N-terminal and carboxyl-terminal extensions of the ROCK kinase domains are essential for catalytic activity.Citation4,Citation8,Citation9 The ROCK kinase domains are located in the N-terminal region, which is followed by a central ~600 amino acid long amphipathic α-helix forming a coiled-coil region ().Citation12 At the carboxyl-terminal region, there is a split pleckstrin homology (PH) domain, which is bisected by an internal cysteine-rich zinc finger-like motif domain (CRD). The two separate PH portions assemble together to form a typical PH domain that is attached by two short linkers to a separate CRD.Citation13 The canonical Rho binding domain (RBD) forms a parallel coiled coil dimer, as revealed by crystal structure determinations, and binds exclusively to the switch I and switch II regions of GTP-bound active RhoA and RhoC.Citation14,Citation15 Two additional Rho-interacting domains were identified that can tightly interact with RhoA, which may cooperatively contribute to binding.Citation16 Crystal structure studies revealed that ROCK has two dimerization domains: the ~70 residue N-terminal dimerization regionCitation8,Citation9 and the coiled-coil helical regions.Citation12 Charged residues in the coiled-coil might function as a hinge that allows the N-terminal kinase domains to interact with C-terminal inhibitory regions. Structural determination of full-length ROCK protein crystals will ultimately reveal how the various domains interact and the mechanism of auto-inhibition.
Figure 1. ROCK functional domains. Protein domains and their indicated positions were taken from the National Center for Biotechnology Information (NCBI; http://www.ncbi.nlm.nih.gov/protein) for human ROCK1 (NP_005397.1) and ROCK2 (NP_004841.2)
Regulation of ROCK Activity
Although ROCK1 and ROCK2 have highly related functional domains and significant amino acid identity, they are regulated both by common means as well as mechanisms unique to ROCK1 or ROCK2.
Conventional activation
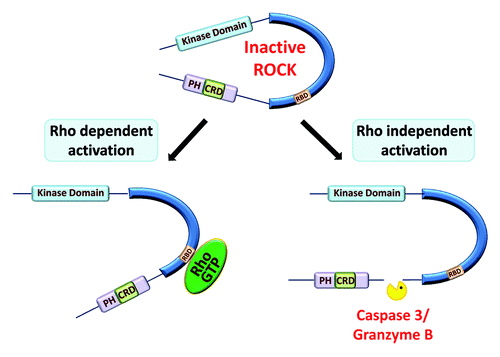
The carboxyl-terminal region of ROCK acts as an auto-inhibitory region, since deletion of this portion results in constitutive activation of either kinase in vitro and in vivo.Citation17,Citation18 Inhibition may occur either because the active site of the kinase domain is blocked by interaction with portions of the carboxyl terminal region or due to stabilization of a catalytically incompetent conformation.Citation8 The Ras superfamily includes the Rho-GTPase proteins RhoA and RhoC, which are the most well characterized ROCK regulators. Interaction of activated, GTP-bound Rho proteins with the RBD have been reported to elevate kinase specific activity by inducing conformational changes that disrupt the negative regulatory interactions between the kinase domain and the auto-inhibitory carboxyl terminal region ().Citation2,Citation4,Citation5 However, structural studies of the holoenzyme, either in isolation or associated with active Rho proteins, have not been reported to confirm this model. An additional possibility is that the recruitment of ROCK proteins to sites of elevated Rho activity may be equally or even more important for the transduction of active Rho signaling. In the context of apoptosis, ROCK1 is activated by cleavage and removal of the auto-inhibitory domain by caspases,Citation19,Citation20 while ROCK2 may similarly be activated by Granzyme B-mediated cleavage, which also leads to concomitant caspase activation and ROCK1 activation.Citation21 Both of these events result in the generation of Rho-independent constitutively active kinase fragments that lead to unconstrained actin-myosin contraction, resulting in membrane blebbing, apoptotic body formation, and packaging of nuclear materials into blebs and apoptotic bodies.Citation19,Citation22,Citation23 In a non-apoptotic scenario in endothelial cells, thrombin was reported to activate caspase 2 and increase ROCK2 expression, resulting in increased levels of caspase 2-mediated proteolytic cleavage and activation of ROCK2 and consequent microparticle formation.Citation24
Figure 2. Modes of ROCK activation. In the inactive state, the carboxyl terminal of ROCK acts as an auto-inhibitory region. Binding of active Rho-GTP to the Rho binding domain (RBD) disrupts the negative regulation, thus activating the kinase. ROCK proteins may also be activated in a Rho-independent manner by removal of the inhibitory carboxyl terminal of ROCK1 and ROCK2 by caspase 3 or granzyme B mediated cleavage, respectively.
Phosphorylation
Crystal structure analyses revealed that the kinase domain had a catalytically competent conformation without phosphorylation or conformational input from RhoA binding to the RBD.Citation8 Unlike other AGC family kinases that require activation loop phosphorylation,Citation6 the ROCK kinase domains do not appear to require phosphorylation for activity. However, additional kinases may regulate ROCK signaling by phosphorylating other regions. For example, Polo-like kinase-1 works synergistically with RhoA to maximally activate ROCK2 by phosphorylating at any one of four conserved sites Thr-967, Ser-1099, Ser-1133, and Ser-1374.Citation25 Phosphorylation of ROCK2 on Tyr-722 inhibits activity by decreasing RhoA binding,Citation26 while Shp-2 mediated dephosphorylation increases RhoA responsiveness.Citation27 Autophosphorylation by ROCK2 on S1366Citation28 and by ROCK1 on S1333Citation29 is a reflection of the kinase activation, although these sites do not regulate catalytic activity. However, phosphorylation on these sites might contribute to protein subcellular localization.Citation30 Large scale phosphoproteomics studies have identified numerous phosphorylations on human ROCK1 (45 sites reported on www.phosphosite.org) and ROCK2 (43 sites), although the majority of them have not been confirmed by independent means. The frequency of some observed phosphorylations (e.g., ROCK1 Tyr-913 and Ser-1341, or ROCK2 Ser-1379) suggests that they have physiological significance, which will likely be determined in the future.
Negative regulation
Although Rho proteins generally activate ROCK, other GTP-binding proteins have been found to negatively regulate ROCK signaling. Gem was shown to interact with ROCK1 in the coiled-coil region adjacent to the RBD, thereby inhibiting phosphorylation of MLC and the MYPT1 subunit of MLC phosphatase.Citation31 Rad1, another small GTP-binding protein was also reported to mimic Gem in inhibiting actomyosin contractility by blocking ROCK2, resulting in reduced cell rounding and neurite retraction.Citation31 In contrast to the highly related RhoA, B and C proteins that can stimulate stress fiber formation through ROCK, RhoE decreases stress fiber formationCitation32,Citation33 by binding to the N-terminal region of ROCK1, but not ROCK2, thus physically interfering with ROCK1 kinase activity.Citation34-Citation36 Interestingly, protein kinase PDK1 competitively binds to a similar region of ROCK1 as RhoE, thereby antagonizing the negative regulation of ROCK1 by Rho E.Citation37 Recently, Coronin1B was identified as an attenuator of ROCK2 signaling by directly binding to the PH domain.Citation38 Identification of additional interactions with similar ROCK regulators may highlight mechanisms that modulate ROCK activity locally without global effects on this signaling pathway.
Expression, Localization, and Downstream Targets
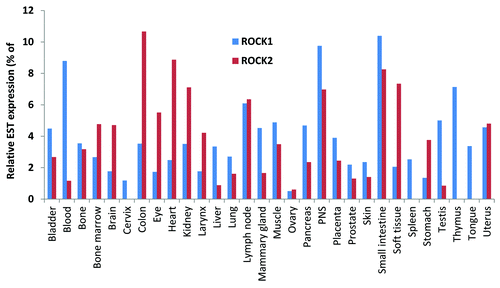
ROCK proteins were initially reported to be ubiquitously expressed throughout embryogenesis and in adult tissues.Citation4,Citation5 Analysis of ROCK1 and ROCK2 expressed sequence tag (EST) distribution using the Tissue-specific Gene Expression and Regulation (TiGer) databaseCitation39 revealed that their distribution patterns were similar and that there were few specific organ and/or tissues with expression levels that were dramatically higher than another (). Notably, there was significant ROCK1 expression in the thymus and blood, with little to no ROCK2 expression detected.
Figure 3. Tissue distribution of ROCK1 and ROCK2 determined from expressed sequence tags (EST). Relative expression levels were derived from the Tissue-specific Gene Expression and Regulation (TiGer) database (http://bioinfo.wilmer.jhu.edu/tiger). The expression levels were normalized with tissue-library size. Each value for a gene in a tissue is a ratio of observed ESTs to the expected one in this tissue. The expected number of ESTs is the product of total ESTs of the gene and the fraction of total ESTs in the tissue among all ESTs in 30 tissues. To depict tissue expression profiles, the normalized expression levels were graphed as percentages from only those tissues having values > 0.
