Abstract
Earlier studies have shown adjudin to cause aspermatogenesis by depleting virtually all germ cells from the seminiferous epithelium, leading to transient infertility; spermatogenesis and fertility were re-established several weeks later after germ cell differentiation was reinitiated by spermatogonia. While adjudin is known to exert its initial effects at the apical ectoplasmic specialization (a testis-specific atypical anchoring junction), thereby perturbing spermatid adhesion, its molecular target at this site is not known. Herein, we report the production of a specific antibody against adjudin after this compound was conjugated to an adjuvant (i.e., keyhole limpet hemocyanin) to maximize immune response in rabbits. This antibody was utilized for co-immunoprecipitation by using an affinity resin to pull-down the binding partners of adjudin. Using this approach coupled with mass spectrometry and immunoblotting, we show testin (a protein largely restricted to the apical ES in the adult testis) and actin-myosin to be molecular targets of adjudin. These findings provide a platform for future functional studies, not only to better understand the molecular mechanism behind adjudin-induced germ cell loss from the seminiferous epithelium, but also to understand the molecular basis of spermiation.
Introduction
Adjudin, 1-(2,4-dichlorobenzyl)-1H-indazole-3-carbohydrazide, is an anti-spermatogenic compound being actively investigated as a male contraceptive for potential human use.Citation1,Citation2 In contrast to hormonal approaches which disrupt spermatogenesis by interfering with the hypothalamic-pituitary-testicular axis,Citation3–Citation5 adjudin exerts its effects behind the blood-testis barrier (BTB) in a specialized microenvironment known as the adluminal compartment. The most obvious phenotype following administration of adjudin to adult rats is the loss of developing germ cells from the seminiferous epithelium, most notably elongating/elongated spermatids, followed by round spermatids and spermatocytes but not spermatogonia residing outside of the BTB.Citation1,Citation2,Citation6 This leads to a transient loss in fertility; fertility is restored after spermatogonia reinitiate germ cell proliferation and differentiation and cells repopulate the seminiferous epithelium.Citation6 While adjudin is known to exert its initial effects at the apical ectoplasmic specialization (apical ES, a testis-specific anchoring junction found between Sertoli cells and step 8–19 spermatids in the rat testis), the molecular target(s) of adjudin remains unknown. This important information, if it were available, would provide additional insight on the molecular basis behind the restricted action of adjudin in the testis, which is still not completely understood. While both acute and subchronic toxicity studies performed by licensed toxicologists following FDA guidelines have shown adjudin to exert its effects primarily in the testis by depleting germ cells from the seminiferous epithelium without affecting cell adhesion in other organs,Citation7 adjudin was not specifically uptaken by the testis. Instead, [3H]-adjudin was distributed rather uniformly amongst different organs, less than 0.05% of [3H]-adjudin was recovered from the testis, and [3H]-adjudin was metabolically cleared by ∼24 hours after administration.Citation6 If our hypothesis—which asserts that adjudin affects adhesion in the testis because of the presence of this unique structure—holds ground, then adjudin should interact with an apical ES-specific protein(s) to trigger ES disassembly. This is the question that we address in this study.
The ES is an actin-based cell-cell anchoring junction that is found at two important sites within the seminiferous epithelium: (1) at the Sertoli cell-elongating/elongated spermatid juncture (defined as the apical ES) and (2) at the Sertoli-Sertoli cell interface at the BTB (defined as the basal ES).Citation8,Citation9 Once the apical ES is assembled at the Sertoli cell—step 8 spermatid interface during spermiogenesis, it becomes the only anchoring device to persist until late spermatids are released from the epithelium at spermiation.Citation8,Citation10 The basal ES at the BTB, on the other hand, co-exists with other junction types, namely tight junctions (TJs), desmosomes and gap junctions (GJs).Citation11 It is also worth noting that both the apical and the basal ES share nearly identical ultrastructural features when examined by electron microscopy. These are typified by tightly packed bundles of non-contractile actin filaments that are sandwiched in between cisternae of endoplasmic reticulum and the Sertoli cell plasma membrane.Citation8,Citation12–Citation14 Interestingly, these ultrastructural features are not found within spermatids so that the apical ES can be ‘loosely’ defined as a hemi-junction; we say this because even though the ES is a hemi-junction, its adhesion in vitro is stronger than that of the desmosome which is present between Sertoli cells and all germ cells up to, but not including, step 8 spermatids.Citation15,Citation16 Moreover, not all multi-protein complexes found at the apical ES (e.g., α6β1-integrin-laminin-333 and junctional adhesion molecule-C-zonula occludens-1) are necessarily found at the basal ES and vice versa.Citation8,Citation17,Citation18 Lastly, many proteins such as cadherin, coxsackievirus and adenovirus receptor (CAR), and connexin 43 that constitute functionality to other junction types (i.e., adherens junctions, TJs and GJs, respectively) are also found at the apical ES.Citation11,Citation16,Citation19–Citation21
Testin is yet another example of a protein found predominantly at the apical ES.Citation22–Citation27 Interestingly, testin expression was shown to be transiently induced several-fold whenever apical ES function was disrupted, leading to aspermatogenesis and whenever spermatogenesis was programmed to return to normal.Citation28 Studies by gene profiling using testes from rats treated with adjudin versus methylcellulose (control) have also identified testin as one of the most induced genes;Citation29 the only other gene to be induced more than testin was wingless-type MMTV integration site family, member 4 (Wnt4) (Mruk DD, Cheng CY, unpublished observation). Similarly, gamendazole, an analog of adjudin, was also shown to stimulate testin expression in the adult testis by 3.2-, 17.6- and 52.4-fold versus control rats by 4, 12, and 24 hours, respectively, in a gene profiling study.Citation53 Collectively, these findings illustrate that the apical ES is a testis-specific hybrid junction and that it is the primary cellular target of adjudin. Herein, we report findings emanating from a series of experiments that identify the molecular targets of adjudin at the apical ES. These results should be helpful in the future study of adjudin (or its analogs) as a non-hormonal male contraceptive. These findings may also help us better understand the process of spermiation.
Results
Effects of adjudin on germ cell adhesion to Sertoli cells in vitro.
