Abstract
Intestines are organs that not only digest food and absorb nutrients, but also provide a defense barrier against pathogens and noxious agents ingested. Tight junctions (TJs) are the most apical component of the junctional complex, providing one form of cell-cell adhesion in enterocytes and playing a critical role in regulating paracellular barrier permeability. Alteration of TJs leads to a number of pathophysiological diseases causing malabsorption of nutrition and intestinal structure disruption, which may even contribute to systemic organ failure. Claudins are the major structural and functional components of TJs with at least 24 members in mammals. Claudins have distinct charge-selectivity, either by tightening the paracellular pathway or functioning as paracellular channels, regulating ions and small molecules passing through the paracellular pathway. In this review, we have discussed the functions of claudin family members, their distribution and localization in the intestinal tract of mammals, their alterations in intestine-related diseases and chemicals/agents that regulate the expression and localization of claudins as well as the intestinal permeability, which provide a therapeutic view for treating intestinal diseases.
Claudin Distributions and Functions in Intestines
Intestinal epithelial integrity is vital for nutrition absorption and host defense against pathogens. The gastrointestinal (GI) tract can be divided into the small intestine that includes duodenum, jejunum and ileum and the large intestine that contains cecum and colon. Both intestines share a general structure with four layers: mucosa, submucosa, muscularis externa and serosa. Intestinal epithelium (enterocytes) is in the mucosa layer. Tight junctions (TJs) are the most apical structure present in the junctional complex between the epithelial cells. They form a circumferential belt around the epithelial sheet, separating the cell membrane into apical (the side toward the lumen) and basolateral (the side facing the extracellular matrix) domains. The permeability of TJs varies in different segments of intestines. Well-formed TJs are characterized by high transepithelial electrical resistance (TER) and low solute permeability. The permeability of TJs can be determined by measuring the paracellular fluxes of ions and small molecules such as 3H-mannitol and FITC-dextran. The permeability of TJs changes under different physiological and pathological conditions.
There are three main families of TJ proteins: the claudin family, the occludin family and the IgG-like family of junctional adhesion molecules (JAMs).Citation1 Claudins are the main determinants of barrier properties of the TJs. Up to now, at least 24 members of the claudin family have been identified. Claudins are transmembrane proteins containing two extracellular domains and one intracellular domain with N- and C-termini facing the cytoplasm.Citation2 Many claudins have distinct charge-selectivity. Some claudins act by plugging the paracellular pathway while others function as paracellular channels. For example, claudin-2 and -15 are cation-selective while claudin-17 is anion-selective.Citation3 The incorporation and association of occludin, claudins and JAMs into TJ strands require local clustering of scaffolding proteins, an important group of which is ZO proteins, such as ZO-1, ZO-2 and ZO-3.Citation4
Differential expression patterns of claudin members in the GI tract are likely to contribute to local diversity of TER and paracellular ion flow. In human, real-time RT-PCR reveals that claudin-2 and -15 are predominantly expressed in the proximal parts of the GI tract.Citation5 By immunofluorescence staining, claudin-1 is expressed and mainly localized at the apex of epithelial cells with a typical reticular pattern in the colon.Citation6,Citation7 Claudin-2 is detected in both villus and crypt cells of the small intestine but restricted to undifferentiated crypt cells in the colon. Claudin-3, -4, -7 and -8 are predominantly expressed in the distal parts (colon, sigmoid and rectum) of the GI tract. Claudin-12 shows an ubiquitous expression pattern throughout the entire GI tract.Citation5 Claudin-4 and -7 are detected in both the lateral membrane of the cell surface and the TJs.Citation8
In mice, mRNAs of claudins 1–5, 7–15, 17 and 18 are all detected in GI tract with claudin-2, -3, -7 and -15 being the most highly expressed while claudin-1, -5, -9, -10 and -11 being weakly expressed.Citation9,Citation10 Both claudin-7 mRNA and protein are strongly expressed in the duodenum, jejunum, ileum and colon.Citation11 Claudin-8 mRNA and protein are moderately expressed in the ileum and colon, but were absent in the jejunum and duodenum. Claudin-12 mRNA and protein are highly expressed in the ileum and are moderately observed in the jejunum and colon and barely detected in the duodenum. Claudin-13 mRNA and protein are observed in colon, but undetectable in the rest of the intestines. The expression of claudin-15 mRNA and protein is high in the duodenum and jejunum, but is decreased from the ileum to colon.Citation12 Claudin expression profiles in both human and mice are shown in .
Table 1. Expression of claudins in mammalian intestines
In terms of sub-cellular localization, claudin-2 protein is localized in the deep crypt of the distal colon while claudin-10 is localized in the entire crypt.Citation9 Claudin-7 is expressed along apical and basolateral cell surfaces of epithelial cells in small intestines, while claudin-8 is distributed on basolateral membranes of epithelial cells in the ileum and colon.Citation11,Citation12 Claudin-12 is localized at the apical-most tips of lateral membranes of epithelial cells in the jejunum, ileum and colon, but not in the duodenum. Claudin-13 is distributed at the apical membranes of colonic epithelial cells and claudin-15 is localized at the apical membranes of epithelial cells in both the small and large intestines.Citation12,Citation13
Peyer's patches are important sites for mucosal immune responses in intestines, and it contains follicle-associated epithelium (FAE) structure. Claudin-1 is detected in the TJs of intestinal lymphoid FAE in mice;Citation14 claudin-2 is only weakly expressed on the crypt side of the FAE compared with stronger expression on the crypt side of villous epithelial cells; claudin-3 is found throughout the dome of the FAE; claudin-4 is preferentially expressed in the apex region of the FAE in mice.Citation15
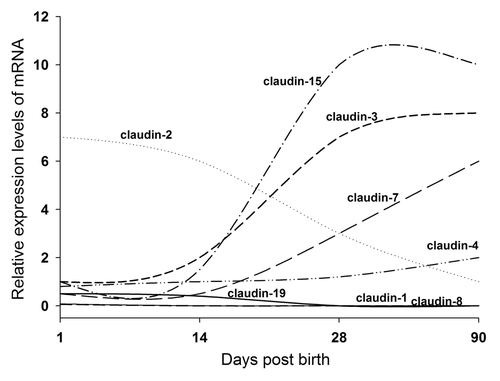
The expression of claudins also changes during the postnatal development. During the development, rodent intestinal barrier function matures in the first 3 weeks of life due to the establishment of the intestinal flora. Commensal bacterial colonization induces intestinal barrier function maturation by promoting claudin-3 expression in mice.Citation16 In mice, by RT-PCR, claudin-19 is only detected at days 1, 14 and 28. Claudin-2 expression decreases continuously from birth to 90 d by 10-fold, while claudin-3, -4, -7 and -15 increase in the range of 2- to 20-fold. Claudin-1 and -8 fall dramatically after day 1 and claudin-12 doubles between day 14 and 28 ().Citation9
Figure 1. Claudin profiling during postnal intestinal development in mice (adapted from Holmes et al., 2006).