The biological actions of kinases do not solely reflect activation status, but are also influenced by the proteins’ subcellular localization. Several studies examined the subcellular localization of the two ROCK isoforms. Early studies on ROCK2 localization reported a predominantly cytosolic distribution.Citation1,Citation3 Overexpression of an active RhoA mutant resulted in the recruitment of ROCK2 to both internal and peripheral cell membranes, where it was observed to be largely associated with actin microfilaments.Citation1,Citation40 Further studies on ROCK2 subcellular distribution revealed accumulation at the cleavage furrow, implicating its role in the formation of contractile ring during cytokinesis.Citation41,Citation42 In addition, ROCK2 has been reported to localize in the nucleus of growing cells,Citation43 stress fibers,Citation44,Citation45 and filamentous vimentin network in serum-starved cellsCitation46 as well as to the centrosomes.Citation47 Data from two independent antibodies reported by the Human Protein Atlas (www.proteinatlas.org) support a largely cytoplasmic localization for ROCK2. Subcellular localization of ROCK1 has not been as well as characterized, although data from three antibodies show a predominantly cytoplasmic expression in many cell types (www.proteinatlas.org). Several lines of evidence also point to ROCK1 association with the plasma membraneCitation48,Citation49 and centrosomes.Citation50 Interestingly, Shroom, a key player in apical cell constriction in neurulation, binds to ROCK2 and regulates its distribution to apical cell junctions to achieve a localized activation.Citation51,Citation52
Activated ROCK proteins phosphorylate a divergent group of downstream targets involved in many biological processes, and given the high similarity in their kinase domains it seems likely that the two isoform would phosphorylate numerous common substrates. From the several studies aimed at identifying ROCK substrates, a consensus amino acid motif for phosphorylation in most cases was found to be R/KXXS/T or R/KXS/T (R, Arginine; K, Lysine; S, Serine; T, Threonine; X, any amino acid), although some non-canonical sites have also been identified by peptide phosphorylation screening.Citation53 ROCK signaling has been implicated in various cellular functions downstream of Rho; one of the primary roles defined for ROCK is the regulation of actin-myosin cytoskeletal organization. Phosphorylated myosin II regulatory light chains (MLC) promote actomyosin contractility by activating myosin ATPase, thus enabling its interaction with F-actin to generate a contractile force.Citation54 ROCK phosphorylation of MLC at Ser-19 and Thr-18 occurs at the same sites that are phosphorylated by MLC kinase.Citation55 However, depletion of Ca2+ to block activation of MLC kinase (MLCK) resulted in decreased MLC phosphorylation in response to G-protein activation in airway smooth muscle cells, indicating that ROCK may largely contribute to elevating MLC phosphorylation indirectly by inhibiting dephosphorylation.Citation56,Citation57 Moreover, MLC phosphorylation to induce stress fiber and focal adhesion assembly is spatially regulated by MLCK, MRCK, and ROCK, where ROCK is involved in the formation of stress fibers in the center of fibroblasts, while MLCK and MRCK function at the periphery.Citation58,Citation59 MLC dephosphorylation is mediated by a phosphatase complex (PP1M) that is composed of a PP1cδ catalytic subunit, the myosin binding subunit MYPT1 that modulates the targeting of myosin to the phosphatase, and a small regulatory subunit M20.Citation60 Phosphorylation of MYPT1 by ROCK on Thr-695 and Thr-850 leads to the inhibition of MLC dephosphorylation by dissociating myosin from the phosphatase, thereby allowing increased net MLC phosphorylation and subsequent activation of myosin ATPase.Citation61-Citation64 In addition, ROCK kinases also regulate actin filament dynamics by phosphorylating LIM kinases 1 and 2Citation65 on activation loop Thr-508Citation66 and Thr-505Citation44,Citation67 respectively. Activated LIM kinases phosphorylate and inactivate the actin severing protein cofilin, resulting in a net increase in the number of actin filaments within cells.Citation68,Citation69 Collectively, ROCK activation leads to a concerted series of events that promote actin-myosin mediated contractile force generation and consequent morphological changes.
ROCK Functions
At the time that the two kinases were identified nearly 20 years ago, attention was focused on their roles in the regulation of the actin-myosin cytoskeleton. Since then the biological roles of ROCK have been studied extensively in many different contexts. Although not exhaustive, major functions will be discussed below.
Actin organization: Formation of stress fibers and focal adhesion complexes
Stress fibers are contractile apparatuses in cells that are composed of bundles of F-actin and myosin II which are especially prominent in cultured cells, but have also been shown to be manifested in cells in vivo.Citation70,Citation71 These actin-myosin fibers are linked to discrete points on the inner plasma membrane called focal adhesions, where dynamic protein complexes that contribute to cell adhesion to the extracellular matrix are localized.Citation72 Formation of stress fibers and focal adhesion complexes by MLC phosphorylation was one of the first functions identified for ROCK.Citation73 Expression of constitutively active ROCK consistently induced the formation of stress fibers and focal adhesions, whereas kinase dead and the N-terminal truncated ROCK mutants induced disassembly of stress fibers and focal adhesion complexes, accompanied by cell spreading.Citation4,Citation18,Citation73 Although both ROCK isoforms appear to contribute to focal adhesion formation and microfilament bundling, siRNA knockdown experiments have revealed some differences in their functions. In fibroblasts, ROCK1 and ROCK2 play distinct roles in the subcellular sites of MLC phosphorylation and in the assembly of fibronectin matrices at the cell surface during actin cytoskeleton mediated extracellular matrix assembly.Citation74,Citation75 Knockdown of ROCK1 in keratinocytes blocked focal adhesion maturation, which decreased cell adhesion to fibronectin, while ROCK2 knockdown reduced turnover, leading to the formation of large focal adhesions with increased stability that promoted fibronectin adhesion.Citation76,Citation77 Using mouse embryo fibroblasts (MEFs) deleted for ROCK1 or ROCK2, it was observed that ROCK1 regulates peripheral actomyosin ring formation through MLC phosphorylation and ROCK2 stabilizes the cytoskeleton through cofilin phosphorylation following treatment with the chemotherapeutic drug doxorubicin.Citation78 These results indicate that ROCK1 and ROCK2 may have different functions in cells, likely due to differences in the way that there are regulated and subtle differences in localization that result from non-overlapping patterns of protein binding.
The biological consequences of increased ROCK signaling do not fully recapitulate the effects of Rho activation, indicating a requirement for additional Rho effector proteins to work cooperatively with ROCK. This has been well demonstrated in the context of stress fiber assembly where ROCK induced stress fibers are thicker than those induced by RhoA.Citation4,Citation18,Citation73 Rho activates one of its effectors mDia1 (mammalian homolog of Drosophila Diaphanous), which interacts with profilin, thus transforming the condensed stress fibers to thinner actin fibers, which remains disorganized in the absence of ROCK activity.Citation79 These studies indicate the necessity for mDia to act in concert with ROCK kinases following Rho activation in stress fiber assembly. In addition, the ability of Rho to promote adherens junctions stability is via mDia1, which antagonizes the destabilizing effects of ROCK-mediated actomyosin contractility, consistent with the concerted actions of these two pathways being necessary to transduce Rho signaling.
Apoptosis
The role of ROCK in regulating the morphological events during the execution phase of apoptosis is well recognized.Citation80 During this phase, typically cells rapidly shrink due to actin-myosin contractile force, which provides the power that drives dynamic membrane blebbing, nuclear disintegration, and fragmentation of apoptotic cells into apoptotic bodies. These apoptotic bodies package the cellular and nuclear fragments that will be recognized and phagocytosed by neighboring or specialized cells. Earlier studies revealed a role for caspase in membrane blebbing and apoptotic body formationCitation81 and several studies implicated the importance of actin-myosin cytoskeleton remodeling and MLC phosphorylation for these processes.Citation82-Citation85 It was subsequently determined that during the apoptosis execution phase, caspase-3 cleaves ROCK1 to remove the auto-inhibitory C-terminal domain, which results in constitutive ROCK1 activation and subsequent induction of plasma membrane blebbing through MLC phosphorylation and ensuing contractile force generation.Citation19,Citation20 The importance of ROCK1 for apoptotic blebbing has been shown in numerous additional cell types, including cardiac myocytes, lymphoma cells, and non-small cell lung carcinoma cells.Citation86-Citation88 ROCK1 cleavage also facilitates redistribution of fragmented DNA into blebs and apoptotic bodiesCitation19 as well as disruption of nuclear integrity.Citation22 These two events also contribute to the leaking of damage associated molecular pattern (DAMP) proteins, such as nucleosomal histones, during the early stages of rapid membrane blebbing and apoptotic body release prior to secondary necrosis.Citation23 In addition, ROCK mediated actomyosin contraction has been reported to be necessary for two events during the execution phase of apoptosis, namely the externalization of N-acetyl glucosamine, a phagocytic marker and fragmentation of the Golgi apparatus.Citation89 In the specialized case of cell death induced by natural killer cells, Granzyme B cleaves ROCK2 at an analogous position to the caspase-cleavage site on ROCK1, leading to constitutive ROCK2 activation that is sufficient to promote caspase-independent membrane blebbing.Citation21 However, given that Granzyme B also activates caspases leading to ROCK1 activation, there are no obvious situations where ROCK2 would be activated without concomitant ROCK1 activation in apoptotic cells.