As previously reported, adjudin is known to affect the adhesion of germ cells to Sertoli cells in the seminiferous epithelium.Citation19,Citation28,Citation30 We have established an in vitro assay using Sertoli-germ cell co-cultures to examine the effects of adjudin on germ cell adhesion. This assay also aims to facilitate rapid screening of second and third generation adjudin analogs (or other compounds having a similar mechanism of action) in order to avoid excessive use of laboratory animals, which would be needed to establish initial efficacy. In brief, Sertoli cells were cultured at 0.5 × 106 cells/cm2 for 4 days to allow the establishment of a functional TJ permeability barrier as assessed by transepithelial electrical resistance (TER) measurements.Citation31 As shown in , TJs (see blue arrowheads) were found to co-exist with basal ES, a structure typified by the presence of actin filament bundles (see green arrowheads) sandwiched in between cisternae of endoplasmic reticulum (ER) and apposing Sertoli cell plasma membranes (see orange arrowheads). Desmosomes (see red arrowheads), characterized by the presence of electron-dense material, were also found at the Sertoli-Sertoli cell interface (). Together, these junctions constitute the Sertoli cell barrier/BTB (). On day 4, germ cells were isolated from adult rat testes, seeded onto the Sertoli cell epithelium and allowed to establish adhesion with Sertoli cells ( and C). shows adhesion between a step 9–10 spermatid and a Sertoli cell. A functional ES, typified by the presence of actin filament bundles (see green arrowheads) sandwiched in between the ER and apposing Sertoli cell-spermatid plasma membranes (see orange arrowheads) is visible in this micrograph as well. Also, Sertoli cells cultured in vitro were found to have microvilli (–C and see black arrow). The acrosome (Ac) and spermatid nucleus (Nu) are noted in . An in vitro assay was performed by fluorescently labeling germ cells and allowing them to adhere to the Sertoli cell epithelium, after which adjudin (1 µg/ml) was added. Adjudin was shown to perturb germ cell adhesion within 1 hour (). Since adjudin is less effective in disrupting adhesion between Sertoli cells and step 1–7 spermatids, spermatocytes and spermatogonia,Citation32,Citation33 it could not detach all germ cells from the Sertoli cell epithelium within 96 hours (). Alternatively, a higher concentration of adjudin may be needed to dislodge these germ cells. These findings are consistent with earlier in vivo studies, which demonstrated round spermatids and spermatocytes to detach from the epithelium by ∼3 and 6.5 days, respectively, versus elongating/elongated spermatids by ∼6 hr, after adjudin treatment.Citation32
Specificity of the anti-adjudin antibody.
To identify the molecular target(s) of adjudin in the seminiferous epithelium, affinity chromatography was used. For this reason, we raised a specific antibody against anti-adjudin. illustrates the conjugation scheme for linking adjudin to keyhole limpet hemocyanin (KLH) where KLH served as an adjuvant. KLH was initially modified with succinimidyl 4-formylbenzoate (SFB) to incorporate a benzaldehyde moiety at its N-terminus. This, in turn, reacted readily with the hydrazide group of adjudin () to form a stable KLH-adjudin conjugate via the hydrazone linkage (). This conjugate was then used as an immunogen for anti-adjudin antibody production in two rabbits, one male and one female.Citation32 As shown in the adjudin radioimmunoassay (RIA), adding increasing concentrations of unlabeled (i.e., cold) adjudin generated a complete displacement curve with a 50% displacement at ∼10 ng and a sensitivity of ∼0.35 ng adjudin (). Because lonidamine is structurally similar to adjudin (i.e., presence of carboxylic acid in the indazole ring of lonidamine instead of carbohydrazide in adjudin, versus ), lonidamine generated a parallel displacement curve. However, lonidamine failed to completely displace the binding of [3H]-adjudin to its antibody, illustrating the specificity of this anti-adjudin antibody. Equally important, adjudin is known to share structural similarities with other steroids such as testosterone and estradiol-17β. While [3H]-adjudin was found to bind to dextran-coated charcoal, both testosterone and estradiol-17β at up to 100 ng per assay tube failed to compete with the binding of [3H]-adjudin to its antibody, illustrating the lack of cross-reactivity. In conclusion, this anti-adjudin antibody is highly specific to adjudin.
Anti-adjudin antibody protects the seminiferous epithelium from the destructive effects of adjudin on germ cell depletion in vivo.
Competitive binding assays based on the use of Staphylococcus aureus cells (formalin fixed) instead of dextrancoated charcoal absorption also confirmed that [3H]-adjudin bound to adjudin antiserum and that this binding was displaced in the presence of excess unlabelled adjudin (). The use of pre-immune serum in place of adjudin antiserum yielded no detectable [3H]-adjudin binding (). These results were confirmed and expanded in the next in vivo experiment, which illustrated that adjudin-mediated germ cell loss from the seminiferous epithelium was effectively blocked by intratesticular injection of anti-adjudin IgG (i–iii versus). However, injection of normal rabbit IgG (isolated from pre-immune serum) could not block the adverse effects of adjudin on Sertoli-germ cell adhesion (iii). i is a cross-section of the testis from control rats and ii is a cross section of the testis from rats 21 days after being treated with adjudin (50 mg/kg b.w., by gavage). Because entry of anti-adjudin IgG (Mr 150 kDa) into the seminiferous epithelium was likely excluded by the BTB,Citation34,Citation35 we believe that anti-adjudin encased the periphery of seminiferous tubules, thereby preventing the entry of adjudin into the adluminal compartment. This protected germ cell adhesion from the destructive effects of adjudin.
Actin-myosin and testin are molecular targets of adjudin.
Earlier studies from our laboratory have shown adjudin to exert its effects mostly at the apical ES,Citation2,Citation19,Citation36 an actin-based hybrid junction found uniquely in the testis.Citation8,Citation37 Moreover, studies using immunofluorescenceCitation9,Citation38 and electron microscopyCitation39 have shown the actin cytoskeleton to be affected by adjudin. To confirm that actin is a cellular target of adjudin, we opted to use a biochemical approach (i.e., co-immunoprecipitation). In brief, antiadjudin IgG was allowed to form a stable immunocomplex with Protein A-Sepharose, which was packed onto an Econo-Column. Thereafter, lysates of testes obtained from rats treated with adjudin were re-circulated through the column for several hours. This allowed adjudin interacting proteins to form a stable complex with the anti-adjudin IgG-Protein A Sepharose. After washing the column extensively, proteins were eluted with a low pH buffer. Proteins were fractionated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), and their identities were determined by mass spectrometry () and immunoblotting using corresponding specific antibodies ( and ). Using these approaches, actin (42 kDa) at the apical ES was shown to be one of the primary targets of adjudin ( and ). While adjudin may also target actin at the basal ES, we do not think this is the case because BTB integrity was unaffected during this time,Citation11 and it remained unaffected until ∼6 weeks following adjudin treatment when the BTB was found to be transiently disrupted.Citation40 Myosin (Mr ∼160 kDa) was also identified as an adjudin target in the testis ().
In addition to actin and myosin, testin (35 and 37 kDa) was also pulled-down as a molecular target of adjudin as demonstrated by co-immunoprecipitation and immunoblotting (). The interaction of adjudin with actin and testin was found to be specific since unlabeled adjudin displaced the binding of [3H]-adjudin to actin or testin IgG in competitive binding assays ( and ). This latter finding is not entirely unexpected because testin is a constituent of the apical ES,Citation27,Citation41 and its presence at the apical ES was confirmed by a study using autoradiography at the electron microscopy level.Citation26
Discussion
As discussed above, the apical ES is a unique junction that is restricted to the Sertoli cell-elongating/elongated spermatid interface. It is typified by the presence of actin filament bundles sandwiched in between cisternae of the ER and apposing Sertoli cell-spermatid plasma membranes.Citation9,Citation13,Citation19,Citation42 This unique arrangement of actin filament bundles appears to contribute to the remarkable adhesive strength of the apical ES since the force required to pull a pre-step 8 spermatid was found to be weaker than that required to pull a post-step 8 spermatid from the Sertoli cell epithelium in vitro as assessed by a micropipette pressure transducing system.Citation15 Specifically, the mean force that was required to detach spermatocytes, pre-step 8 spermatids and post-step 8 spermatids from the Sertoli cell epithelium was 5.25 × 10−7 pN, 4.73 × 10−7 pN and 8.82 × 10−7 pN,Citation15 respectively, illustrating that the apical ES is almost twice as stable as the desmosome in terms of adhesion. Moreover, the inclusion of adjudin into these co-cultures significantly weakened the apical ES; hence, less force was needed to pull post-step 8 spermatids from the Sertoli cell epithelium.Citation33 These in vitro findings are important because they confirm earlier in vivo observations which illustrated the apical ES to be the primary cellular target of adjudin in the seminiferous epithelium.Citation32 Unfortunately, the molecular mechanism behind these observations continues to remain largely unknown. Herein, we utilized a specific anti-adjudin antibody and co-immunoprecipitation to show that actin-myosin and testin bind adjudin, which destabilizes actin filament bundles as visualized by electron microscopyCitation39 and leads to premature germ cell loss (i.e., pseudo-spermiation). This postulate was further supported by recent findings that showed a reduction in epidermal growth factor receptor pathway substrate 8 (EPS8, a protein that caps the barbed ends of and bundles actin filamentsCitation37,Citation43,Citation44) and a mis-localization of actin related protein 3 (ARP3, a component of the ARP2/3 complex, which together with neuronal Wiskott-Aldrich syndrome protein [N-WASP] and ARP2/3 complex subunits 1 to 5 [ARPC 1–5] form a regulatory complex to induce branching of the actin networkCitation37,Citation45,Citation46) at the apical ES. The net result of these changes is the destabilization of cell adhesion and loss of germ cells.