The rat colon shows the highest epithelial resistance, followed by duodenum, jejunum and ileum. Duodenum and colon have the strongest expression of “tightening” claudin-1, -3, -4, -5 and -8 and the lowest expression of claudins mediating ion permeability, mainly claudin-2, -7 and -12, compared with jejunum and ileum.Citation17 Claudin-3, -4 and -5 are all detectable in ileum, but only claudin-3 and -5 are detected in jejunum in rats. Claudin-4 expression is also found in the enteric neurons in rat distal colon.Citation18 The localization of claudins can be junctional, lateral or show a gradient in junctional and/or lateral distribution along the crypt-to-villus surface axis. Claudin-2 is restricted to crypts in the small and large intestines. Claudin-3 localizes both at the TJs and the lateral membrane and increases from the crypt to surface cells of the colon. Claudin-4 expression is highest in the lateral membrane of the villus surface epithelial cells in both the small and large intestines. Claudin-5 is strictly located at the junctions of both endothelial and epithelial cells and shows no gradation along the crypt-to-villus surface axis in any part of the intestines. Claudin-2 shows a crypt-to-villus decrease.Citation19
In dogs, there are high expressions of claudin-3 and -5 and a weak expression of claudin-7 in the duodenum. In the colon, there are high expressions of claudin-2 and -3 and weak expressions of claudin-5 and -7 proteins by western blot. The localization detected by immunofluorescence microscopy shows that the duodenum and colon have the staining for claudin-3 and -5 in the most apical region and claudin-7 in the basolateral region.Citation20
Studies of claudin mutations in humans and gene-knockout in mice have revealed specific roles for a number of claudins in the TJ barrier function, selective ion permeability, as well as their related pathological phenotypes.
Claudin-7 forms a protein complex with claudin-1 and integrin α2 at the basolateral surface in mouse intestines. Knockout of claudin-7 in mice (Cldn7−/−) has severe intestinal defects resembling inflammatory bowel disease (IBD) syndrome that includes mucosal ulcerations, epithelial cell sloughing and inflammation, which lead to the death of the mice. At the molecular level, Cldn7−/− intestines produce significantly higher levels of cytokines, NF-κB p65 and cyclooxygenase 2 (COX-2) as well as matrix metalloproteinase (MMP)-3 and -7.Citation11 Claudin-8 is found to be involved in regulating paracellular Na+ permeability, protecting the leakage of Na+ into the intestinal lumen.Citation21 Claudin-2 and -12 contribute to Ca2+ absorption in intestinal epithelial cells. The expression of claudin-2 and -12 could be induced by an active form of vitamin D3, 1α, 25-dihydroxyvitamin D3.Citation22,Citation23 The small intestine is responsible for nutrient absorption and the absorption of monosacchrides, amino acids and vitamin C is coupled directly to Na+ absorption. It has been reported that claudin-15 knockout mice are born and grow normally but with an enlarged upper small intestinal phenotype, megaintestine, in that the upper small intestine is approximately 2 times larger than normal in length and diameter. Knockout of claudin-15 does not alter the expression of other types of claudins, such as claudin-1, -2, -3, -4, -7, -12, -18, -20 and -23.Citation24 Double knockout of claudin-2 and -15 reduces the paracellular flow of Na+ from intestinal submucosa into the lumen and decreases the absorption of glucose, amino acids and fats, thus leads to the malnutrition and death of the mice 25 d after birth.Citation25 Claudin-16 may be responsible for the defective absorption of Ca2+ in the intestines causing primary hypercalciuria.Citation26
The function of claudins in intestines just begins to be unraveled in recently years. It is expected that new discovery will be made when more claudin-specific gene deletion models become available in the near future.