Although the role of ROCK1 as a critical effector of morphological changes during the execution phase of apoptosis is well established, ROCK activity has generally been found not to be required for the initiation and propagation of the apoptotic program. For example, inhibition of ROCK activity does not affect caspase activation or cytochrome c release in response to several forms of apoptotic stimuli.Citation19,Citation20,Citation23 However, recent studies have revealed that the precise role of ROCK in apoptosis is highly dependent on cell type and form of apoptotic stimuli. For example, inhibition of ROCK activity resulted in disruption of actin stress fibers, leading to apoptosis in airway epithelial cells but not in NIH3T3 fibroblasts.Citation90 Similarly, ROCK inhibition resulted in death in a variety of other cell types including hepatic stellate cells,Citation91 glioma cells,Citation92 pancreatic stellate cells,Citation93 and airway smooth muscle cells,Citation94 indicating that ROCK activity contributes to cell survival in specific contexts. Interestingly, screens for small molecular weight inhibitors that promoted human embryonic stem cell survival identified the ROCK inhibitor Y27632 as the most effective compound.Citation95,Citation96 Since these initial findings, other ROCK inhibitors have been determined to have similar protective effects on stem cells from additional tissue origins and species.Citation97 So effective is ROCK inhibition at promoting stem cell survival that the inclusion of compounds such as Y27632 has become part of standard stem cell culture protocols. Protective effects of ROCK inhibition on cell survival have also been demonstrated in a variety of animal models. Treatment with ROCK inhibitor Y-27632 significantly reduced cardiomyocyte apoptosis during acute myocardial ischemia and/or reperfusion injury,Citation98 while the ROCK inhibitor fasudil reduced lipopolysaccharide induced hepatocellular apoptosis.Citation99 The conclusion that can be made from these studies is that ROCK may be pro-apoptotic or anti-apoptotic depending on intrinsic properties of the cell and external conditions. Recent studies have highlighted a role for ROCK1 as a regulator of the crosstalk between apoptosis and autophagy. ROCK1 phosphorylates Beclin-1 to induce autophagy under stress conditions and has also been shown to regulate the size of autophagosomes.Citation100,Citation101 Because of these dual roles in regulating survival and/or apoptosis, ROCK expression levels and associated regulators of actin-myosin contractility would be expected to have the potential to be increased or decreased in different cancers. Consistent with this possibility, LIMK2, which is a downstream effector of ROCK signaling, was found to be significantly and progressively downregulated in human colorectal cancer as a result of promoter methylation.Citation102
ROCK in development
ROCK1 and ROCK2 have been reported to have overlapping expression patterns in developing embryos and are highly enriched in the cardiac mesoderm, lateral plate mesoderm, and the neural plate in chick and mouse embryos where they play vital roles in various embryonic morphogenetic events, including cell migration, differentiation, and axis formation.Citation103 Many experiments that sought to elucidate the roles of ROCK signaling in development have been performed with small molecule inhibitors that block both kinases with equal potencyCitation104; therefore these studies do not provide any information on the specific functions of each ROCK protein. Genetic deletion of ROCK1 or ROCK2 in mice made it possible to dissect the roles of these proteins in development. A consistent abnormality in ROCK1 and ROCK2 knockout mice on a C57BL/6N strain background is failure of closure of eyelids and ventral body wall giving rise to eyes open at birth and omphalocele phenotypes.Citation105,Citation106 Formation of organized actomyosin cables in the eyelid epithelial sheets and the actomyosin-mediated closure of umbilical rings were impaired in both knockout models.Citation105,Citation106 Despite the two isoforms having highly conserved kinase domains, they apparently cannot compensate for each other in these tissues. ROCK1 and ROCK2 double heterozygous mice also displayed the same phenotype, albeit at a lower frequency, suggesting that the observed phenotypes are the result of insufficient gene dosage with both isoforms cooperatively regulating the movement of epithelial sheets for eyelid and ventral body wall closure.Citation106 Yet, ROCK1 or ROCK2 knockout mice and double heterozygous mice that survived continued to develop normally, and were fertile and apparently healthy, suggesting that ROCK1 and ROCK2 are particularly important during development when cell movement is required for the formation of tissues and organs.Citation105,Citation106
Interestingly, ROCK2 deletion on a mixed genetic background of C57BL/6N and 129/Sv strains exhibited placental dysfunction, intrauterine growth retardation, and 90% embryonic death in utero.Citation107 On the same C57BL/6N strain background, the survival rate of ROCK2 knockouts was less than was observed for ROCK1 deletion, due to an additional defect in the placental labyrinth layer.Citation106 Similarly, differences in strain background also had an effect in ROCK1 knockouts, as the eyelids open at birth and omphalocele phenotypes were not observed in mice with FVB strain backgrounds.Citation108,Citation109 These studies indicate that strain differences, and by inference additional genetic modifiers that have yet to be identified, affect the manifestation of these phenotypes in ROCK1 or ROCK2 deleted mice. The lack of phenotypes in other tissues suggests that both homologs can functionally compensate for each other during development or that there is a lower requirement for total ROCK protein. However, neural crest specific expression of dominant negative form of ROCK resulting in severe craniofacial defects was reported recently.Citation110 The different phenotypes resulting from the deletion of one or the other ROCK isoform strongly suggests that the two genes have non-redundant roles. The development of tissue-specific conditional knockouts for ROCK1 and ROCK2 singly or in combination will shed more light on the specific functions of each protein in development, adult tissue homeostasis, and pathophysiological conditions such as cancer.
Cell proliferation and cytokinesis
Numerous studies have highlighted the importance of ROCK in the regulation of cell proliferation, which may be due to a role in mediating cytoskeletal tension or may be mediated by cytoskeleton-independent pathways. In accordance with this, ROCK inhibitors have been shown to have antiproliferative effects in some cell types including airway and prostatic smooth muscle cells and cardiac myocytes.Citation94,Citation111,Citation112 Conversely, active ROCK can induce proliferation in some cell types including fibroblasts, in which modulation of the levels of specific cell cycle regulators appears to play an important role.Citation113 The influence of ROCK activity on the levels of cell cycle regulatory proteins also has been implicated in the proliferation of corneal epithelial cellsCitation114 and gastric cells.Citation115
Although ROCK activity may promote proliferation in some cells types, in other contexts ROCK appears to have anti-proliferative functions. For example, inhibition of ROCK activity in human keratinocytes resulted in increased proliferation and decreased terminal differentiation, while conditional ROCK2 activation had the opposite effects, suggesting a role for ROCK in the regulation of keratinocyte fate.Citation116
During cytokinesis, cells undergo dramatic reorganization of the cytoskeleton and are divided in two daughters through the actions of the actin-myosin rich contractile ring. Shortly after the identification of ROCK kinases, it was observed that dominant negative ROCK2 inhibited cleavage furrow formation in Xenopus embryos and in mammalian cells, resulting in multinucleation.Citation117 Further studies revealed the accumulation of ROCK2 at the cleavage furrow during cytokinesis, where it was an important contributor to MLC phosphorylation.Citation41,Citation118 However, although ROCK2 inhibition did not completely arrest cytokinesis, it did result in prolonged cleavage furrow ingression, suggesting that ROCK2 makes indispensable contributions to the normal progression of cytokinesis.Citation118 Interestingly, ROCK kinases do not appear to be responsible for the phosphorylation of ezrin/radixin/moesin (ERM) proteins localized at the cleavage furrow, suggesting that there must be other cleavage furrow kinases that may play complementary and/or redundant roles in cytokinesis.Citation118,Citation119 Taken together, ROCK may have a general positive role as a promoter of cell proliferation in many cell types, with some exceptions in specialized contexts.
Therapeutic Implications: Insights from Genetically Modified Mouse Models
There is growing evidence that abnormal ROCK activity contributes to a variety of pathological conditions. As a result, the development of ROCK inhibitors has gained considerable interest in the pharmaceutical industry.Citation120 Currently, inhibitors that are widely used for studies in various disease models, such as Y-27632, H1152P, and fasudil, are non-isoform selective ROCK inhibitors that target the ATP-dependent kinase domain. The extensive potential therapeutic uses for ROCK inhibitors have been reviewed elsewhere.Citation97,Citation121 Fasudil is currently the only ROCK inhibitor approved for human use, which has been used in Japan since 1995 for treatment of cerebral vasospasm following subarachnoid hemorrhage.Citation97
Pharmacological inhibitor studies have contributed greatly to our understanding of ROCK biology. Although there is increasing evidence of distinct functions for each ROCK isoform, ROCK inhibitors typically are not isoform selective, likely due to the high degree of homology between the kinase domains. Therefore, it is difficult to attribute specific functions to either of the two ROCK isoforms based on inhibitor studies. These inhibitors may also have possible off-target effects since at higher concentrations they may also inhibit other serine/threonine kinases such as PKA and PKC.Citation122 Hence there is a need for ROCK1 and ROCK2 isoform specific genetically-modified (GM) mouse models to understand the individual functions of each protein and the interplay between them in the context of various pathological conditions.
Extensive studies using ROCK inhibitors have shown that ROCK signaling plays important roles in cardiac hypertrophy and subsequent development of cardiac fibrosis.Citation123,Citation124 In contrast to these studies, haploinsufficiency or targeted deletion of the ROCK1 gene did not prevent cardiac hypertrophy induced by angiotensin or pressure overload.Citation125,Citation126 However, it was recently reported that cardiac specific ROCK2 deletion prevented angiotensin induced hypertrophy, suggesting that ROCK2 and not ROCK1 was an important mediator of the hypertrophic process.Citation127 Despite their apparent isoform specific roles in cardiac hypertrophy, both targeted ROCK1 and ROCK2 mouse models exhibited decreased cardiac fibrosis and cardiomyocyte apoptosis that occurs in response to pathological hypertrophy.Citation86,Citation125-Citation127 Hence, these genetic models helped in identifying isoform specific roles in cardiac hypertrophy leading to heart failure. It is worth noting that pharmacological inhibition of ROCK activity conferred a protective effect against renal fibrosis as well.Citation128,Citation129 However, ROCK1 deletion did not prevent renal fibrosis in a mouse model of obstructive kidney disease.Citation130 This may imply that both ROCK1 and ROCK2 are required for the protective effect or it might be a case of off-target inhibition of other proteins by ROCK inhibitors.
Along with its well-established role in cardiovascular biology, ROCK proteins have been implicated in other disease pathologies including insulin resistance. Insulin signaling is essential for glucose uptake and metabolism. Targeting the ROCK pathway using inhibitors like fasudil and Y-27632 reduced blood pressure and enhanced glucose tolerance in obese rats, suggesting that ROCK kinases were responsible for impairment of insulin signaling.Citation131 However, ROCK was shown as a positive regulator of glucose metabolism in normal mice since treatment with Y-27632 caused insulin resistance by reducing insulin mediated glucose uptake in skeletal muscle.Citation132 In support of this data, mice with ROCK1 deletion caused insulin resistance in skeletal muscle.Citation109 Interestingly, it was recently shown that adipose specific ROCK1 deletion in mice resulted in enhanced insulin signaling, suggesting that ROCK1 deletion had a protective effect against insulin resistance in this tissue.Citation133 These data suggests that there are tissue or cell type specific roles for ROCK1 in regulating glucose metabolism. Further genetic studies are required to determine the contribution of the ROCK2 isoform in insulin signaling and glucose homeostasis. The number of complete ROCK1, or tissue-specific ROCK1 or ROCK2 GM models in various disease contexts has been increasing over the recent years and this has been summarized in . However, the incidence of embryonic and perinatal lethality observed in ROCK2 knockout mice has impeded extensive investigation of this isoform.Citation106,Citation107 Overall, these genetic models have highlighted the need for inhibitors that could specifically target ROCK1 or ROCK2. Efforts to develop isoform-specific ROCK inhibitors are underway, with the ROCK2 selective inhibitor SLx-2119 already showing promise in cancer xenograft models.Citation134
Table 1. ROCK1 and ROCK2 Genetically Modified Mouse Models
Additionally, refined mouse models that express conditionally active ROCK in a tissue selective manner have made it possible to examine how ROCK activation may contribute to disease initiation and progression.Citation135 For example, conditionally-active ROCK2 expressed in mouse keratinocytes elevated collagen deposition that increased tissue stiffness, which in turn resulted in activation of β-catenin transcriptional activity that promoted interfollicular basal keratinocyte hyperproliferation and skin thickening.Citation136 Future experiments with tissue-specific expression of conditionally-active ROCK proteins will help characterize how elevated signaling through this pathway contributes to disease pathogenesis.