Similar to lonidamine, adjudin is a small non-steroidal molecule,Citation47 and studies have shown the hypothalamic-pituitary-testicular axis not to be affected by adjudin (or its analog, gamendazole) because serum luteinizing hormone (LH), follicle-stimulating hormone (FSH) and testosterone levels did not change.Citation28,Citation48 The only exception to this finding is that elevated LH and FSH levels were noted in men 1 and 3 weeks after lonidamine treatment, but testosterone, prolactin and thyroid-stimulating hormone (TSH) levels all remained unchanged.Citation49 In another study, five cancer patients being treated with lonidamine for 4–8 weeks exhibited a significant decrease in their serum testosterone levels.Citation50 Thus, although lonidamine and adjudin are structurally similar, their mechanisms of action are somewhat different. Also, it remains to be determined if adjudin has any chemotherapeutic properties, similar to lonidamine. The fact that adjudin can be pulled-down with dextran-coated charcoal seemingly suggests that it shares physico-chemical properties, at least partly, with steroids. Furthermore, the anti-adjudin antibody was shown to be highly specific to adjudin; it did not recognize testosterone or estradiol-17β, and despite its structural similarity to lonidamine, it only partially cross-reacted with lonidamine. Based on the findings reported in this study, we believe that adjudin binds to testin at the apical ES, which in turn affects cell adhesion mediated by several multi-protein complexes (e.g., integrin-laminin, cadherin-catenin, JAM-ZO-1, CAR-ZO-1 or nectin-afadin). EPS8 and ARP3 may also be involved, that is, they may regulate some aspect of protein internalization, which would be needed for apical ES disassembly.
In this context, the precise role of testin in these cellular events is not yet known. What is known is that testin is a signaling molecule whose expression surges during apical ES disassemblyCitation23,Citation28,Citation51 (its level is also highest at late stage VIII of the seminiferous epithelial cycleCitation27) and that testin binds actin strongly Mruk DD and Cheng CY, unpublished observations). Testin expression is also largely restricted to the testis, as well as to the ovary.Citation23,Citation24 This brings into question the exact role of testin in actin dynamics. While actin is present in all cells, adjudin does not affect adhesion in other organs.Citation6,Citation7 Although a speculation, testin may be responsible for the non-contractile nature of actin at the apical ES,Citation11 and non-contractility (i.e., rigidity) of actin filaments at the apical ES may also make this structure more susceptible to compounds such as adjudin by a yet unknown mechanism. Alternatively, the testin-actin protein complex may be functioning as a platform to recruit EPS8 and ARP3 (or other proteins) to the apical ES. The observation that adjudin also binds myosin, a motor protein that itself binds to actin, is also important because myosins are responsible for actin-based motility and endocytosis.Citation52
In summary, we have identified actin-myosin and testin as molecular targets of adjudin at the apical ES. Because testin is an ES protein, these findings explain the restricted action of adjudin in the testis. Adjudin apparently exerts its effects by binding to testin since adjudinCitation28,Citation29 or its analog, gamendazole,Citation53 are known to upregulate testin expression in the testis, in turn perturbing actin, affecting adhesion and resulting in germ cell loss. Nevertheless, additional research is needed to better understand adjudin's mechanism of action in the testis.
Materials and Methods
Animals.
Sprague-Dawley (outbred) rats were purchased from Charles River Laboratories. New Zealand white rabbits were obtained from Harlan Laboratories. All animals were housed at The Rockefeller University Comparative Bioscience Center at 22 ± 1°C with constant light:dark cycles of 12 hour:12 hour with free access to standard chow and water. The use of rats and rabbits was approved by The Rockefeller University Animal Care and Use Committee with protocol numbers 03040, 06018 and 09016.
Electron microscopy of Sertoli-germ cell co-cultures.
Sertoli cells were isolated from the testes of 20-day-old rats.Citation54,Citation55 By this postnatal day, Sertoli cells had ceased to divide and were fully differentiated.Citation56 Also, these cells are known to functionally mimic Sertoli cells isolated from adult rat testes.Citation57,Citation58 Sertoli cells were plated on MatrigelTM-(BD Biosciences) coated 60-mm dishes at 0.5 × 106 cells/cm2 and cultured in serum-free Dulbecco's modified Eagle medium: Ham's nutrient mixture F-12 (DMEM/F-12 at 1:1, [v/v]) supplemented with growth factors and antibiotics as described.Citation54,Citation55 Approximately 36 hours after plating, cultures were treated with a hypertonic buffer (20 mM Tris pH 7.4 at 22°C) to lyse contaminating germ cells,Citation59 resulting in a Sertoli cell purity of >98%. Also, there was negligible contamination by Leydig and peritubular myoid cells when cell specific markers were used for RT-PCR and immunoblotting experiments as previously described.Citation60 A functional TJ permeability barrier and ultrastructural features corresponding to TJs, basal ES and desmosomes which mimicked the BTB in vivo when examined by electron microscopy were also noted in these Sertoli cell cultures by day 4. On day 4, total germ cells were isolated from adult rat testes.Citation61 Successive passages through glass wool were used to remove step 12–19 spermatids, and isolated germ cells were then incubated with 1 µM gelsolin (an actin-severing protein,Citation62 Sigma-Aldrich) in DMEM/F-12 for 15 min to remove residual actin filaments which may have remained attached to spermatids during the isolation procedure. Germ cell purity was >95% with negligible somatic cell contamination,Citation61 and germ cells were co-cultured with Sertoli cells within 1 hour of their isolation. On day 6 (i.e., ∼48 hours after plating germ cells onto the Sertoli cell epithelium), co-cultures were fixed in 2.5% glutaraldehyde [v/v]/2.5% paraformaldehyde [w/v] in 0.1 M sodium cacodylate buffer pH 7.5 at 22°C. Sertoli cells cultured alone for 6 days were also processed for electron microscopy as described.Citation26 Cells were post-fixed in 1% OsO4 [v/v] in 0.1 M sodium cacodylate buffer and stained with uranyl acetate. After dehydration in ascending grades of ethanol, cells were detached from dishes with propylene oxide and subsequently embedded in EPON (Electron Microscopy Sciences). Silver sections were generated with an ultramicrotome, and images were obtained with a JEOL 100CDXII electron microscope at 80 kV. Electron microscopy was performed at the Bio-imaging Resource Center, The Rockefeller University.