Regulation of Claudin Expressions in Intestines
The expression levels of claudins in intestines are under the regulation of a number of genes and/or proteins. Cathepsin L belongs to the cysteine protease class of the papain superfamily that is involved in intracellular and extracellular protein degradations. Inhibition of intracellular cathepsin L activity results in a rapid upregulation of claudin-1 protein accumulation in IEC-6/Cdx2L1 cell line, an epithelial cell line conditionally expressing Cdx2, a transcription factor that initiates intestinal epithelial differentiation. Mutant mice defective in cathepsin L activity display an elevated level of intestinal claudin-1 and -2 expression, which may be responsible for the intestinal neoplasia in mice.Citation27 Claudin-1 gene is also under the regulation of β-catenin. Claudin-1 expression decreases significantly in response to the reduction of β-catenin in intestines.Citation28 The trefoil factor family 3 (TFF3) plays an important role in the protection and repair of the GI mucosa. Overexpression of TFF3 in HT29/B6 cells, a human colon carcinoma cell line, increases the cellular level of claudin-1 and decreases the amount of claudin-2 accompanied by an increase in the TER in confluent monolayers of these cells.Citation29 Claudin-2 expression is under the positive regulation of Cdx2.Citation30 The 5′-flanking region of the claudin-2 gene contains binding sites for intestine-specific Cdx homeodomain proteins and hepatocyte nuclear factor (HNF)-1, which are conserved in human and mouse. Both Cdx1 and Cdx2 activate the claudin-2 promoter in the human intestinal colorectal adenocarcinoma epithelial cell line Caco-2, which is a widely used model for studying the human intestinal barrier. HNF-1α augments the Cdx2-induced but not Cdx1-induced transcriptional activation of the human claudin-2 promoter.Citation30 Claudin-2 expression in the intestine is also regulated by the transcriptional factor GATA-4, which is undetectable in the colon.Citation31 Claudin-2, -3, -7 and -15 have been found to be recruited to the TJs in the mouse intestinal epithelial cells by EpCAM. Mutation of EpCAM in mice leads to the downregulation of these claudins and the disruption of TJ strands.Citation32
Sodium proton exchangers (NHEs) constitute a large family of integral membrane protein transporters that are responsible for the counter-transport of protons and sodium ions across lipid bilayers. Mucosa from Na+/H+ exchanger 2 knockout mice subjected to complete mesenteric ischemia displays a shift of occludin and claudin-1 membrane expression to cytoplasm and shows the disruption of occludin and claudin-1 localization patterns following injury.Citation33 Expression of claudin-2 may be under the regulation of NHE3. Knockout of NHE3 in mice decreases the mRNA expression of claudin-2 and -15.Citation34 Aquaporin 3 (AQP3) knockdown significantly enhances the paracellular permeability and decreases the expression of claudin-1 and occludin by western blots.Citation35
Protein tyrosine phosphatase, non-receptor type 2 (PTPN2) is an inflammatory bowel disease (IBD) candidate gene. Mutation of PTPN2 causes IBD. McCole has reported that PTPN2 protects the epithelial barrier function by restricting the capacity of IFN-γ to increase epithelial permeability and prevent the induction of the pore-forming protein, claudin-2 expression.Citation36 Toll-like receptors (TLRs) are a class of proteins that play a key role in the innate immune system as well as in the digestive system. TLR2−/− mice suffer the impaired epithelial barrier function with colonic mucosal ulcerations, bleeding and increased cell death after bacterial pathogen Citrobacter rodentium infection compared with the wild-type (WT) mice. Claudin-3 is mislocalized in TLR2−/− intestine after infection in that it relocates from the normally lateral distribution to the cytoplasm in the enterocytes.Citation37 IL-10 knockout mice (IL-10−/−) exhibit a significant increase in the cumulative permeation of mannitol through the colonic mucosa and a significant decrease in TER compared with WT mice. Histological analysis shows that spontaneous colitis develops in all IL-10−/− mice with mucosal ulceration, erosion and neutrophil infiltration in the lamina propria. Molecular analysis reveals the decreased expression and redistribution of claudin-1, ZO-1 and occludin in IL-10−/− mice. Probiotic Lactobacillus plantarum treatment on IL-10−/− mice greatly improves colitis in that the paracellular permeability is reduced and the expression and distribution of claudin-1, ZO-1 and occludin is restored in IL-10−/− mice.Citation38
FoxO4 is a member of the forkhead box transcription factor O (FoxO) subfamily. FoxO4 inhibits the transcriptional activity of NF-кB and protects the mice against colonic injury and inflammation. FoxO4 deficiency in mice results in an increase in intestinal epithelial permeability and the downregulation of ZO-1 and claudin-1.Citation39 Guanylyl Cyclase C (GCC) signaling is a critical mediator of intestinal fluid homeostasis. Knockout of GCC in mice results in increased intestinal permeability. Claudin-2 expression is found to be reduced in GCC deficient intestine.Citation40 Lin et al. have reported that mice deficient in GCC exhibits the intestinal barrier hyper-permeability associated with the reduced junctional proteins occludin and claudin-4. The same observation is also found in Caco-2 cells. This regulation is possibly through the upregulation of AKT1 pathway. Restoration of occludin and claudin-4 is associated with the reconstitution of intestinal barrier integrity by reducing AKT1 expression in GCC knockout mice.Citation41 Claudin-1 and -4 can be phosphorylated by PKCθ and they can also form endogenous complexes with PKCθ, which has the stabilizing effect on the monolayer barrier dynamics in Caco-2 cells.Citation42
Loss of smad5 leads to the de-regulation of claudin-1 and -2 expression in intestines.Citation43 Knockdown of PTEN significantly inhibits the polarization, functional differentiation and brush border development in Caco-2/15 cells, a stable clone of the parent Caco-2 cells. A strong reduction in claudin-1, -3, -4 and -8 is also observed in addition to a decrease in TER.Citation44,Citation45 Claudin-4 has been found to be associated with cellular prion protein (PrP(c)) that is located at cell-cell junctions. Knockout of PrP(c) in mice greatly increases the paracellular permeability. Knockdown of PrP(c) in Caco-2/TC7 enterocytes, a stable clone of the parent Caco-2 cells, decreases the expression of claudin-4, as well as other TJ proteins, such as occludin, ZO-1 and tricellulin at cell contacts.Citation46 The protein C (PC) pathway is a well-characterized coagulation system. PC deficient mice develop spontaneous intestinal inflammation and show increased intestinal permeability. Structural analysis of epithelial TJ molecules reveals that lack of PC leads to decreased JAM-A and claudin-3 expression and altered pattern of ZO-1 expression.Citation47 Knockout of COX-2 significantly increases epithelial permeability and reduces the expression of claudin-1, ZO-1 and occludin in the ileum following cecal ligation and puncture in mice.Citation48 The TJ-associated gene claudin-10 is upregulated, whereas claudin-1 and -5 are downregulated in Mucin Muc2 knockout mice compared with the WT.Citation49
Claudin expression levels are also under the regulation of other proteins, such as desmoglein 2,Citation50 matriptase,Citation51 connexin 26,Citation52 suppression of tumorigenicity-14 (ST14),Citation53 and JAM-A.Citation54 Proteins and their regulatory effect on the expression of claudins in intestines are summarized in . The search for the effective regulators of claudin genes/proteins is an ongoing task and has high clinical relevance.