Conclusion and Future Directions
Since their discovery, studies have identified numerous ROCK substrates involved in diverse cellular processes. It has become increasingly evident that the two ROCK homologs have common as well as non-redundant functions, and that their downstream signaling may lead to different effects depending on several factors including cell type and microenvironmental factors. Conditional and tissue specific deletion of ROCK1 or ROCK2 will provide further insights into the distinct or shared functions of each protein. ROCK inhibitors like fasudil are already in use or in clinical trials for a number of pathological conditions including cerebral vasospasm, hypertension, atherosclerosis, and aortic stiffness. Given the accumulating evidence of the potential roles of ROCK in additional pathologies such as cancer, it seems rational to direct future studies toward unraveling the tissue specific functions of each homolog. These studies will help determine whether it would be advantageous to develop ROCK inhibitors with greater selectivity toward one or the other protein, such as the ROCK2 selective inhibitor SLx-2119.Citation137
Disclosure of Potential Conflicts of Interest
No potential conflict of interest was disclosed.
Acknowledgments
This work was supported by Cancer Research UK.
References
- Leung T, Manser E, Tan L, Lim L. A novel serine/threonine kinase binding the Ras-related RhoA GTPase which translocates the kinase to peripheral membranes. J Biol Chem 1995; 270:29051 - 4; http://dx.doi.org/10.1074/jbc.270.49.29051; PMID: 7493923
- Ishizaki T, Maekawa M, Fujisawa K, Okawa K, Iwamatsu A, Fujita A, Watanabe N, Saito Y, Kakizuka A, Morii N, et al. The small GTP-binding protein Rho binds to and activates a 160 kDa Ser/Thr protein kinase homologous to myotonic dystrophy kinase. EMBO J 1996; 15:1885 - 93; PMID: 8617235
- Matsui T, Amano M, Yamamoto T, Chihara K, Nakafuku M, Ito M, Nakano T, Okawa K, Iwamatsu A, Kaibuchi K. Rho-associated kinase, a novel serine/threonine kinase, as a putative target for small GTP binding protein Rho. EMBO J 1996; 15:2208 - 16; PMID: 8641286
- Leung T, Chen XQ, Manser E, Lim L. The p160 RhoA-binding kinase ROK alpha is a member of a kinase family and is involved in the reorganization of the cytoskeleton. Mol Cell Biol 1996; 16:5313 - 27; PMID: 8816443
- Nakagawa O, Fujisawa K, Ishizaki T, Saito Y, Nakao K, Narumiya S. ROCK-I and ROCK-II, two isoforms of Rho-associated coiled-coil forming protein serine/threonine kinase in mice. FEBS Lett 1996; 392:189 - 93; http://dx.doi.org/10.1016/0014-5793(96)00811-3; PMID: 8772201
- Schackmann RCJ, van Amersfoort M, Haarhuis JHI, Vlug EJ, Halim VA, Roodhart JML, Vermaat JS, Voest EE, van der Groep P, van Diest PJ, et al. Cytosolic p120-catenin regulates growth of metastatic lobular carcinoma through Rock1-mediated anoikis resistance. J Clin Invest 2011; 121:3176 - 88; http://dx.doi.org/10.1172/JCI41695; PMID: 21747168
- Pelosi M, Marampon F, Zani BM, Prudente S, Perlas E, Caputo V, Cianetti L, Berno V, Narumiya S, Kang SW, et al. ROCK2 and its alternatively spliced isoform ROCK2m positively control the maturation of the myogenic program. Mol Cell Biol 2007; 27:6163 - 76; http://dx.doi.org/10.1128/MCB.01735-06; PMID: 17606625
- Jacobs M, Hayakawa K, Swenson L, Bellon S, Fleming M, Taslimi P, Doran J. The structure of dimeric ROCK I reveals the mechanism for ligand selectivity. J Biol Chem 2006; 281:260 - 8; http://dx.doi.org/10.1074/jbc.M508847200; PMID: 16249185
- Yamaguchi H, Kasa M, Amano M, Kaibuchi K, Hakoshima T. Molecular mechanism for the regulation of rho-kinase by dimerization and its inhibition by fasudil. Structure 2006; 14:589 - 600; http://dx.doi.org/10.1016/j.str.2005.11.024; PMID: 16531242
- Heikkila T, Wheatley E, Crighton D, Schroder E, Boakes A, Kaye SJ, Mezna M, Pang L, Rushbrooke M, Turnbull A, et al. Co-crystal structures of inhibitors with MRCKβ, a key regulator of tumor cell invasion. PLoS One 2011; 6:e24825; http://dx.doi.org/10.1371/journal.pone.0024825; PMID: 21949762
- Elkins JM, Amos A, Niesen FH, Pike AC, Fedorov O, Knapp S. Structure of dystrophia myotonica protein kinase. Protein Sci 2009; 18:782 - 91; http://dx.doi.org/10.1002/pro.82; PMID: 19309729
- Samuel MS, Olson MF. Dying alone: a tale of rho. Cell Stem Cell 2010; 7:135 - 6; http://dx.doi.org/10.1016/j.stem.2010.07.002; PMID: 20682437
- Wen W, Liu W, Yan J, Zhang M. Structure basis and unconventional lipid membrane binding properties of the PH-C1 tandem of rho kinases. J Biol Chem 2008; 283:26263 - 73; http://dx.doi.org/10.1074/jbc.M803417200; PMID: 18640982
- Shimizu T, Ihara K, Maesaki R, Amano M, Kaibuchi K, Hakoshima T. Parallel coiled-coil association of the RhoA-binding domain in Rho-kinase. J Biol Chem 2003; 278:46046 - 51; http://dx.doi.org/10.1074/jbc.M306458200; PMID: 12954645
- Dvorsky R, Blumenstein L, Vetter IR, Ahmadian MR. Structural insights into the interaction of ROCKI with the switch regions of RhoA. J Biol Chem 2004; 279:7098 - 104; http://dx.doi.org/10.1074/jbc.M311911200; PMID: 14660612
- Blumenstein L, Ahmadian MR. Models of the cooperative mechanism for Rho effector recognition: implications for RhoA-mediated effector activation. J Biol Chem 2004; 279:53419 - 26; http://dx.doi.org/10.1074/jbc.M409551200; PMID: 15475352
- Amano M, Chihara K, Nakamura N, Kaneko T, Matsuura Y, Kaibuchi K. The COOH terminus of Rho-kinase negatively regulates rho-kinase activity. J Biol Chem 1999; 274:32418 - 24; http://dx.doi.org/10.1074/jbc.274.45.32418; PMID: 10542285
- Ishizaki T, Naito M, Fujisawa K, Maekawa M, Watanabe N, Saito Y, Narumiya S. p160ROCK, a Rho-associated coiled-coil forming protein kinase, works downstream of Rho and induces focal adhesions. FEBS Lett 1997; 404:118 - 24; http://dx.doi.org/10.1016/S0014-5793(97)00107-5; PMID: 9119047
- Coleman ML, Sahai EA, Yeo M, Bosch M, Dewar A, Olson MF. Membrane blebbing during apoptosis results from caspase-mediated activation of ROCK I. Nat Cell Biol 2001; 3:339 - 45; http://dx.doi.org/10.1038/35070009; PMID: 11283606
- Sebbagh M, Renvoizé C, Hamelin J, Riché N, Bertoglio J, Bréard J. Caspase-3-mediated cleavage of ROCK I induces MLC phosphorylation and apoptotic membrane blebbing. Nat Cell Biol 2001; 3:346 - 52; http://dx.doi.org/10.1038/35070019; PMID: 11283607
- Sebbagh M, Hamelin J, Bertoglio J, Solary E, Bréard J. Direct cleavage of ROCK II by granzyme B induces target cell membrane blebbing in a caspase-independent manner. J Exp Med 2005; 201:465 - 71; http://dx.doi.org/10.1084/jem.20031877; PMID: 15699075
- Croft DR, Coleman ML, Li S, Robertson D, Sullivan T, Stewart CL, Olson MF. Actin-myosin-based contraction is responsible for apoptotic nuclear disintegration. J Cell Biol 2005; 168:245 - 55; http://dx.doi.org/10.1083/jcb.200409049; PMID: 15657395
- Wickman GR, Julian L, Mardilovich K, Schumacher S, Munro J, Rath N, Zander SA, Mleczak A, Sumpton D, Morrice N, et al. Blebs produced by actin-myosin contraction during apoptosis release damage-associated molecular pattern proteins before secondary necrosis occurs. Cell Death Differ 2013; 20:1293 - 305; http://dx.doi.org/10.1038/cdd.2013.69; PMID: 23787996
- Sapet C, Simoncini S, Loriod B, Puthier D, Sampol J, Nguyen C, Dignat-George F, Anfosso F. Thrombin-induced endothelial microparticle generation: identification of a novel pathway involving ROCK-II activation by caspase-2. Blood 2006; 108:1868 - 76; http://dx.doi.org/10.1182/blood-2006-04-014175; PMID: 16720831
- Lowery DM, Clauser KR, Hjerrild M, Lim D, Alexander J, Kishi K, Ong SE, Gammeltoft S, Carr SA, Yaffe MB. Proteomic screen defines the Polo-box domain interactome and identifies Rock2 as a Plk1 substrate. EMBO J 2007; 26:2262 - 73; http://dx.doi.org/10.1038/sj.emboj.7601683; PMID: 17446864