In vitro germ cell binding assays.
Sertoli cells isolated from the testes of 20-day-old rats were plated on Matrigel™-coated 24-well dishes (Nunc) at 0.5 × 106 cells/cm2 and cultured in DMEM/F-12 for 4 days as previously described with a hypotonic treatment performed ∼36 hours after plating.Citation59 Thereafter, germ cells isolated from the testes of 90-day-old rats were fluorescently labeled with 1,1′-dioctadecyl-6,6′di(4-sulfophenyl)-3,3,3′,3′-tetramethylindocarbocyanine [SP-DiIC18(3), Molecular Probes/Invitrogen)]. Successive passages through glass wool were employed to remove step 12–19 spermatids. Germ cells (1 × 106 cells/1 ml) were labeled with SP-DiIC18(3) [2 µM, diluted from a 20 µM stock solution prepared in DMSO] at 35°C for 5 min. This was followed by an additional incubation at 4°C for 15 min. Germ cell viability was not affected during this labeling procedure, and only viable germ cells were fluorescently labeled. Thereafter, fluorescently labeled germ cells were successively washed with DMEM/F-12, added onto the Sertoli cell epithelium at a Sertoli:germ cell ratio of 1:3, and allowed to adhere to Sertoli cells for 24–36 hours. The following day, co-cultures were washed twice, and DMEM/F-12 containing adjudin (1 µg/ml, diluted from a 1 mg/ml stock solution prepared in ethanol) was added into wells, after which time DMEM/F-12 was no longer changed. At specific time points, co-cultures in triplicate wells were terminated by washing three times to remove unbound germ cells, and fluorescence (530 nmEX/590 nmEM) was quantified with a GENios cytofluorometer (Phenix Research Products).
Production of adjudin antiserum.
Adjudin antiserum was produced by a standard protocol.Citation63 In brief, adjudin was cross-linked to keyhole limpet hemocyanin (KLH) as follows. First, KLH was reacted with succinimidyl 4-formylbenzoate (SFB, Pierce Biotechnology) to yield an aldehyde group at its N-terminus. Second, this aldehyde group was reacted readily with the hydrazide group in adjudin to form a stable hydrazone bond. This conjugate with a 1:1 molar ratio of KLH:adjudin served as the immunogen, which was subsequently emulsified in Freund's Adjuvant and injected subcutaneously at three different sites into two New Zealand rabbits (1 male and 1 female, ∼4 kg b.w. at the time of immunization) with ∼1 µmol adjudin per site (i.e., ∼3 µmol adjudin per immunization per rabbit). At 6- and 8-week intervals, animals received a booster injection containing the same amount of immunogen as was used for the initial immunization. Thereafter, blood was collected from the marginal vein in the ear to obtain antiserum.Citation64 Anti-adjudin IgG was prepared by (NH4)2SO4 precipitation and DEAE-chromatography.Citation64–Citation66 We routinely obtained ∼2 mg purified (as assessed by SDS-PAGE and Coomassie blue staining) anti-adjudin IgG per ml antiserum.
Characterization of adjudin antiserum.
Adjudin antiserum was partially characterized by two methods. First, adjudin antiserum was used for competitive binding assays. In brief, binding buffer (10 mM NaH2PO4, pH 7.4 at 22°C containing 0.15 M NaCl, 0.5% BSA [w/v], 0.2% gelatin [w/v] and 0.05% NaN3 [w/v]), adjudin antiserum (1:50–1:2,000) and [3H]-adjudin (15 × 106 cpm) were combined in reaction tubes to a final volume of 500 µl. Samples were vortexed and incubated at 4°C for 36 hours. Thereafter, Staphylococcus aureus (Sac) cells (formalin-fixed) were added to separate bound from free [3H]-adjudin, and samples were incubated at 4°C for 1 hour. Samples were centrifuged and radioactivity quantified in pellets. As a control, pre-immune serum was used instead of adjudin antiserum. [3H]-Adjudin (specific activity, 580 mCi/mmol) was obtained from PerkinElmer.Citation6
Second, anti-adjudin IgG was administered to adult rats (∼300 gm b.w.) to determine if it could protect the testis from the adverse effects of adjudin on Sertoli-germ cell adhesion. Animals were divided into four groups: group 1-received anti-adjudin IgG (by intratesticular injection) plus adjudin (50 mg/kg b.w. by gavage; n = 5), group 2-received non-immune rabbit IgG plus adjudin (n = 3), group 3-received adjudin only (n = 3) and group 4-control, received 0.5% methylcellulose [w/v] (n = 3). In brief, 100 µg anti-adjudin or non-immune rabbit IgG suspended in 75 µl sterile PBS, pH 7.4 at 22°C was injected intratesticularly into the right testis with a 29-gauge needle-syringe combination. (Note: the left testis received no injection of IgG). This was followed immediately by oral administration of adjudin (50 mg/kg b.w. suspended at 20 mg/ml in 0.5% methyl cellulose [w/v]) to induce germ cell loss from the seminiferous epithelium.Citation28,Citation30 This time point was designated as day 0. Thereafter, rats received two additional injections of anti-adjudin or non-immune rabbit IgG (100 µg each) on days 3 and 6. These days were chosen because the most extensive loss of germ cells from the seminiferous epithelium occurred during this time. All animals in this experiment were sacrificed on day 21. Testes were removed, embedded in paraffin and stained with hematoxylin and eosin (H&E).
Adjudin radioimmunoassay (RIA).
An adjudin RIA was established as follows. The appropriate antiserum dilution was estimated in pilot experiments using various dilutions ranging from 1:10 to 1:1,000. A 1:50 dilution was selected because it yielded a ∼30% binding, which was almost completely displaced by unlabeled adjudin. A standard curve was prepared in triplicate using unlabeled adjudin (or lonidamine) diluted in ethanol ranging from 0.1 to 300 ng per assay tube to assess their ability to displace [3H]-adjudin from binding to the anti-adjudin antibody. RIA buffer (0.05 M Tris-HCl pH 8.0 at 22°C containing 0.1 M NaCl, 0.1% NaN3 [w/v] and 0.1% gelatin [w/v]), cold adjudin (dissolved in ethanol at 1 mg/ml) and adjudin antiserum (1:50) were combined in reaction tubes to a final volume of 600 µl. Samples were vortexed and incubated at room temperature for 30 min. Thereafter, 100 µl [3H]-adjudin (∼2 × 104 cpm) was added into each tube, samples vortexed and incubated at 60°C in a shaking water bath. Samples were cooled down at 4°C before adding 300 µl ice-cold dextran-coated charcoal suspension (0.5% activated charcoal [w/v] in RIA buffer containing 0.05% dextran T70 [w/v]) to each tube. Samples were vortexed and placed on an ice-water bath for 10 min, followed by centrifugation at 2,500 g for 10 min at 4°C. Supernatants were carefully decanted into scintillation vials and ∼5-ml of Ready Safe scintillation fluid (Beckman) was added. Radioactivity was quantified in supernatants with a Packard Tri-Carb 2900TR β-counter. Non-specific binding was assessed by using RIA buffer containing 0.1% gelatin [w/v]. Assays were computer-analyzed by fitting the standard curve to a four-parameter logistic function and unknowns were interpolated from the resultant curve using the software program SigmaPlot (version 12, Systat Software Inc.). The minimal detectable dose was ∼0.35 ng adjudin per assay tube and 50% displacement was at 9.5 ng adjudin. The intra- and inter-assay coefficient of variation was 8% and 12%, respectively.