Table 2. Regulation of claudin expression in intestines by transcription factors or other protein modulators
Claudins are Modulated in the Disease State of Intestines
The intestinal barrier plays a critical role in the transport of nutrients and macromolecules. At the same time, it has to provide an effective barrier to harmful macromolecules and microorganisms.Citation55 Defects in this barrier function have been observed in intestinal disorders, such as IBD that includes ulcerative colitis (UC) and Crohn disease (CD), food allergies and celiac diseases. TJs are essential components of the physical intercellular barrier that separates and prevents the mixing of luminal contents with the abdomen. Compromised epithelial barrier function and TJ alterations are hallmarks of a number of GI disorders. Luminal antigen uptake occurs via TJ discontinuities and epithelial gross lesions, which is likely to induce many other changes to the epithelium besides simply TJ barrier alterations.
In IBD, epithelial barrier function is impaired, which contributes to diarrhea by a leak flux mechanism and triggers inflammation by an increased luminal antigen uptake. Studies show that claudin-1 protein expression is significantly reduced in IBD patients and its expression is correlated with the duration of the IBD symptoms.Citation56 Further studies reveal that the expression of claudin-1 is downregulated in epithelial cells immediately adjacent to transmigrating neutrophils in IBD.Citation57 The localization of claudin-1 is also irregularly distributed and disappeared from intercellular junctions in the samples from irritable bowel syndrome (IBS) patients.Citation6 There are conflicts on the expression levels of claudin-3 and -4 in IBD patients. Claudin-3 and -4 are present throughout normal colonic epithelium, but are unchanged, reduced or redistributed in the disease surface epithelium.Citation58 Some studies show reduced expression of claudin-3 with no changes in claudin-4 expression,Citation59 while others report stable claudin-3 expression with reduced claudin-4 expression at protein levels,Citation8,Citation60 or without any changes,Citation58,Citation61 or changes only in the cellular localization.Citation58 IBD-associated diarrhea may result from NF-кB-mediated TJ proteins occludin, claudin-1 and ZO-1 internalization, thus increasing the paracellular permeability.Citation62 Disruption of the epithelial barrier is also associated with the internalization of claudin-4 to a sub-apical cytoplasmic compartment.Citation56
Epithelial barrier function is impaired in UC. Claudin-2 is undetectable in normal colon, but it is strongly expressed along the inflamed crypt epithelium. A 956 ± 252% increase in claudin-2 expression is observed in mucosal biopsy specimens from human patients with UC.Citation63 Mennigen et al. have demonstrated that expressions of claudin-1, -3, -4 and -5 are all decreased in acute colitis.Citation64 Urinary claudin-3 can be used as an early noninvasive diagnostic marker for intestinal TJ loss. It is correlated with reduced claudin-3 staining in colonic tissues from biopsies.Citation65 T helper (Th) 2 cytokines, such as interleukin (IL)-13 and tumor necrosis factor (TNF)-α, are important factors in UC. IL-6, a pleiotropic cytokine, is elevated in IBD patients. IL-6 treatment increases TJ permeability by stimulating the expression of channel-forming claudin-2 in intestinal epithelial Caco-2 cells.Citation66 IL-13 stimulates epithelial apoptosis as well as upregulates claudin-2 in UC.Citation67,Citation68 This activation is through the signal transducer and activator of transcription 6 (STAT6) pathway.Citation69 Myosin light-chain kinase (MLCK) activation that is often observed in IBD patients can also trigger claudin-2 synthesis and increase paracellular cation flux.Citation70 Claudin-7 is detected in both the lateral membrane of the cell surface and the TJs by immunofluorescence staining and its signal is decreased in active UC biopsies collected from the rectum of patients. Downregulation of claudin-7 may lead to an altered TJ structure and thus the impaired epithelial function in active UC.Citation8 Chronic inflammation in mucosal tissues can influence the epithelial barrier function via pro-inflammatory cytokines such as interferon (IFN)-γ and TNF-α. IFN-γ induces a time-dependent increase in paracellular permeability that is associated with the internalization of claudin-1, occludin and JAM-A.Citation71
The epithelium in inflamed intestinal segments of patients with CD is characterized by a reduction of TJ strands, strand breaks and alterations of TJ protein content and composition.Citation72 CD patients show significantly increased intestinal permeability. In the intestines of patients with active CD, freeze fracture electron microscopic analysis reveals that occludin and the sealing TJ proteins claudin-5 and -8 are all downregulated and redistributed off the TJ, whereas the pore-forming TJ protein claudin-2 is strongly upregulated, which constitute the molecular basis of TJ changes.Citation59 Das et al. have also reported that claudin-4, -5 and -8 are redistributed from the TJ to the subjunctional lateral membrane in CD patients.Citation73
The mRNA of claudin-12 is significantly upregulated in the ileum of CD patients while the claudin-2 mRNA is significantly reduced by 5-fold in the sigmoid colon compared with healthy controls. The mRNA expression levels of claudin-12 and -4 are significantly downregulated in CD patients in the colon compared with the nearby unaffected tissue. Although significant differences are not observed, there is a trend of downregulation of claudin-3 in the colon of CD patients.Citation5 Portitz et al. have reported an increase of claudin-1 in human UC specimens and unchanged claudin-1 expression in CD patients. Thus it is suggested that claudin-1 may be used to differentiate between UC and CD cases.Citation74
Various animal models are established to study the mechanisms causing IBD. An upregulation of claudin-2 is observed in dinitrobenzenesulfonic acid (DNBS)-induced colitis in mice.Citation75 The 2,4,6-trinitrobenzene sulphonic acid (TNBS)-induced colitic rats show damaged intestinal mucosa and decreased expression of claudin-1, -3, -5 and -8 in TJ membrane fractions. The n-3 polyunsaturated fatty acids (PUFAs) can prevent the redistribution of these TJ proteins and elevate the expression of claudin-1, -5 and -8.Citation76 DSS-treated mice/rats show colitis-like syndrome with inflammatory response and mucosal damage and are often used as a model to study IBD. Immunohistochemistry staining as well as western blots show the decreased signals of colonic claudin-1, -3, -5, -7 and -8 after DSS treatment in mice, while claudin-2 expression is significantly increased after DSS treatment. These changes are restored and the intestinal damage is attenuated after AMD3100 treatment. AMD3100 is a CXCR4 antagonist. CXCR4 is constitutively expressed in intestinal epithelial cells and its expression is increased in the specimens of UC patients.Citation77 SAMP1/YitFc (SAMP) mouse strain is a spontaneous model of IBD, closely resembling CD. SAMP mice show the disrupted barrier function accompanied by the aberrant expression of claudin-2 and occludin.Citation78