- Sinning JM, Losch J, Walenta K, Böhm M, Nickenig G, Werner N. Circulating CD31+/Annexin V+ microparticles correlate with cardiovascular outcomes. Eur Heart J 2011; 32:2034 - 41; http://dx.doi.org/10.1093/eurheartj/ehq478; PMID: 21186238
- Lee HH, Chang ZF. Regulation of RhoA-dependent ROCKII activation by Shp2. J Cell Biol 2008; 181:999 - 1012; http://dx.doi.org/10.1083/jcb.200710187; PMID: 18559669
- Rautou PE, Vion AC, Amabile N, Chironi G, Simon A, Tedgui A, Boulanger CM. Microparticles, vascular function, and atherothrombosis. Circ Res 2011; 109:593 - 606; http://dx.doi.org/10.1161/CIRCRESAHA.110.233163; PMID: 21852557
- Chuang HH, Liang SW, Chang ZF, Lee HH. Ser1333 phosphorylation indicates ROCKI activation. J Biomed Sci 2013; 20:83; http://dx.doi.org/10.1186/1423-0127-20-83; PMID: 24168723
- Ishiuchi T, Takeichi M. Willin and Par3 cooperatively regulate epithelial apical constriction through aPKC-mediated ROCK phosphorylation. Nat Cell Biol 2011; 13:860 - 6; http://dx.doi.org/10.1038/ncb2274; PMID: 21685893
- Ward Y, Yap SF, Ravichandran V, Matsumura F, Ito M, Spinelli B, Kelly K. The GTP binding proteins Gem and Rad are negative regulators of the Rho-Rho kinase pathway. J Cell Biol 2002; 157:291 - 302; http://dx.doi.org/10.1083/jcb.200111026; PMID: 11956230
- Guasch RM, Scambler P, Jones GE, Ridley AJ. RhoE regulates actin cytoskeleton organization and cell migration. Mol Cell Biol 1998; 18:4761 - 71; PMID: 9671486
- Nobes CD, Lauritzen I, Mattei MG, Paris S, Hall A, Chardin P. A new member of the Rho family, Rnd1, promotes disassembly of actin filament structures and loss of cell adhesion. J Cell Biol 1998; 141:187 - 97; http://dx.doi.org/10.1083/jcb.141.1.187; PMID: 9531558
- Riento K, Guasch RM, Garg R, Jin B, Ridley AJ. RhoE binds to ROCK I and inhibits downstream signaling. Mol Cell Biol 2003; 23:4219 - 29; http://dx.doi.org/10.1128/MCB.23.12.4219-4229.2003; PMID: 12773565
- Riento K, Ridley AJ. Rocks: multifunctional kinases in cell behaviour. Nat Rev Mol Cell Biol 2003; 4:446 - 56; http://dx.doi.org/10.1038/nrm1128; PMID: 12778124
- Riento K, Totty N, Villalonga P, Garg R, Guasch R, Ridley AJ. RhoE function is regulated by ROCK I-mediated phosphorylation. EMBO J 2005; 24:1170 - 80; http://dx.doi.org/10.1038/sj.emboj.7600612; PMID: 15775972
- Pinner S, Sahai E. PDK1 regulates cancer cell motility by antagonising inhibition of ROCK1 by RhoE. Nat Cell Biol 2008; 10:127 - 37; http://dx.doi.org/10.1038/ncb1675; PMID: 18204440
- Rana MK, Worthylake RA. Novel mechanism for negatively regulating Rho-kinase (ROCK) signaling through Coronin1B protein in neuregulin 1 (NRG-1)-induced tumor cell motility. J Biol Chem 2012; 287:21836 - 45; http://dx.doi.org/10.1074/jbc.M112.346114; PMID: 22563075
- Liu X, Yu X, Zack DJ, Zhu H, Qian J. TiGER: a database for tissue-specific gene expression and regulation. BMC Bioinformatics 2008; 9:271; http://dx.doi.org/10.1186/1471-2105-9-271; PMID: 18541026
- Vandenabeele P, Galluzzi L, Vanden Berghe T, Kroemer G. Molecular mechanisms of necroptosis: an ordered cellular explosion. Nat Rev Mol Cell Biol 2010; 11:700 - 14; http://dx.doi.org/10.1038/nrm2970; PMID: 20823910
- Kosako H, Goto H, Yanagida M, Matsuzawa K, Fujita M, Tomono Y, Okigaki T, Odai H, Kaibuchi K, Inagaki M. Specific accumulation of Rho-associated kinase at the cleavage furrow during cytokinesis: cleavage furrow-specific phosphorylation of intermediate filaments. Oncogene 1999; 18:2783 - 8; http://dx.doi.org/10.1038/sj.onc.1202633; PMID: 10348354
- Inada H, Togashi H, Nakamura Y, Kaibuchi K, Nagata K, Inagaki M. Balance between activities of Rho kinase and type 1 protein phosphatase modulates turnover of phosphorylation and dynamics of desmin/vimentin filaments. J Biol Chem 1999; 274:34932 - 9; http://dx.doi.org/10.1074/jbc.274.49.34932; PMID: 10574968
- Tanaka T, Nishimura D, Wu RC, Amano M, Iso T, Kedes L, Nishida H, Kaibuchi K, Hamamori Y. Nuclear Rho kinase, ROCK2, targets p300 acetyltransferase. J Biol Chem 2006; 281:15320 - 9; http://dx.doi.org/10.1074/jbc.M510954200; PMID: 16574662
- Katoh K, Kano Y, Amano M, Onishi H, Kaibuchi K, Fujiwara K. Rho-kinase--mediated contraction of isolated stress fibers. J Cell Biol 2001; 153:569 - 84; http://dx.doi.org/10.1083/jcb.153.3.569; PMID: 11331307
- Kawabata S, Usukura J, Morone N, Ito M, Iwamatsu A, Kaibuchi K, Amano M. Interaction of Rho-kinase with myosin II at stress fibres. Genes Cells 2004; 9:653 - 60; http://dx.doi.org/10.1111/j.1356-9597.2004.00749.x; PMID: 15265008
- Sin WC, Chen XQ, Leung T, Lim L. RhoA-binding kinase alpha translocation is facilitated by the collapse of the vimentin intermediate filament network. Mol Cell Biol 1998; 18:6325 - 39; PMID: 9774649
- Ma Z, Kanai M, Kawamura K, Kaibuchi K, Ye K, Fukasawa K. Interaction between ROCK II and nucleophosmin/B23 in the regulation of centrosome duplication. Mol Cell Biol 2006; 26:9016 - 34; http://dx.doi.org/10.1128/MCB.01383-06; PMID: 17015463
- Glyn MC, Lawrenson JG, Ward BJ. A Rho-associated kinase mitigates reperfusion-induced change in the shape of cardiac capillary endothelial cells in situ. Cardiovasc Res 2003; 57:195 - 206; http://dx.doi.org/10.1016/S0008-6363(02)00616-8; PMID: 12504829
- Stroeken PJ, Alvarez B, Van Rheenen J, Wijnands YM, Geerts D, Jalink K, Roos E. Integrin cytoplasmic domain-associated protein-1 (ICAP-1) interacts with the ROCK-I kinase at the plasma membrane. J Cell Physiol 2006; 208:620 - 8; http://dx.doi.org/10.1002/jcp.20699; PMID: 16741948
- Chevrier V, Piel M, Collomb N, Saoudi Y, Frank R, Paintrand M, Narumiya S, Bornens M, Job D. The Rho-associated protein kinase p160ROCK is required for centrosome positioning. J Cell Biol 2002; 157:807 - 17; http://dx.doi.org/10.1083/jcb.200203034; PMID: 12034773
- Flynn PG, Helfman DM. Non-muscle myosin IIB helps mediate TNF cell death signaling independent of actomyosin contractility (AMC). J Cell Biochem 2010; 110:1365 - 75; http://dx.doi.org/10.1002/jcb.22653; PMID: 20564232
- Ehrenschwender M, Siegmund D, Wicovsky A, Kracht M, Dittrich-Breiholz O, Spindler V, Waschke J, Kalthoff H, Trauzold A, Wajant H. Mutant PIK3CA licenses TRAIL and CD95L to induce non-apoptotic caspase-8-mediated ROCK activation. Cell Death Differ 2010; 17:1435 - 47; http://dx.doi.org/10.1038/cdd.2010.36; PMID: 20379197
- Kang JH, Jiang Y, Toita R, Oishi J, Kawamura K, Han A, Mori T, Niidome T, Ishida M, Tatematsu K, et al. Phosphorylation of Rho-associated kinase (Rho-kinase/ROCK/ROK) substrates by protein kinases A and C. Biochimie 2007; 89:39 - 47; http://dx.doi.org/10.1016/j.biochi.2006.08.003; PMID: 16996192
- Vicente-Manzanares M, Ma X, Adelstein RS, Horwitz AR. Non-muscle myosin II takes centre stage in cell adhesion and migration. Nat Rev Mol Cell Biol 2009; 10:778 - 90; http://dx.doi.org/10.1038/nrm2786; PMID: 19851336
- Amano M, Ito M, Kimura K, Fukata Y, Chihara K, Nakano T, Matsuura Y, Kaibuchi K. Phosphorylation and activation of myosin by Rho-associated kinase (Rho-kinase). J Biol Chem 1996; 271:20246 - 9; http://dx.doi.org/10.1074/jbc.271.34.20246; PMID: 8702756
- Iizuka K, Yoshii A, Samizo K, Tsukagoshi H, Ishizuka T, Dobashi K, Nakazawa T, Mori M. A major role for the rho-associated coiled coil forming protein kinase in G-protein-mediated Ca2+ sensitization through inhibition of myosin phosphatase in rabbit trachea. Br J Pharmacol 1999; 128:925 - 33; http://dx.doi.org/10.1038/sj.bjp.0702864; PMID: 10556927
- Swärd K, Dreja K, Susnjar M, Hellstrand P, Hartshorne DJ, Walsh MP. Inhibition of Rho-associated kinase blocks agonist-induced Ca2+ sensitization of myosin phosphorylation and force in guinea-pig ileum. J Physiol 2000; 522:33 - 49; http://dx.doi.org/10.1111/j.1469-7793.2000.0033m.x; PMID: 10618150