Identification of the cellular targets of adjudin.
Adjudin (100 mg/kg b.w.) was administered orally to adult rats (∼300 gm b.w., n = 3). Animals were sacrificed 4 hours after administration when the level of adjudin was highest in the testis.Citation6 Also, this time point was selected because it preceded the loss of elongated spermatids from the seminiferous epithelium which occurred at ∼6.5 hours.Citation32 Testes from three different rats were combined and used to prepare lysate in lysis buffer (50 mM Tris, pH 7.4 at 22°C containing 0.15 M NaCl, 10% glycerol [v/v], 1% NP-40 [v/v], 1 mM NaF, 1 mM Na3VO4, 1 mM AEBSF, 1 mM EDTA, 150 µM bestatin, 10 µg/ml E-64, 1 µM leupeptin and 1 µM aprotinin) as previously described Citation67,Citation68 and used for the following experiment. In brief, an Econo-Column (1.5 × 10 cm, i.d., BIO-RAD) was packed with Protein A-Sepharose (Amersham Biosciences/GE Life Sciences) and washed with PBS pH 7.4 at 22°C at a flow rate of ∼20 ml/hour. Anti-adjudin IgG was immobilized onto Sepharose beads by re-circulation and the column was washed extensively to remove unbound IgG. Thereafter, ∼500 mg testis lysate was re-circulated for 3–4 hours at room temperature, and the column was washed with ten column volumes of PBS pH 7.4 at 22°C. Bound proteins were eluted with elution buffer (0.2 M glycine, pH 2.5 at 22°C) and designated as adjudin binding proteins. The pH of this eluted sample was reconstituted to 7.4 using Tris, the sample was concentrated using a Microcon centrifugal filter unit (Mr cut-off at 10 kDa) and an aliquot was resolved by SDS-PAGE for Coomassie blue gel staining. Proteins were subjected to in-gel digestion by trypsin and identified by liquid chromatography-mass spectrometry (LC-MS) which was performed at the Proteomics Resource Center, The Rockefeller University. Results obtained from LC-MS experiments were confirmed by SDS-PAGE and immunoblotting, which were performed by standard protocols.Citation67,Citation68
Competitive binding assays.
Competitive binding assays were performed to validate the pull-down of testin and actin by anti-adjudin IgG. In brief, binding buffer (see above), testis lysate (0 and 4 hours and 4 day post-adjudin treatment), [3H]-adjudin (30 × 104 cpm) and anti-actin IgG (1–2 µl; Santa Cruz Biotechnology; cat # sc-7210, lot # G1404 or cat # sc-1616, lot # K0405) or anti-testin IgG (1–2 µl)Citation22,Citation23 in the absence (to assess total binding) or presence (to assess displacement, thereby monitoring binding specificity) of 5 µg unlabeled adjudin (1 mg/ml stock prepared in ethanol). All samples were combined in a final volume of 500 µl. Samples were vortexed and incubated at 4°C for 36 hours. Thereafter, Sac cells were added and samples were incubated at 4°C for 1 hour. Samples were centrifuged and radioactivity quantified in pellets in a β-counter with 1 ml of scintillation fluid. As a control, rabbit IgG was used in place of anti-actin or anti-testin IgG. Non-specific binding was assessed by using binding buffer containing 0.5% BSA [w/v].
General methods.
Protein concentrations were determined by the bicinchoninic acid protein assay (Thermo Scientific). Paraffin embedment of testes and H&E staining were performed using routine protocols. Light microscopy was carried out by using a Nikon Eclipse 90i microscope with a built-in DS-Fi1 12.5 MPx digital camera with Nikon NIS-Elements imaging software package (version 3.2). Statistical analyses were performed by Student's t-test or two-way ANOVA followed by Tukey's post hoc test using InStat software package (version 3.01; GraphPad Software, Inc.). p < 0.5 was taken as statistically significant.
Figures and Tables
Figure 1 Use of Sertoli and germ cell co-cultures to assess the effects of adjudin on disrupting germ cell adhesion in vitro. (A) This is a typical Sertoli cell epithelium in which cells were cultured for 4 days. A functional Sertoli cell barrier, manifested by the presence of TJs (see blue arrowheads) and basal ES, is visible between adjacent Sertoli cells. The basal ES is typified by the presence of actin filament bundles (see green arrowheads) sandwiched in between cisternae of endoplasmic reticulum (ER) and apposing Sertoli cell plasma membranes (see orange arrowheads). Desmosomes, typified by the presence of electron-dense material, are also visible (see red arrowheads). (B) A germ cell attaches to the Sertoli cell epithelium ∼48 hours after seeding germ cells (Nu, Sertoli cell nucleus). (C) This is a step 9–10 spermatid with a functional apical ES, typified by the presence of actin filament bundles (see green arrowheads) sandwiched in between cisternae of ER and apposing Sertoli cell-spermatid plasma membranes (see orange arrowheads). Both the acrosome (Ac) and condensed general material within the nucleus (Nu) are visible. Microvilli (A–C) are also seen in all micrographs (see black arrows). (D) Germ cells, such as those shown in (B and C) were fluorescently labeled and added onto the Sertoli cell epithelium (A) at time 0. After Sertoli-germ cell adhesion was established, adjudin (1 µg/ml) was added into triplicate wells per time point to assess its effects on germ cell adhesion (see Materials and Methods). A loss in germ cell adhesion was detected 1 hour (hr) after treatment when the amount of fluorescence remaining in co-cultures was quantified by cytofluorometry. Error bars represent mean ± SD from at least three different experiments. *p < 0.05; ns, not significant. (two-way ANOVA followed by Tukey's post-hoc test). Bar in A = 0.5 µm, B = 4 µm, C = 0.3 µm.
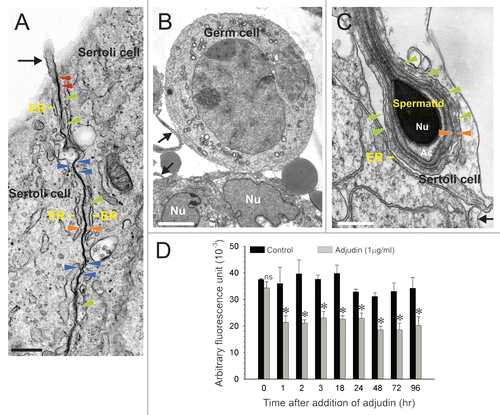
Figure 2 Conjugation of adjudin to KLH (keyhole limpet hemocyanin, an adjuvant), and its use as an immunogen for anti-adjudin antibody production. KLH was first reacted with SFB (succinimidyl 4-formylbenzoate, a heterobifunctional crosslinker) to generate a benzaldehyde at its N-terminus. This modified KLH was then reacted spontaneously in physiological buffer with the hydrazide group in adjudin, forming a stable KLH-adjudin conjugate via a hydrazone linkage.