Gluten-intolerant diseases include celiac disease and gluten-sensitive disease. Celiac disease is an autoimmune enteropathy triggered by the ingestion of gluten. Gluten-sensitive individuals develop GI symptoms similar to those in celiac disease, but the overall clinical symptom is generally less severe. Gluten-sensitivity displays significantly reduced intestinal permeability and significantly increased expression of claudin-4 at the mRNA level compared with the healthy controls in adults.Citation79 Biopsies from proximal and distal parts of duodenum from children with celiac disease show that claudin-2 and -3 expression are significantly increased in the severe form of celiac disease in bulb and in distal duodenum in comparison to controls, which may be, at least in part, responsible for increased permeability observed in celiac disease patients.Citation80 In celiac disease patients, pore-forming claudin-2 and -15 are upregulated and the tightening claudin-3, -5 and -7 and occludin are downregulated by western blot.Citation81 Patients with refractory celiac disease unresponsive to the treatments show increased expression of claudin-4 and -5 and decreased expression of pore-forming claudin-2.Citation82 Recent studies have indicated that schizophrenia may be also related to the celiac disease in that the celiac disease causes an alteration of gut permeability that may allow the exogenous psychosis-causing substances to enter the body thus causing the development of schizophrenia and other mental conditions.Citation83 Collagenous colitis is an inflammatory disease of unknown etiology with diarrhea as the leading symptom. A pronounced decrease in NaCl absorption is observed in collagenous colitis, which may be due to the downregulation of occludin and claudin-4.Citation60
Besides IBD, there are a number of diseases that have been found to be related to intestinal dysfunction. The in vitro culture model of distal duodenum biopsies from nine patients with food allergy is established to study the food allergy. Peptic-tryptic digest of wheat gliadin, wheat albumins and apple proteins are applied on the culture of biopsy tissues to induce food allergy. For the allergic tissues, after exposure to food allergens, claudin-1 expression appears to be reduced and exhibits breaks at the cell-cell regions.Citation84 Acute pancreatitis can induce intestinal injury and reduce the expression of claudin-1 at both mRNA and protein levels in the intestinal tissues. Studies of colonic biopsies indicate that both minimized and conventional cardiopulmonary bypass procedures cause moderate mucosal damage, an increase in epithelial cell proliferation and a decrease in expression of TJ protein claudin-4.Citation85 Other diseases, such as uremia and chronic kidney disease (CKD) can affect intestinal barrier function and cause systemic inflammation. CKD greatly reduces the protein expressions of claudin-1, occludin and ZO-1 in the colonic mucosa, showing the thickened colonic wall and heavy infiltration of mononuclear leukocytes in the intestinal lamina propria.Citation86 Patients with liver cirrhosis show the intestinal barrier dysfunction and hyperpermeability. Assimakopoulos et al. have recently reported that liver cirrhosis patients show significantly reduced expression of occludin and claudin-1 in duodenum as compared with healthy controls by immunohistochemical analysis. As a result, liver cirrhosis patients present significantly higher endotoxin values in peripheral blood as compared with healthy controls. Disruption of TJs could be due to the cytokine secretion, such as TNF-α and IFN-γ, in cirrhosis patients.Citation87 Iron can be toxic to the intestines in that it increases cell monolayer permeability and causes partial delocalization of claudin-4 from the plasma membrane to an intracellular compartment.Citation88 Expression of claudin-2 is induced by iron-deficiency in rats’ duodenum.Citation89 High fat diet is also shown to increase small intestinal permeability that is possibly due to decreases in claudin-1, claudin-3 and occludin expression in the small intestine.Citation90 Intestinal hyper-permeability is often seen in patients with obstructive jaundice. Occludin, claudin-1 and -7 are significantly decreased whereas claudin-4 is significantly increased in jaundiced patients and their distributions are altered as well. Patients with malignant obstructive jaundice show loose microvillus and wider cell junctions with reduced expression of claudin-1 and increased expression of claudin-4 in the duodenum.Citation91 Strangulated intestinal obstruction (STR-IO) patients show increased expression levels of ZO-1, occludin and claudin-1 at both mRNA and protein levels in the colonic tissues.Citation7 Chronic metabolic acidosis (CMA) enhances the mRNA expressions of claudin-2, -3, -6, -8, -11, -12, -14, -19 and -22 in Sprague-Dawley rats with chronic metabolic acidosis induced by NH4Cl.Citation92,Citation93 Hemorrhagic shock (HS) leads to the intestinal barrier loss as well as the loss of claudin-3 and ZO-1 in rats.Citation94 Beutheu et al. have shown that claudin-1 and occludin expression levels are reduced during the acute phase of mucositis compared with control rats. During the recovery phase, their expression levels are restored.Citation95 Wang et al. have reported that the side-stream smoking increases mouse intestinal bacteria and upregulates the expression of claudin-3 and ZO-2 in mouse large intestine.Citation96
The intestinal epithelial barrier function is often disrupted in many surgical diseases, including trauma, shock, burn injury and the other surgically critical illness, resulting in the increased intestinal permeability and subsequent translocation of bacteria and/or endotoxin from the GI tract.Citation97,Citation98 Intestinal surgical trauma can transiently compromise the protein levels of claudin-1 and E-cadherin in intestines.Citation99 Ischemia/reperfusion (I/R) injury of the intestine is the leading cause of organ dysfunction after restoration of blood flow in many diverse events, including shock and intestinal transplantation. I/R injury can cause intestinal mucosal lesions in rats and redistribute occludin, ZO-1, claudin-1 and -3 from the cell membrane to the cytoplasm in intestinal epithelial cells as shown by immunofluorescent microscopy.Citation100 In addition, mRNA expression levels of claudin-1 and -7 are significantly decreased from the beginning of reperfusion and are recovered to the control level by 24 h. On the other hand, claudin-4 mRNA expression level is significantly increased from the start of the reperfusion and then reaches to the control level 24 h after reperfusion. The protein expression levels of claudin-1, -2, -4 and -7 are significantly decreased until 1 h after reperfusion.Citation101 The expression level of nitric oxide (NO) is increased during intestinal I/R. NO participates in opening TJ by downregulating the protein expression of claudin-1, -2, -4 and -7.Citation102
Claudin-1 is predominantly localized in the cytoplasm of the ileum in mice, and its expression is markedly elevated in cytoplasm following burn injury, which may contribute to the histological damage of intestinal mucosa after burn injury.Citation103 EtOH combined with burn injury results in no change of claudin-1 protein content but its phosphorylation on tyrosine is decreased following EtOH and burn injury.Citation104 summarizes the changes of claudin expression in intestines in currently known disease status. We conclude that the majority of the claudins are downregulated in the disease with only pore-forming claudin-2 being consistently upregulated in several diseases.