- Totsukawa G, Yamakita Y, Yamashiro S, Hartshorne DJ, Sasaki Y, Matsumura F. Distinct roles of ROCK (Rho-kinase) and MLCK in spatial regulation of MLC phosphorylation for assembly of stress fibers and focal adhesions in 3T3 fibroblasts. J Cell Biol 2000; 150:797 - 806; http://dx.doi.org/10.1083/jcb.150.4.797; PMID: 10953004
- Ando K, Fukuhara S, Moriya T, Obara Y, Nakahata N, Mochizuki N. Rap1 potentiates endothelial cell junctions by spatially controlling myosin II activity and actin organization. J Cell Biol 2013; 202:901 - 16; http://dx.doi.org/10.1083/jcb.201301115; PMID: 24019534
- Hartshorne DJ, Hirano K. Interactions of protein phosphatase type 1, with a focus on myosin phosphatase. Mol Cell Biochem 1999; 190:79 - 84; http://dx.doi.org/10.1023/A:1006917032557; PMID: 10098973
- Kimura K, Ito M, Amano M, Chihara K, Fukata Y, Nakafuku M, Yamamori B, Feng J, Nakano T, Okawa K, et al. Regulation of myosin phosphatase by Rho and Rho-associated kinase (Rho-kinase). Science 1996; 273:245 - 8; http://dx.doi.org/10.1126/science.273.5272.245; PMID: 8662509
- Feng J, Ito M, Ichikawa K, Isaka N, Nishikawa M, Hartshorne DJ, Nakano T. Inhibitory phosphorylation site for Rho-associated kinase on smooth muscle myosin phosphatase. J Biol Chem 1999; 274:37385 - 90; http://dx.doi.org/10.1074/jbc.274.52.37385; PMID: 10601309
- Kawano Y, Fukata Y, Oshiro N, Amano M, Nakamura T, Ito M, Matsumura F, Inagaki M, Kaibuchi K. Phosphorylation of myosin-binding subunit (MBS) of myosin phosphatase by Rho-kinase in vivo. J Cell Biol 1999; 147:1023 - 38; http://dx.doi.org/10.1083/jcb.147.5.1023; PMID: 10579722
- Velasco G, Armstrong C, Morrice N, Frame S, Cohen P. Phosphorylation of the regulatory subunit of smooth muscle protein phosphatase 1M at Thr850 induces its dissociation from myosin. FEBS Lett 2002; 527:101 - 4; http://dx.doi.org/10.1016/S0014-5793(02)03175-7; PMID: 12220642
- Scott RW, Olson MF. LIM kinases: function, regulation and association with human disease. J Mol Med (Berl) 2007; 85:555 - 68; http://dx.doi.org/10.1007/s00109-007-0165-6; PMID: 17294230
- Ohashi K, Hosoya T, Takahashi K, Hing H, Mizuno K. A Drosophila homolog of LIM-kinase phosphorylates cofilin and induces actin cytoskeletal reorganization. Biochem Biophys Res Commun 2000; 276:1178 - 85; http://dx.doi.org/10.1006/bbrc.2000.3599; PMID: 11027607
- Sumi T, Matsumoto K, Nakamura T. Specific activation of LIM kinase 2 via phosphorylation of threonine 505 by ROCK, a Rho-dependent protein kinase. J Biol Chem 2001; 276:670 - 6; http://dx.doi.org/10.1074/jbc.M007074200; PMID: 11018042
- Arber S, Barbayannis FA, Hanser H, Schneider C, Stanyon CA, Bernard O, Caroni P. Regulation of actin dynamics through phosphorylation of cofilin by LIM-kinase. Nature 1998; 393:805 - 9; http://dx.doi.org/10.1038/31729; PMID: 9655397
- Adams PD, Sellers WR, Sharma SK, Wu AD, Nalin CM, Kaelin WG Jr.. Identification of a cyclin-cdk2 recognition motif present in substrates and p21-like cyclin-dependent kinase inhibitors. Mol Cell Biol 1996; 16:6623 - 33; PMID: 8943316
- Sugimoto K, Fujii S, Yamashita K. Expression of stress fibers in bullfrog mesothelial cells in response to tension. Exp Cell Res 1991; 196:353 - 61; http://dx.doi.org/10.1016/0014-4827(91)90271-U; PMID: 1893944
- Murakami T, Ishikawa H. Stress fibers in situ in proximal tubules of the rat kidney. Cell Struct Funct 1991; 16:231 - 40; http://dx.doi.org/10.1247/csf.16.231; PMID: 1913854
- Schiller HB, Fässler R. Mechanosensitivity and compositional dynamics of cell-matrix adhesions. EMBO Rep 2013; 14:509 - 19; http://dx.doi.org/10.1038/embor.2013.49; PMID: 23681438
- Amano M, Chihara K, Kimura K, Fukata Y, Nakamura N, Matsuura Y, Kaibuchi K. Formation of actin stress fibers and focal adhesions enhanced by Rho-kinase. Science 1997; 275:1308 - 11; http://dx.doi.org/10.1126/science.275.5304.1308; PMID: 9036856
- Yoneda A, Multhaupt HA, Couchman JR. The Rho kinases I and II regulate different aspects of myosin II activity. J Cell Biol 2005; 170:443 - 53; http://dx.doi.org/10.1083/jcb.200412043; PMID: 16043513
- Yoneda A, Ushakov D, Multhaupt HA, Couchman JR. Fibronectin matrix assembly requires distinct contributions from Rho kinases I and -II. Mol Biol Cell 2007; 18:66 - 75; http://dx.doi.org/10.1091/mbc.E06-08-0684; PMID: 17065553
- Surma M, Wei L, Shi J. Rho kinase as a therapeutic target in cardiovascular disease. Future Cardiol 2011; 7:657 - 71; http://dx.doi.org/10.2217/fca.11.51; PMID: 21929346
- Hohenberger P, Eing C, Straessner R, Durst S, Frey W, Nick P. Plant actin controls membrane permeability. Biochim Biophys Acta 2011; 1808:2304 - 12; http://dx.doi.org/10.1016/j.bbamem.2011.05.019; PMID: 21669183
- Shi J, Wu X, Surma M, Vemula S, Zhang L, Yang Y, Kapur R, Wei L. Distinct roles for ROCK1 and ROCK2 in the regulation of cell detachment. Cell Death Dis 2013; 4:e483; http://dx.doi.org/10.1038/cddis.2013.10; PMID: 23392171
- Watanabe N, Kato T, Fujita A, Ishizaki T, Narumiya S. Cooperation between mDia1 and ROCK in Rho-induced actin reorganization. Nat Cell Biol 1999; 1:136 - 43; http://dx.doi.org/10.1038/11056; PMID: 10559899
- Coleman ML, Olson MF. Rho GTPase signalling pathways in the morphological changes associated with apoptosis. Cell Death Differ 2002; 9:493 - 504; http://dx.doi.org/10.1038/sj.cdd.4400987; PMID: 11973608
- Zhang J, Reedy MC, Hannun YA, Obeid LM. Inhibition of caspases inhibits the release of apoptotic bodies: Bcl-2 inhibits the initiation of formation of apoptotic bodies in chemotherapeutic agent-induced apoptosis. J Cell Biol 1999; 145:99 - 108; http://dx.doi.org/10.1083/jcb.145.1.99; PMID: 10189371
- Mills JC, Stone NL, Erhardt J, Pittman RN. Apoptotic membrane blebbing is regulated by myosin light chain phosphorylation. J Cell Biol 1998; 140:627 - 36; http://dx.doi.org/10.1083/jcb.140.3.627; PMID: 9456322
- Mills JC, Stone NL, Pittman RN. Extranuclear apoptosis. The role of the cytoplasm in the execution phase. J Cell Biol 1999; 146:703 - 8; http://dx.doi.org/10.1083/jcb.146.4.703; PMID: 10459006
- Cotter TG, Lennon SV, Glynn JM, Green DR. Microfilament-disrupting agents prevent the formation of apoptotic bodies in tumor cells undergoing apoptosis. Cancer Res 1992; 52:997 - 1005; PMID: 1737363
- Suarez-Huerta N, Mosselmans R, Dumont JE, Robaye B. Actin depolymerization and polymerization are required during apoptosis in endothelial cells. J Cell Physiol 2000; 184:239 - 45; http://dx.doi.org/10.1002/1097-4652(200008)184:2<239::AID-JCP12>3.0.CO;2-R; PMID: 10867649
- Chang J, Xie M, Shah VR, Schneider MD, Entman ML, Wei L, Schwartz RJ. Activation of Rho-associated coiled-coil protein kinase 1 (ROCK-1) by caspase-3 cleavage plays an essential role in cardiac myocyte apoptosis. Proc Natl Acad Sci U S A 2006; 103:14495 - 500; http://dx.doi.org/10.1073/pnas.0601911103; PMID: 16983089
- Parent N, Sané AT, Droin N, Bertrand R. Procaspase-2S inhibits procaspase-3 processing and activation, preventing ROCK-1-mediated apoptotic blebbing and body formation in human B lymphoma Namalwa cells. Apoptosis 2005; 10:313 - 22; http://dx.doi.org/10.1007/s10495-005-0805-7; PMID: 15843892
- Zihni C, Mitsopoulos C, Tavares IA, Baum B, Ridley AJ, Morris JD. Prostate-derived sterile 20-like kinase 1-alpha induces apoptosis. JNK- and caspase-dependent nuclear localization is a requirement for membrane blebbing. J Biol Chem 2007; 282:6484 - 93; http://dx.doi.org/10.1074/jbc.M608336200; PMID: 17158878
- Orlando KA, Pittman RN. Rho kinase regulates phagocytosis, surface expression of GlcNAc, and Golgi fragmentation of apoptotic PC12 cells. Exp Cell Res 2006; 312:3298 - 311; http://dx.doi.org/10.1016/j.yexcr.2006.06.033; PMID: 16904666