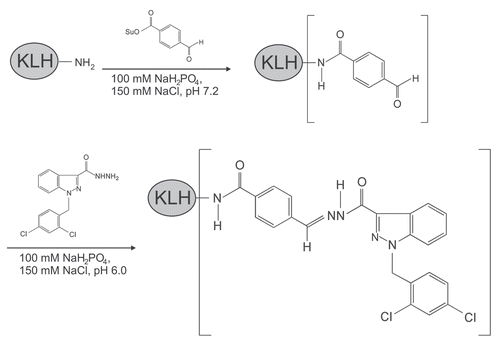
Figure 3 Specificity analysis of anti-adjudin antiserum. Structural formulae of adjudin (A) and lonidamine (B). (C) Displacement of [3H]-adjudin binding to anti-adjudin anti-serum by unlabelled (i.e., cold) adjudin, lonidamine, testosterone or estradiol-17β. Adjudin competed with the binding of [3H]-adjudin to its antibody. While lonidamine shares structural similarities with adjudin, it generated a parallel but incomplete displacement curve. Both testosterone and estradiol-17β failed to compete with the binding of [3H]-adjudin to its antibody.
![Figure 3 Specificity analysis of anti-adjudin antiserum. Structural formulae of adjudin (A) and lonidamine (B). (C) Displacement of [3H]-adjudin binding to anti-adjudin anti-serum by unlabelled (i.e., cold) adjudin, lonidamine, testosterone or estradiol-17β. Adjudin competed with the binding of [3H]-adjudin to its antibody. While lonidamine shares structural similarities with adjudin, it generated a parallel but incomplete displacement curve. Both testosterone and estradiol-17β failed to compete with the binding of [3H]-adjudin to its antibody.](/cms/asset/b2487503-9805-449f-83a3-26f148ea5f42/kspe_a_10916449_f0003.gif)
Figure 4 A study to assess the ability of the anti-adjudin antibody to protect adjudin-induced germ cell loss from the seminiferous epithelium in rat testes. (A) Adjudin competed with the binding of [3H]-adjudin to its antibody in a competitive binding assay. (B) Anti-adjudin IgG blocked the effects of adjudin on Sertoli-germ cell adhesion. (i) Cross-section of the control testis. (ii) Cross-section of the testis 21 days after treatment with adjudin (50 mg/kg b.w.). (iii) Cross-section of the testis 21 days after treatment with non-immune rabbit IgG and adjudin (50 mg/kg b.w.). (iv) Cross-section of the testis 21 days after treatment with anti-adjudin IgG and adjudin. All cross-sections were stained with H&E. Boxed areas in (i–iv) represent magnified views, and these are shown to the right of each image. Bar in (Bi) [also applies to (Bii–iv)] = 125 µm; bar in magnified views = 75 µm. Error bars represent mean ± SD from four different experiments. *p < 0.05. (two-way ANOVA followed by Tukey's post-hoc test).
![Figure 4 A study to assess the ability of the anti-adjudin antibody to protect adjudin-induced germ cell loss from the seminiferous epithelium in rat testes. (A) Adjudin competed with the binding of [3H]-adjudin to its antibody in a competitive binding assay. (B) Anti-adjudin IgG blocked the effects of adjudin on Sertoli-germ cell adhesion. (i) Cross-section of the control testis. (ii) Cross-section of the testis 21 days after treatment with adjudin (50 mg/kg b.w.). (iii) Cross-section of the testis 21 days after treatment with non-immune rabbit IgG and adjudin (50 mg/kg b.w.). (iv) Cross-section of the testis 21 days after treatment with anti-adjudin IgG and adjudin. All cross-sections were stained with H&E. Boxed areas in (i–iv) represent magnified views, and these are shown to the right of each image. Bar in (Bi) [also applies to (Bii–iv)] = 125 µm; bar in magnified views = 75 µm. Error bars represent mean ± SD from four different experiments. *p < 0.05. (two-way ANOVA followed by Tukey's post-hoc test).](/cms/asset/a2e087da-7e32-4b58-b9ef-bbaf579a4237/kspe_a_10916449_f0004.gif)
Figure 5 Identification of actin and myosin as molecular targets of adjudin in the rat testis by co-immunoprecipitation and mass spectrometry. (A) Actin and myosin were found to bind adjudin as determined by co-immunoprecipitation using affinity chromatography with an anti-adjudin IgG-Protein A resin, coupled with SDS-PAGE, Coomassie blue gel staining and mass spectrometry. M, molecular weight marker. (B) Sequences corresponding to actin (i) and myosin (ii) as obtained by mass spectrometry.
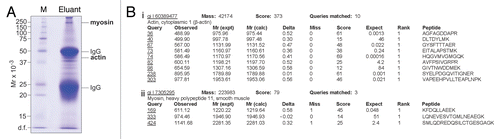
Figure 6 Characterization of actin as a molecular target of adjudin. (A) Adjudin binds to actin (42 kDa) as determined by co-immunoprecipitation. Aliquots (lane 1, 10 µl; lane 2, 30 µl) of the eluant () were resolved by SDS-PAGE and proteins were transferred to nitrocellulose for immunoblotting using anti-actin IgG. Ctrl, testis lysate (20 µg). (B) The interaction of adjudin with actin was specific since unlabeled (i.e., cold) adjudin displaced the binding of [3H]-adjudin to actin IgG in a competitive binding assay. Error bars represent mean ± SD from three different experiments. *p < 0.05; ns, not significant. (Student's t-test).
![Figure 6 Characterization of actin as a molecular target of adjudin. (A) Adjudin binds to actin (42 kDa) as determined by co-immunoprecipitation. Aliquots (lane 1, 10 µl; lane 2, 30 µl) of the eluant (Fig. 5A) were resolved by SDS-PAGE and proteins were transferred to nitrocellulose for immunoblotting using anti-actin IgG. Ctrl, testis lysate (20 µg). (B) The interaction of adjudin with actin was specific since unlabeled (i.e., cold) adjudin displaced the binding of [3H]-adjudin to actin IgG in a competitive binding assay. Error bars represent mean ± SD from three different experiments. *p < 0.05; ns, not significant. (Student's t-test).](/cms/asset/9cf8bd90-fb49-4bdd-b1f8-f6e734218702/kspe_a_10916449_f0006.gif)
Figure 7 Characterization of testin as a molecular target of adjudin. (A) Adjudin binds to testin (35 and 37 kDa) as determined by co-immunoprecipitation. Aliquots (lane 1, 10 µl; lane 2, 30 µl) of the eluant () were resolved by SDS-PAGE and proteins were transferred to nitrocellulose for immunoblotting using a monospecific anti-testin antibody.Citation22,Citation23 Ctrl, testis lysate (100 µg). (B) The interaction of adjudin with testin was specific since unlabeled (i.e., cold) adjudin displaced the binding of [3H]-adjudin to testin IgG in a competitive binding assay. Error bars represent mean ± SD from three different experiments. *p < 0.05. (Student's t-test).