Table 3. Changes in claudin expression in intestines in diseases
Claudins and Colorectal Cancer
The function of TJ proteins is compromised not only in a number of intestinal diseases but also in colorectal cancer. Claudin-1 is weakly expressed at the apical boarder of the lateral membrane of normal enterocytes, but is strongly expressed at cell-cell boundaries as well as in the cytoplasm of colorectal cancer cells.Citation28 Huo et al. have also indicated that the expression of claudin-1 at the mRNA and protein levels is found to be increased in colorectal cancer tissue in comparison to that in the normal tissue specimens. The mRNA level of claudin-1 is correlated with tumor depth. Claudin-1 protein may therefore be one of the major factors involved in colorectal tumorigenesis.Citation105 The increased expression of claudin-2 may participate in colorectal carcinogenesis.Citation106 Claudin-3 is overexpressed in human cancerous colorectal tissues and may be used as a biomarker to differentiate cancerous colorectal tissues from normal colorectal tissues.Citation107 Using tissue biopsy samples, Mees et al. have also found that colorectal carcinoma in human exhibits significantly elevated expression levels of claudin-1, -3, -4 compared with normal mucosa, which suggests that these proteins may be potential markers for diagnosing colorectal carcinoma.Citation108 Claudin-7 overexpression is found in colorectal cancer. Its overexpression is under the regulation of Tcf-4 and Sox-9.Citation109 Claudin-8 is downregulated in colorectal adenoma samples compared with the normal intestinal tissues.Citation110 Plasma markers for enterocyte damage (I-FABP, I-BABP) and urinary presence of TJ protein claudin-3 can be used to assess the gut mucosal barrier.Citation111
Effects of Bacteria/Viruses on Claudin-Mediated Intestinal Functions
A number of bacteria and viruses can cause the infection of intestines and alter the intestinal permeability. While some of the bacteria have beneficial effects to the intestines, it has been recognized that bacteria and/or endotoxin translocation and increased gut permeability play a very important role in the setting of severe complications such as systemic inflammatory response syndrome, sepsis, multiple organ dysfunction syndrome and multiple organ failure.Citation112,Citation113
It is known that Enteropathogenic Escherichia coli (EPEC) infection disrupts TJs in mice. Occludin and claudin-1 are displaced from TJ membrane microdomains to cytoplasm after EPEC infection using sucrose density gradient centrifugation and western blot analysis.Citation114 It has been reported that the micro integral membrane protein (MIMP) can protect the intestinal barrier from injury by EPEC infection since MIMP-expressing NCM460 epithelial cells (NCM460/MIMP) show significantly higher immunostaining signals of occludin, claudin-1, JAM-1 and ZO-1 compared with parental NCM460 cells.Citation115 The associations between ZO-1, occludin and claudin-1 are progressively decreased after EPEC infection in human intestinal epithelial T84 cells, resulting in a loss of barrier function.Citation116
Enteroaggregative Escherichia coli (EAEC)-infected T84 cells exhibit irregular shapes and some cells become elongated and/or enlarged. Infection of EAEC on intestinal T84 cells also induces a decrease in TER and dissociation of claudin-1 from the TJs.Citation117
Enterohemorrhagic Escherichia coli (EHEC) infection leads to microvillous effacement of mouse colonocytes. EHEC infection results in the alteration of TJ proteins claudin-2 and -3. After infection, claudin-3 immunostaining is clearly diminished from the lateral membranes. However, claudin-2 staining is progressively increased within the cytoplasm. Quantitative real-time PCR shows that EHEC alters the mRNA transcription of claudin-2 and -3. Most notably, claudin-2 expression is significantly increased, which correlates with increased intestinal permeability.Citation118
Yersinia enterocolitica is a common cause of acute gastroenteritis. Exposure of human colonic HT-29/B6 cells to Y. enterocolitica results in a decrease in TER. After infection, claudin-3, -4 and -8 are redistributed from TJ into the cytoplasm. In addition, the expression of claudin-2, -3, -8, -10 and ZO-1 is diminished by western blot analysis.Citation119
Campylobacter jejuni is a leading cause of human enterocolitis. Infection of polarized T84 monolayers with C. jejuni causes a time-dependent decrease in TER. Lipid rafts are submembrane domains that are preferentially partitioned in the apical membrane of polarized epithelium. The loss of TJ barrier function has been correlated with translocation of lipid raft-associated TJ proteins.Citation120 In the uninfected control T84 cells, the majority of claudin-1 is found in lipid rafts. Upon infection, T84 cells exhibit a concomitant increase in raft-associated claudin-1.Citation121 Infection of HT-29/B6 cells with bacteria strains Campylobacter concisus impairs the epithelial barrier function characterized by a time- and dose-dependent decrease in TER. The expression level of barrier-forming TJ protein claudin-5 is only 66 ± 8% at the protein level and 49 ± 16% at the mRNA level compared with the control cells after infection. However, there is no distribution change of claudin-5 observed.Citation122
Humen et al. have reported recently that Giardia intestinalis trophozoite promotes an adhesion-dependent decrease in TER accompanied by a rearrangement of functional TJ-associated occludin and delocalization of claudin-1 in human intestinal Caco-2/TC7 cells.Citation123 Entamoeba histolytica produces and secretes Prostaglandin E(2) (PGE(2)), an inflammatory molecule that decreases barrier integrity of TJs. Loss of mucosal barrier integrity corresponds with the increased Na+ permeability across TJs. PGE(2) also dissociates claudin-4 from cell membrane to cytoplasm.Citation124