- Moore M, Marroquin BA, Gugliotta W, Tse R, White SR. Rho kinase inhibition initiates apoptosis in human airway epithelial cells. Am J Respir Cell Mol Biol 2004; 30:379 - 87; http://dx.doi.org/10.1165/rcmb.2003-0019OC; PMID: 12933355
- Iwamoto H, Nakamuta M, Tada S, Sugimoto R, Enjoji M, Nawata H. A p160ROCK-specific inhibitor, Y-27632, attenuates rat hepatic stellate cell growth. J Hepatol 2000; 32:762 - 70; http://dx.doi.org/10.1016/S0168-8278(00)80245-7; PMID: 10845663
- Cechin SR, Dunkley PR, Rodnight R. Signal transduction mechanisms involved in the proliferation of C6 glioma cells induced by lysophosphatidic acid. Neurochem Res 2005; 30:603 - 11; http://dx.doi.org/10.1007/s11064-005-2747-4; PMID: 16176063
- Masamune A, Kikuta K, Satoh M, Satoh K, Shimosegawa T. Rho kinase inhibitors block activation of pancreatic stellate cells. Br J Pharmacol 2003; 140:1292 - 302; http://dx.doi.org/10.1038/sj.bjp.0705551; PMID: 14581180
- Takeda N, Kondo M, Ito S, Ito Y, Shimokata K, Kume H. Role of RhoA inactivation in reduced cell proliferation of human airway smooth muscle by simvastatin. Am J Respir Cell Mol Biol 2006; 35:722 - 9; http://dx.doi.org/10.1165/rcmb.2006-0034OC; PMID: 16858009
- Watanabe K, Ueno M, Kamiya D, Nishiyama A, Matsumura M, Wataya T, Takahashi JB, Nishikawa S, Nishikawa S, Muguruma K, et al. A ROCK inhibitor permits survival of dissociated human embryonic stem cells. Nat Biotechnol 2007; 25:681 - 6; http://dx.doi.org/10.1038/nbt1310; PMID: 17529971
- Samuel MS, Olson MF. Dying alone: a tale of rho. Cell Stem Cell 2010; 7:135 - 6; http://dx.doi.org/10.1016/j.stem.2010.07.002; PMID: 20682437
- Olson MF. Applications for ROCK kinase inhibition. Curr Opin Cell Biol 2008; 20:242 - 8; http://dx.doi.org/10.1016/j.ceb.2008.01.002; PMID: 18282695
- Bao W, Hu E, Tao L, Boyce R, Mirabile R, Thudium DT, Ma XL, Willette RN, Yue TL. Inhibition of Rho-kinase protects the heart against ischemia/reperfusion injury. Cardiovasc Res 2004; 61:548 - 58; http://dx.doi.org/10.1016/j.cardiores.2003.12.004; PMID: 14962485
- Thorlacius K, Slotta JE, Laschke MW, Wang Y, Menger MD, Jeppsson B, Thorlacius H. Protective effect of fasudil, a Rho-kinase inhibitor, on chemokine expression, leukocyte recruitment, and hepatocellular apoptosis in septic liver injury. J Leukoc Biol 2006; 79:923 - 31; http://dx.doi.org/10.1189/jlb.0705406; PMID: 16641138
- Gurkar AU, Chu K, Raj L, Bouley R, Lee SH, Kim YB, Dunn SE, Mandinova A, Lee SW. Identification of ROCK1 kinase as a critical regulator of Beclin1-mediated autophagy during metabolic stress. Nat Commun 2013; 4:2189; http://dx.doi.org/10.1038/ncomms3189; PMID: 23877263
- Mleczak A, Millar S, Tooze SA, Olson MF, Chan EY. Regulation of autophagosome formation by Rho kinase. Cell Signal 2013; 25:1 - 11; http://dx.doi.org/10.1016/j.cellsig.2012.09.010; PMID: 22975682
- Lourenço FC, Munro J, Brown J, Cordero J, Stefanatos R, Strathdee K, Orange C, Feller SM, Sansom OJ, Vidal M, et al. Reduced LIMK2 expression in colorectal cancer reflects its role in limiting stem cell proliferation. Gut 2014; 63:480 - 93; http://dx.doi.org/10.1136/gutjnl-2012-303883; PMID: 23585469
- Shi J, Zhang L, Wei L. Rho-kinase in development and heart failure: insights from genetic models. Pediatr Cardiol 2011; 32:297 - 304; http://dx.doi.org/10.1007/s00246-011-9920-0; PMID: 21327630
- Wei L, Roberts W, Wang L, Yamada M, Zhang S, Zhao Z, Rivkees SA, Schwartz RJ, Imanaka-Yoshida K. Rho kinases play an obligatory role in vertebrate embryonic organogenesis. Development 2001; 128:2953 - 62; PMID: 11532918
- Shimizu Y, Thumkeo D, Keel J, Ishizaki T, Oshima H, Oshima M, Noda Y, Matsumura F, Taketo MM, Narumiya S. ROCK-I regulates closure of the eyelids and ventral body wall by inducing assembly of actomyosin bundles. J Cell Biol 2005; 168:941 - 53; http://dx.doi.org/10.1083/jcb.200411179; PMID: 15753128
- Thumkeo D, Shimizu Y, Sakamoto S, Yamada S, Narumiya S. ROCK-I and ROCK-II cooperatively regulate closure of eyelid and ventral body wall in mouse embryo. Genes Cells 2005; 10:825 - 34; http://dx.doi.org/10.1111/j.1365-2443.2005.00882.x; PMID: 16098146
- Thumkeo D, Keel J, Ishizaki T, Hirose M, Nonomura K, Oshima H, Oshima M, Taketo MM, Narumiya S. Targeted disruption of the mouse rho-associated kinase 2 gene results in intrauterine growth retardation and fetal death. Mol Cell Biol 2003; 23:5043 - 55; http://dx.doi.org/10.1128/MCB.23.14.5043-5055.2003; PMID: 12832488
- Zhang Y-MM, Bo J, Taffet GE, Chang J, Shi J, Reddy AK, Michael LH, Schneider MD, Entman ML, Schwartz RJ, et al. Targeted deletion of ROCK1 protects the heart against pressure overload by inhibiting reactive fibrosis. FASEB J 2006; 20:916 - 25; http://dx.doi.org/10.1096/fj.05-5129com; PMID: 16675849
- Lee DH, Shi J, Jeoung NH, Kim MS, Zabolotny JM, Lee SW, White MF, Wei L, Kim YB. Targeted disruption of ROCK1 causes insulin resistance in vivo. J Biol Chem 2009; 284:11776 - 80; http://dx.doi.org/10.1074/jbc.C900014200; PMID: 19276091
- Phillips HM, Papoutsi T, Soenen H, Ybot-Gonzalez P, Henderson DJ, Chaudhry B. Neural crest cell survival is dependent on Rho kinase and is required for development of the mid face in mouse embryos. PloS One 2012; 7:e37685; http://dx.doi.org/10.1371/journal.pone.0037685; PMID: 22629443
- Zhao Z, Rivkees SA. Rho-associated kinases play an essential role in cardiac morphogenesis and cardiomyocyte proliferation. Dev Dyn 2003; 226:24 - 32; http://dx.doi.org/10.1002/dvdy.10212; PMID: 12508221
- Yahiaoui L, Villeneuve A, Valderrama-Carvajal H, Burke F, Fixman ED. Endothelin-1 regulates proliferative responses, both alone and synergistically with PDGF, in rat tracheal smooth muscle cells. Cell Physiol Biochem 2006; 17:37 - 46; http://dx.doi.org/10.1159/000091462; PMID: 16543720
- Croft DR, Olson MF. The Rho GTPase effector ROCK regulates cyclin A, cyclin D1, and p27Kip1 levels by distinct mechanisms. Mol Cell Biol 2006; 26:4612 - 27; http://dx.doi.org/10.1128/MCB.02061-05; PMID: 16738326
- Chen J, Guerriero E, Lathrop K, SundarRaj N. Rho/ROCK signaling in regulation of corneal epithelial cell cycle progression. Invest Ophthalmol Vis Sci 2008; 49:175 - 83; http://dx.doi.org/10.1167/iovs.07-0488; PMID: 18172090
- Zhang S, Tang Q, Xu F, Xue Y, Zhen Z, Deng Y, Liu M, Chen J, Liu S, Qiu M, et al. RhoA regulates G1-S progression of gastric cancer cells by modulation of multiple INK4 family tumor suppressors. Mol Cancer Res 2009; 7:570 - 80; http://dx.doi.org/10.1158/1541-7786.MCR-08-0248; PMID: 19372585
- McMullan R, Lax S, Robertson VH, Radford DJ, Broad S, Watt FM, Rowles A, Croft DR, Olson MF, Hotchin NA. Keratinocyte differentiation is regulated by the Rho and ROCK signaling pathway. Curr Biol 2003; 13:2185 - 9; http://dx.doi.org/10.1016/j.cub.2003.11.050; PMID: 14680635
- Yasui Y, Amano M, Nagata K, Inagaki N, Nakamura H, Saya H, Kaibuchi K, Inagaki M. Roles of Rho-associated kinase in cytokinesis; mutations in Rho-associated kinase phosphorylation sites impair cytokinetic segregation of glial filaments. J Cell Biol 1998; 143:1249 - 58; http://dx.doi.org/10.1083/jcb.143.5.1249; PMID: 9832553
- Kosako H, Yoshida T, Matsumura F, Ishizaki T, Narumiya S, Inagaki M. Rho-kinase/ROCK is involved in cytokinesis through the phosphorylation of myosin light chain and not ezrin/radixin/moesin proteins at the cleavage furrow. Oncogene 2000; 19:6059 - 64; http://dx.doi.org/10.1038/sj.onc.1203987; PMID: 11146558
- Yokoyama T, Goto H, Izawa I, Mizutani H, Inagaki M. Aurora-B and Rho-kinase/ROCK, the two cleavage furrow kinases, independently regulate the progression of cytokinesis: possible existence of a novel cleavage furrow kinase phosphorylates ezrin/radixin/moesin (ERM). Genes Cells 2005; 10:127 - 37; http://dx.doi.org/10.1111/j.1365-2443.2005.00824.x; PMID: 15676024