![Figure 7 Characterization of testin as a molecular target of adjudin. (A) Adjudin binds to testin (35 and 37 kDa) as determined by co-immunoprecipitation. Aliquots (lane 1, 10 µl; lane 2, 30 µl) of the eluant (Fig. 5A) were resolved by SDS-PAGE and proteins were transferred to nitrocellulose for immunoblotting using a monospecific anti-testin antibody.Citation22,Citation23 Ctrl, testis lysate (100 µg). (B) The interaction of adjudin with testin was specific since unlabeled (i.e., cold) adjudin displaced the binding of [3H]-adjudin to testin IgG in a competitive binding assay. Error bars represent mean ± SD from three different experiments. *p < 0.05. (Student's t-test).](/cms/asset/7850e355-e0d9-40e6-bbaf-593b3f6b09a2/kspe_a_10916449_f0007.gif)
Acknowledgments
This work was supported in part by grants from the National Institutes of Health (NICHD, R01 HD056034 and R01 HD056034-02-S1 to C.Y.C.; U54 HD029990 Project 5 to C.Y.C.; R03 HD061401 to D.D.M.).
References
- Cheng CY, Mruk DD. The biology of spermatogenesis: the past, present and future. Philos Trans R Soc Lond B Biol Sci 2010; 365:1459 - 1463
- Cheng CY, Mruk DD. New frontiers in non-hormonal male contraception. Contraception 2010; 82:476 - 482
- Wang C, Swerdloff RS. Hormonal approaches to male contraception. Curr Opin Urol 2010; 20:520 - 524
- Nieschlag E. Male hormonal contraception. Handb Exp Pharmacol 2010; 198:197 - 223
- O'Donnell L, Meachem SJ, Stanton PG, McLachlan RI. Neill JD. Endocrine regulation of spermatogenesis. Physiology of Reproduction 2006; Amsterdam Elsevier 1017 - 1069
- Cheng CY, Mruk DD, Silvestrini B, Bonanomi M, Wong CH, Siu MKY, et al. AF-2364 [1-(2,4-dichlorobenzyl)-1H-indazole-3-carbohydrazide] is a potential male contraceptive: a review of recent data. Contraception 2005; 72:251 - 261
- Mruk DD, Wong CH, Silvestrini B, Cheng CY. A male contraceptive targeting germ cell adhesion. Nat Med 2006; 12:1323 - 1328
- Cheng CY, Mruk DD. A local autocrine axis in the testes that regulates spermatogenesis. Nat Rev Endocrinol 2010; 6:380 - 395
- Mruk DD, Cheng CY. Cell-cell interactions at the ectoplasmic specialization in the testis. Trends Endocrinol Metab 2004; 15:439 - 447
- O'Donnell L, Nicholls PK, O'Bryan MK, McLachlan RI, Stanton PG. Spermiation: the process of sperm release. Spermatogenesis 2011; 1:14 - 35
- Mruk DD, Cheng CY. Sertoli-Sertoli and Sertoli-germ cell interactions and their significance in germ cell movement in the seminiferous epithelium during spermatogenesis. Endocr Rev 2004; 25:747 - 806
- Toyama Y, Maekawa M, Yuasa S. Ectoplasmic specializations in the Sertoli cells: new vistas based on genetic defects and testicular toxicology. Anat Sci Int 2003; 78:1 - 16
- Vogl AW, Pfeiffer DC, Mulholland D, Kimel G, Guttman J. Unique and multifunctional adhesion junctions in the testis: ectoplasmic specializations. Arch Histol Cytol 2000; 63:1 - 15
- Vogl AW, Vaid KS, Guttman JA. Cheng CY. The Sertoli cell cytoskeleton. Molecular Mechanisms in Spermatogenesis 2008; Austin Landes Bioscience/Springer Science + Business Media LLC 186 - 211
- Wolski KM, Perrault C, Tran-Son-Tay R, Cameron DF. Strength measurement of the Sertoli-spermatid junctional complex. J Androl 2005; 26:354 - 359
- Lie PPY, Cheng CY, Mruk DD. The biology of the desmosome-like junction: a versatile anchoring junction and signal transducer in the seminiferous epithelium. Int Rev Cell Mol Biol 2011; 286:223 - 269
- Yan HHN, Mruk DD, Cheng CY. Junction restructuring and spermatogenesis: the biology, regulation, and implication in male conraceptive development. Curr Top Dev Biol 2008; 80:57 - 92
- Cheng CY, Mruk DD. An intracellular trafficking pathway in the seminiferous epithelium regulating spermatogenesis: a biochemical and molecular perspective. Crit Rev Biochem Mol Biol 2009; 44:245 - 263
- Mruk DD, Silvestrini B, Cheng CY. Anchoring junctions as drug targets: role in contraceptive development. Pharmacol Rev 2008; 60:146 - 180
- Siu MK, Cheng CY. Dynamic cross-talk between cells and the extracellular matrix in the testis. BioEssays 2004; 26:978 - 992
- Yan HH, Mruk DD, Lee WM, Cheng CY. Ectoplasmic specialization: a friend or a foe of spermatogenesis?. BioEssays 2007; 29:36 - 48
- Cheng CY, Bardin CW. Identification of two testosterone-responsive testicular proteins in Sertoli cell-enriched culture medium whose secretion is suppressed by cells of the intact seminiferous tubule. J Biol Chem 1987; 262:12768 - 12779
- Cheng CY, Grima J, Stahler MS, Lockshin RA, Bardin CW. Testins are structurally related Sertoli cell proteins whose secretion is tightly coupled to the presence of germ cells. J Biol Chem 1989; 264:21386 - 21393
- Grima J, Zhu LJ, Zong SD, Catterall JF, Bardin CW, Cheng CY. Rat testin is a newly identified component of the junctional complexes in various tissues whose mRNA is predominantly expressed in the testis and ovary. Biol Reprod 1995; 52:340 - 355
- Grima J, Zhu LJ, Cheng CY. Testin is tightly associated with testicular cell membrane upon its secretion by Sertoli cells whose steady-state mRNA level in the testis correlates with the turnover and integrity of inter-testicular cell junctions. J Biol Chem 1997; 272:6499 - 6509
- Grima J, Wong CCS, Zhu LJ, Zong SD, Cheng CY. Testin secreted by Sertoli cells is associated with the cell surface and its expression correlates with the disruption of Sertoli-germ cell junctions but not the inter-Sertoli tight junction. J Biol Chem 1998; 273:21040 - 21053
- Zong SD, Zhu LJ, Grima J, Aravindan GR, Bardin CW, Cheng CY. Cyclic and postnatal developmental changes of testin in the rat seminiferous epithelium—an immunohistochemical study. Biol Reprod 1994; 51:843 - 851
- Cheng CY, Silvestrini B, Grima J, Mo MY, Zhu LJ, Johansson E, et al. Two new male contraceptives exert their effects by depleting germ cells prematurely from the testis. Biol Reprod 2001; 65:449 - 461
- Xia W, Mruk DD, Lee WM, Cheng CY. Unraveling the molecular targets pertinent to junction restructuring events during spermatogenesis using the adjudin-induced germ cell depletion model. J Endocrinol 2007; 192:563 - 583
- Mok KW, Mruk DD, Lie PPY, Lui WY, Cheng CY. Adjudin, a potential male contraceptive, exerts its effects locally in the seminiferous epithelium of mammalian testes. Reproduction 2011; 141:571 - 580