Bifidobacterium bifidum improves the intestinal integrity in a rat model of necrotizing enterocolitis. Asphyxia and cold stress are applied to the premature newborn rats to induce neonatal necrotizing enterocolitis (NEC). Claudin-3 is significantly increased in NEC rats and is localized mainly in the cytoplasm of the enterocytes. Administration of probiotic B. bifidum normalizes the expression and localization of claudin-3 in the ileum compared with the control animals with NEC, thus it protects against NEC in the neonatal rat model.Citation125Bifidobacterium infantis culture media increase TER and the expression of occludin and ZO-1 while decreasing claudin-2 in T84 cells.Citation126
Lactobacillus plantarum inhibits the intestinal epithelial barrier dysfunction induced by unconjugated bilirubin (UCB). High concentrations of UCB cause cytotoxicity and decrease the TER of the Caco-2 cell monolayer. UCB treatment results in a loss of claudin-1 and -4 from the cell membrane as revealed by immunofluorescence microscopy. Both protein and mRNA levels of claudin-1 and -4 are reduced in UCB-treated cells as compared with the control cells. Probiotic L. plantarum exerts a protective effect against UCB damage to Caco-2 monolayer cells, and it restores the structure and distribution of TJ proteins and increases the mRNA and protein levels of claudin-1 and -4.Citation127L. plantarum also prevents the reduced expression and rearrangement of claudin-1, occludin, JAM-1 and ZO-1 proteins induced by enteroinvasive Escherichia coli (EIEC) infection in Caco-2 cells.Citation128
Salmonella typhimurium regulates the distribution of claudin-1 from the Triton X-100-insoluble fractions to the Triton X-100-soluble fractions. Infection with S. typhimurium is associated with the rapid targeting of TJ complex and loss of barrier function. These events result in the enhanced bacterial translocation and initiation of polymorphonuclear leukocyte (PMN) migration across the intestinal barrier.Citation129 Analysis of duodenal biopsy specimens from patients with chronic giardiasis Giardia lamblia infection shows the downregulation of claudin-1 and increased epithelial apoptosis.Citation130Shigella flexneri serotype 2a has the ability to remove claudin-1 from TJs upon exposure to T84 cell monolayers.Citation131
The neonatal small intestine is susceptible to the damage by endotoxins and LPS is one of the bacteria endotoxins that are widely used by researchers to establish the intestine damage model. Bacteria endotoxins are the main cause of sepsis, which is one of the primary causes of patient death in the intensive care units. Significantly damaged intestinal tissues, inflammatory cell infiltration and hemorrhage are observed in the sepsis patients. During the polymicrobial sepsis, TJ architecture and the protein redistribution in TJ membrane microdomains are altered.Citation132 ZO-1 and claudin-2 expressions are lost from the cell membrane, and their protein expression levels are decreased after LPS treatment in rats. Carbachol, one of the clinical cholinomimetic drugs, decreases the mucosal damage and ameliorates the intestinal epithelial TJ damage induced by LPS in that the gut barrier permeability is reduced, and the ultrastructure disruption of TJs is prevented. Carbachol treatment also significantly increases ZO-1 and claudin-2 protein expression after LPS administration.Citation133 Claudin-1, -3, -4, -5 and -8 are present predominantly in the microvillous surface and along the lateral membranes of the epithelial cells. Polymicrobial sepsis leads to the diffusion and loss of these TJ proteins from the lateral cell boundaries in mice. However, the expression of claudin-2 is markedly upregulated in sepsis by immunofluorescence staining.
Rotavirus infection of Caco-2 cells alters paracellular permeability and causes redistribution of TJ proteins claudin-1, occludin and ZO-1. Claudin-1 redistribution is notably apparent at the onset of the decline in TER.Citation134 Expression of TJ proteins occludin, claudin-4 and -5 is reduced in duodenum biopsy samples from patients with norovirus infection that may lead to epithelial barrier dysfunction.Citation135 HIV can also contribute to the impairment of the intestinal barrier in that the pore-forming claudin-2 is increased while the expression of the sealing claudin-1 is reduced in HIV patients.Citation136
Disruptions of Claudin-Mediated Intestinal Barriers
Many molecules and factors can disrupt intestinal barriers. For examples, platelet-activating factor (PAF) induces downregulation of TJ protein claudin-1 and ZO-1 expression, shifts their localization from the cell membrane to cytoplasm and reduces TER in Caco-2 cells. Intestinal trefoil factor (ITF) can suppress PAF-induced downregulation of TJ proteins and inhibit the abnormal localization and distribution of claudin-1 and ZO-1.Citation137 Mycotoxin ochratoxin A is able to disrupt the barrier function of Caco-2 cells by removal of claudin-3 and -4 from the cell membrane.Citation138 Deoxynivalenol (DON) is a mycotoxin that often appears on cereals used for human and animal nutrition. Using porcine intestinal epithelial cell line (IPEC-J2) as a model, the integrity of cell connections is disrupted as measured by TER, and the expression of ZO-1 and claudin-3 is reduced when DON is applied to the basolateral side of the cells. Interestingly, there is no effect of DON on the IPEC-J2 when it is applied to the apical side.Citation139 DON also contributes to the loss of barrier function of porcine intestinal epithelial cell line (IPEC-1) through the decreased expression of claudin-4 protein, which is involved in the maintenance of the intestinal epithelial cell barrier function. DON-induced impairment of intestinal barrier is through the activation of ERK/MAPK signaling pathway. Inhibition of ERK/MAPK pathway restores the intestinal expression of claudin-4 protein.Citation140 DON increases the paracellular permeability of Caco-2 cell monolayers. This may be through its protein synthesis inhibition role by diminishing the synthesis of claudin-4.Citation141 Irinotecan causes disorders in the intestinal epithelial barrier and induces bacterial translocation. Claudin-1 mRNA in the small intestine is significantly decreased and claudin-1 protein expression in both the small and large intestines is significantly reduced after application of irinotecan.Citation142 Endocannabinoids decreases the mRNA