- Rath N, Olson MF. Rho-associated kinases in tumorigenesis: re-considering ROCK inhibition for cancer therapy. EMBO Rep 2012; 13:900 - 8; http://dx.doi.org/10.1038/embor.2012.127; PMID: 22964758
- Hahmann C, Schroeter T. Rho-kinase inhibitors as therapeutics: from pan inhibition to isoform selectivity. Cell Mol Life Sci 2010; 67:171 - 7; http://dx.doi.org/10.1007/s00018-009-0189-x; PMID: 19907920
- Davies SP, Reddy H, Caivano M, Cohen P. Specificity and mechanism of action of some commonly used protein kinase inhibitors. Biochem J 2000; 351:95 - 105; http://dx.doi.org/10.1042/0264-6021:3510095; PMID: 10998351
- Higashi M, Shimokawa H, Hattori T, Hiroki J, Mukai Y, Morikawa K, Ichiki T, Takahashi S, Takeshita A. Long-term inhibition of Rho-kinase suppresses angiotensin II-induced cardiovascular hypertrophy in rats in vivo: effect on endothelial NAD(P)H oxidase system. Circ Res 2003; 93:767 - 75; http://dx.doi.org/10.1161/01.RES.0000096650.91688.28; PMID: 14500337
- Wang YX, da Cunha V, Martin-McNulty B, Vincelette J, Li W, Choy DF, Halks-Miller M, Mahmoudi M, Schroeder M, Johns A, et al. Inhibition of Rho-kinase by fasudil attenuated angiotensin II-induced cardiac hypertrophy in apolipoprotein E deficient mice. Eur J Pharmacol 2005; 512:215 - 22; http://dx.doi.org/10.1016/j.ejphar.2005.02.024; PMID: 15840407
- Rikitake Y, Oyama N, Wang CY, Noma K, Satoh M, Kim HH, Liao JK. Decreased perivascular fibrosis but not cardiac hypertrophy in ROCK1+/- haploinsufficient mice. Circulation 2005; 112:2959 - 65; PMID: 16260635
- Zhang YM, Bo J, Taffet GE, Chang J, Shi J, Reddy AK, Michael LH, Schneider MD, Entman ML, Schwartz RJ, et al. Targeted deletion of ROCK1 protects the heart against pressure overload by inhibiting reactive fibrosis. FASEB J 2006; 20:916 - 25; http://dx.doi.org/10.1096/fj.05-5129com; PMID: 16675849
- Okamoto R, Li Y, Noma K, Hiroi Y, Liu PY, Taniguchi M, Ito M, Liao JK. FHL2 prevents cardiac hypertrophy in mice with cardiac-specific deletion of ROCK2. FASEB J 2013; 27:1439 - 49; http://dx.doi.org/10.1096/fj.12-217018; PMID: 23271052
- Nagatoya K, Moriyama T, Kawada N, Takeji M, Oseto S, Murozono T, Ando A, Imai E, Hori M. Y-27632 prevents tubulointerstitial fibrosis in mouse kidneys with unilateral ureteral obstruction. Kidney Int 2002; 61:1684 - 95; http://dx.doi.org/10.1046/j.1523-1755.2002.00328.x; PMID: 11967018
- Satoh S, Yamaguchi T, Hitomi A, Sato N, Shiraiwa K, Ikegaki I, Asano T, Shimokawa H. Fasudil attenuates interstitial fibrosis in rat kidneys with unilateral ureteral obstruction. Eur J Pharmacol 2002; 455:169 - 74; http://dx.doi.org/10.1016/S0014-2999(02)02619-5; PMID: 12445583
- Fu P, Liu F, Su S, Wang W, Huang XR, Entman ML, Schwartz RJ, Wei L, Lan HY. Signaling mechanism of renal fibrosis in unilateral ureteral obstructive kidney disease in ROCK1 knockout mice. J Am Soc Nephrol 2006; 17:3105 - 14; http://dx.doi.org/10.1681/ASN.2005121366; PMID: 17005937
- Kanda T, Wakino S, Homma K, Yoshioka K, Tatematsu S, Hasegawa K, Takamatsu I, Sugano N, Hayashi K, Saruta T. Rho-kinase as a molecular target for insulin resistance and hypertension. FASEB J 2006; 20:169 - 71; PMID: 16267124
- Furukawa N, Ongusaha P, Jahng WJ, Araki K, Choi CS, Kim HJ, Lee YH, Kaibuchi K, Kahn BB, Masuzaki H, et al. Role of Rho-kinase in regulation of insulin action and glucose homeostasis. Cell Metab 2005; 2:119 - 29; http://dx.doi.org/10.1016/j.cmet.2005.06.011; PMID: 16098829
- Lee SH, Huang H, Choi K, Lee DH, Shi J, Liu T, Chun KH, Seo JA, Lima IS, Zabolotny JM, et al. ROCK1 isoform-specific deletion reveals a role for diet-induced insulin resistance. Am J Physiol Endocrinol Metab 2014; 306:E332 - 43; http://dx.doi.org/10.1152/ajpendo.00619.2013; PMID: 24326423
- Shifrin V, Annand RR, Flusberg D, McGonigle S, Wong E, Paradise E, Bartolozzi A, Ram S, Foudoulakis H, Kirk B, et al. Effects of SLx-2119, a novel small molecule inhibitor of Rho-associated kinase ROCK (ROK), on growth of human tumor xenografts in nude mice. AACR Meeting Abstracts 2005; 1:158.
- Samuel MS, Munro J, Bryson S, Forrow S, Stevenson D, Olson MF. Tissue selective expression of conditionally-regulated ROCK by gene targeting to a defined locus. Genesis 2009; 47:440 - 6; http://dx.doi.org/10.1002/dvg.20519; PMID: 19391117
- Samuel MS, Lopez JI, McGhee EJ, Croft DR, Strachan D, Timpson P, Munro J, Schröder E, Zhou J, Brunton VG, et al. Actomyosin-mediated cellular tension drives increased tissue stiffness and β-catenin activation to induce epidermal hyperplasia and tumor growth. Cancer Cell 2011; 19:776 - 91; http://dx.doi.org/10.1016/j.ccr.2011.05.008; PMID: 21665151
- Boerma M, Fu Q, Wang J, Loose DS, Bartolozzi A, Ellis JL, McGonigle S, Paradise E, Sweetnam P, Fink LM, et al. Comparative gene expression profiling in three primary human cell lines after treatment with a novel inhibitor of Rho kinase or atorvastatin. Blood Coagul Fibrinolysis 2008; 19:709 - 18; http://dx.doi.org/10.1097/MBC.0b013e32830b2891; PMID: 18832915
- Shi J, Zhang YW, Summers LJ, Dorn GW 2nd, Wei L. Disruption of ROCK1 gene attenuates cardiac dilation and improves contractile function in pathological cardiac hypertrophy. J Mol Cell Cardiol 2008; 44:551 - 60; http://dx.doi.org/10.1016/j.yjmcc.2007.11.018; PMID: 18178218
- Shi J, Zhang YW, Yang Y, Zhang L, Wei L. ROCK1 plays an essential role in the transition from cardiac hypertrophy to failure in mice. J Mol Cell Cardiol 2010; 49:819 - 28; http://dx.doi.org/10.1016/j.yjmcc.2010.08.008; PMID: 20709073
- Vemula S, Shi J, Hanneman P, Wei L, Kapur R. ROCK1 functions as a suppressor of inflammatory cell migration by regulating PTEN phosphorylation and stability. Blood 2010; 115:1785 - 96; http://dx.doi.org/10.1182/blood-2009-08-237222; PMID: 20008297
- Vemula S, Shi J, Mali RS, Ma P, Liu Y, Hanneman P, Koehler KR, Hashino E, Wei L, Kapur R. ROCK1 functions as a critical regulator of stress erythropoiesis and survival by regulating p53. Blood 2012; 120:2868 - 78; http://dx.doi.org/10.1182/blood-2011-10-384172; PMID: 22889758
- Ongusaha PP, Qi HH, Raj L, Kim Y-B, Aaronson SA, Davis RJ, Shi Y, Liao JK, Lee SW. Identification of ROCK1 as an upstream activator of the JIP-3 to JNK signaling axis in response to UVB damage. Sci Signal 2008; 1:ra14; http://dx.doi.org/10.1126/scisignal.1161938; PMID: 19036714
- Duffy P, Schmandke A, Schmandke A, Sigworth J, Narumiya S, Cafferty WB, Strittmatter SM. Rho-associated kinase II (ROCKII) limits axonal growth after trauma within the adult mouse spinal cord. J Neurosci 2009; 29:15266 - 76; http://dx.doi.org/10.1523/JNEUROSCI.4650-09.2009; PMID: 19955379
- Noma K, Rikitake Y, Oyama N, Yan G, Alcaide P, Liu PY, Wang H, Ahl D, Sawada N, Okamoto R, et al. ROCK1 mediates leukocyte recruitment and neointima formation following vascular injury. J Clin Invest 2008; 118:1632 - 44; http://dx.doi.org/10.1172/JCI29226; PMID: 18414683
- Shimizu T, Fukumoto Y, Tanaka S, Satoh K, Ikeda S, Shimokawa H. Crucial role of ROCK2 in vascular smooth muscle cells for hypoxia-induced pulmonary hypertension in mice. Arterioscler Thromb Vasc Biol 2013; 33:2780 - 91; http://dx.doi.org/10.1161/ATVBAHA.113.301357; PMID: 24135024
- Zhou L, Liu F, Huang XR, Liu F, Chen H, Chung AC, Shi J, Wei L, Lan HY, Fu P. Amelioration of albuminuria in ROCK1 knockout mice with streptozotocin-induced diabetic kidney disease. Am J Nephrol 2011; 34:468 - 75; http://dx.doi.org/10.1159/000332040; PMID: 21986457
- Zhou Q, Mei Y, Shoji T, Han X, Kaminski K, Oh GT, Ongusaha PP, Zhang K, Schmitt H, Moser M, et al. Rho-associated coiled-coil-containing kinase 2 deficiency in bone marrow-derived cells leads to increased cholesterol efflux and decreased atherosclerosis. Circulation 2012; 126:2236 - 47; http://dx.doi.org/10.1161/CIRCULATIONAHA.111.086041; PMID: 23011471