- Cheng CY, Mruk DD. Harris JR, Graham JM, Rickwood D. Tight junction assembly. Cell Biology Protocols 2006; New York John Wiley 296 - 299
- Chen YM, Lee NPY, Mruk DD, Lee WM, Cheng CY. Fer kinase/Fer T and adherens junction dynamics in the testis: an in vitro and in vivo study. Biol Reprod 2003; 69:656 - 672
- Wolski KM, Mruk DD, Cameron DF. The Sertolispermatid junctional complex adhesion strength is affected in vitro by adjudin. J Androl 2006; 27:790 - 794
- Koskimies AI, Kormano M, Lahti A. A difference in the immunoglobulin content of seminiferous tubule fluid and rete testis fluid of the rat. J Reprod Fertil 1971; 27:463 - 465
- Setchell BP. Finn CA. Sermatogenesis. The Mammalian Testis 1978; New York Cornell University Press 181 - 232
- Mruk DD. New perspectives in non-hormonal male contraception. Trends Endocrinol Metab 2008; 19:57 - 64
- Cheng CY, Mruk DD. Regulation of spermiogenesis, spermiation and blood-testis barrier dynamics: novel insights from studies on Eps8 and Arp3. Biochem J 2011; 435:553 - 562
- Mruk DD, Lau ASN. RAB13 participates in ectoplasmic specialization dynamics in the rat testis. Biol Reprod 2009; 80:590 - 601
- Wong EWP, Mruk DD, Lee WM, Cheng CY. Par3/Par6 polarity complex coordinates apical ectoplasmic specialization and blood-testis barrier restructuring during spermatogenesis. Proc Natl Acad Sci USA 2008; 105:9657 - 9662
- Mok KW, Mruk DD, Lee WM, Cheng CY. Spermatogonial stem cells alone are not sufficient to re-initiate spermatogenesis in the rat testis following adjudin-induced infertility. Int J Androl 2011; DOI: 101111/j1365-2605201001183x
- Zong SD, Bardin CW, Phillips D, Cheng CY. Testins are localized to the junctional complexes of rat Sertoli and epididymal cells. Biol Reprod 1992; 47:568 - 572
- Toyama Y, Maekawa M, Yuasa S. Ectoplasmic specializations in the Sertoli cell: new vistas based on genetic defects and testicular toxicology. Anat Sci Int 2003; 78:1 - 16
- Disanza A, Carlier MF, Stradal TEB, Didry D, Frittoli E, Confalonieri S, et al. Eps8 controls actin-based motility by capping the barbed ends of actin filaments. Nat Cell Biol 2004; 6:1180 - 1188
- Higgs HN. There goes the neighbourhood: Eps8 joins the barbed-end crowd. Nat Cell Biol 2004; 6:1147 - 1149
- Firat-Karalar EN, Welch MD. New mechanisms and functions of actin nucleation. Curr Opin Cell Biol 2011; 23:4 - 13
- Rottner K, Hanisch J, Campellone KG. WASH, WHAMM and JMY: regulation of Arp2/3 complex and beyond. Trends Cell Biol 2010; 20:650 - 661
- Silvestrini B, Palazzo G, De Gregorio M. Lonidamine and related compounds. Prog Med Chem 1984; 21:111 - 135
- Tash JS, Attardi B, Hild SA, Chakrasali R, Jakkaraj SR, Georg GI. A novel potent indazole carboxylic acid derivative blocks spermatogenesis and is contraceptive in rats after a single oral dose. Biol Reprod 2008; 78:1127 - 1138
- Santiemma V, Tullio G, Iapadre G, Fabbrini A. Effects of lonidamine on pituitary-gonadal axis in man. Oncology 1984; 41:53 - 55
- Evans WK, Sheperd FA, Mullis B. Phase II evaluation of lonidamine in patients with advanced malignancy. Oncology 1984; 41:69 - 77
- Grima J, Cheng CY. Testin induction: the role of cyclic 3′, ′-adenosine monophosphate/protein kinase A signaling in the regulation of basal and lonidamine-induced testin expression by rat Sertoli cells. Biol Reprod 2000; 63:1648 - 1660
- Parson JT, Horwitz AR, Schwartz MA. Cell adhesion: integrating cytoskeletal dynamics and cellular tension. Nat Rev Mol Cell Biol 2010; 11:633 - 643
- Tash JS, Chakrasali R, Jakkaraj SR, Hughes J, Smith SK, Hornbaker K, et al. Gamendazole, an orally active indazole carboxylic acid male contraceptive agent, targets HSP90AB1 (HSP90BHETA) and EEFA1 (eEF1A), and stimulates Il1a transcription in rat Sertoli cells. Biol Reprod 2008; 78:1139 - 1152
- Cheng CY, Mather JP, Byer AL, Bardin CW. Identification of hormonally responsive proteins in primary Sertoli cell culture medium by anion-exchange high performance liquid chromatography. Endocrinology 1986; 118:480 - 488
- Mruk DD, Siu MKY, Conway AM, Lee NPY, Lau ASN, Cheng CY. Role of tissue inhibitor of metallo-proteases-1 in junction dynamics in the testis. J Androl 2003; 24:510 - 523
- Orth JM. Proliferation of Sertoli cells in fetal and post-natal rats: a quantitative autoradiographic study. Anat Rec 1982; 203:485 - 492
- Li JCH, Lee WM, Mruk DD, Cheng CY. Regulation of Sertoli cell myotubularin (rMTM) expression by germ cells in vitro. J Androl 2001; 22:266 - 277
- Lui WY, Lee WM, Cheng CY. Transforming growth factor-β3 regulates the dynamics of Sertoli cell tight junctions via the p38 mitogen-activated protein kinase pathway. Biol Reprod 2003; 68:1597 - 1612
- Galdieri M, Ziparo E, Palombi F, Russo MA, Stefanini M. Pure Sertoli cell cultures: a new model for the study of somatic-germ cell interactions. J Androl 1981; 5:249 - 259
- Lee NPY, Mruk DD, Conway AM, Cheng CY. Zyxin, axin and Wiskott-Aldrich syndrome protein are adaptors that link the cadherin/catenin protein complex to the cytoskeleton at adherens junctions in the seminiferous epithelium of the rat testis. J Androl 2004; 25:200 - 215
- Aravindan GR, Pineau C, Bardin CW, Cheng CY. Ability of trypsin in mimicking germ cell factors that affect Sertoli cell secretory function. J Cell Physiol 1996; 168:123 - 133
- Ono S. Mechanism of depolymerization and severing of actin filaments and its significance in cytoskeletal dynamics. Int Rev Cytol 2007; 258:1 - 82
- Cooper HM, Paterson Y. Production of antibodies 2005; Hoboken John Wiley & Sons
- Cheng CY, Mathur PP, Grima J. Structural analysis of clusterin and its subunits in ram rete testis fluid. Biochemistry 1988; 27:4079 - 4088
- Page M, Thorpe R. Walker JM. Purification of IgG using DEAE-Sepharose chromatography. The Protein Protocols Handbook 2001; Totowa Humana Press, Inc. 987 - 988
- Page M, Thorpe R. Walker JM. Purification of IgG by precipitation with sodium sulfate or ammonium sulfate. The Protein Protocols Handbook 2001; Totowa Humana Press, Inc. 983 - 984
- Lau ASN, Mruk DD. Rab8B GTPase and junction dynamics in the testis. Endocrinology 2003; 144:1549 - 1563
- Sarkar O, Xia W, Mruk DD. Adjudin-mediated junction restructuring in the seminiferous epithelium leads to displacement of soluble guanylate cyclase from adherens junctions. J Cell Physiol 2006; 208:175 - 187