of claudin-1 in Caco-2 cells.Citation143 Exposure to phenol results in decreased TER and increased paracellular flux of FITC-dextran. Delocalization of claudin-1 and ZO-1 from TJs to cytosol correlates with the permeability increase after phenol treatment.Citation144 Prolactin (PRL) can enhance intestinal absorption of Ca2+ and other minerals for fetal development and milk production. PRL markedly stimulates the transcellular and paracellular Ca2+ transport in the duodenum of pregnant and lactating rats as well as in Caco-2 cell monolayers. Claudin-15, which regulates the epithelial cation selectivity and paracellular Ca2+ movement, is required for this PRL-enhanced paracellular transport.Citation145 PRL is found to downregulate the expression of claudin-3 and occludin in IEC-6 crypt cells, a rat small intestine epithelial cell line.Citation146 Cofilin is an actin binding protein and its dephosphorylation corresponds to the TJ opening, TER decrease and claudin-1 dissociation from the cytoskeleton. This suggests that cofilin may serve as a target for TJ permeability regulation in epithelial cells.Citation147 Phosphatidylethanol is produced from ethanol by phospholipase D. It is accumulated after chronic ethanol exposure, which induces claudin-1 endocytosis and disrupts the claudin-1/ZO-1 association.Citation148
Oxidative stress can cause the increased colonic epithelial permeability. For example, hydrogen peroxide (H2O2) treatment changes the localization of claudin-4 from apical TJ to lateral membrane in Caco-2 cells.Citation149 Interestingly, incubation of T84 cells with IL-4 leads to an increased claudin-2 expression with a corresponding decrease in TER and increase in permeability while the IFN-γ treatment has the opposite effects, leading to decreased claudin-2 and increased TER.Citation150 Gliadin is known to cause celiac disease. Treatment of gliadin on Caco-2 cells causes a reorganization of actin filaments and reduces the expression of the TJ protein occludin, claudin-3 and -4. Immunofluorescence staining of these TJ proteins reveals a decrease in plasma membrane staining and an increase in punctate, cytosolic staining after gliadin treatment.Citation151 Chitosan can transiently and reversibly open the TJs between Caco-2 cells, thus enhancing the paracellular permeability. Chitosan treatment induces claudin-4 degradation and redistribution in the cells, leading to the opening of TJs. Chitosan could potentially be used to mediate the transepithelial drug delivery.Citation152 Nanoparticle-mediated drug delivery is a fast-growing research field. Si-nanowire-coated silica microparticles alter TJ permeability and decrease the width of ZO-1 and claudin-1 at the TJ in Caco-2 cells in culture.Citation153 Vitamin D can suppress the expression of claudin-3 and change the permeability of the TJs, thus enhancing the paracellular Ca2+ transport.Citation154 Miltefosine (hexadecylphosphocholine) is the first effective oral agent for the treatment of visceral leishmaniasis. Its oral administration alters the distribution of claudin-1 and translocates it from the intercellular junctions to the cytoplasm.Citation155 Glutamine deprivation markedly decreases TER and claudin-1 expression.Citation156
Clostridium perfringens enterotoxin (CPE) is responsible for the GI symptoms of the second-most-common bacterial food-borne illness. On the other hand, CPE is also currently being explored to be used as an anti-cancer therapeutic or enhancing drug delivery agent.Citation157 C-terminal CPE (C-CPE) can bind and modulate claudin structures, thus enhancing mucosal absorption. Members of the claudin family can serve as CPE receptors and convey CPE sensitivity. Human claudin-3 and -4 are two functional CPE receptors. Recently, claudin-8 and -14 have also been shown to be able to convey the cytotoxic effect of CPE on mammalian intestines although the efficiency is 2-to-10-fold less than that of claudin-4. Specific residues responsible for modulating the CPE binding activities have been identified.Citation157 Binding of C-CPE to claudin-4 enhances jejunal absorption of dextran.Citation158 Besides CPE and C-CPE, Matsuhisa et al. have developed a C-CPE mutant that has a broad binding specificity to claudin-1, -2, -4 and -5, which could enhance the jejunal absorption of dextran. The C-CPE mutant binder exhibits a more potent jejunal absorption-enhancing effect than that of C-CPE.Citation159 The treatment of non-cytotoxic recombinant CPE variant is able to partially inhibit CPE-induced histologic damage, suggesting that non-cytotoxic recombinant CPE variants may be useful for protecting against some intestinal effects of CPE.Citation160-Citation162 Tyr306 and Leu315 are key residues in C-CPE that are involved in the modulation of claudin-4.Citation163,Citation164 Takahashi et al. have reported that the C-CPE194 binds to claudin-4 and enhances mucosal absorption. Recently, they have created a mutated form of C-CPE194 by substituting asparagine at position 309 and serine at position 313 with alanine, which increases the affinity to claudin-4 by almost 10-fold as compared with C-CPE194. Deletion of 10 amino acids in the N-terminal domain of the double-alanine-substituted mutant further increases the affinity to claudin-4 by nearly 24-fold as compared with C-CPE194. Therefore, these C-CPE mutants may be a promising lead for the development of a clinical TJ modulator.Citation165
Improvements of Intestinal Barrier that Involves Claudins
A number of molecules or chemicals have been found to improve the intestinal barrier and ameliorate the alteration of claudins in pathological status and thus are used to treat the intestinal barrier dysfunction.
Moxibustion may relieve colonic epithelial barrier defect by upregulating the expression of colonic epithelial occludin, claudin-1 and ZO-1 proteins and their mRNAs in the cultivated colonic epithelial cells.Citation166 The fish oil supplement in piglets improves intestinal morphology by increasing villus height and intestinal barrier function and upregulating intestinal TJ proteins including occludin and claudin-1. Under the inflammatory condition triggered by LPS, fish oil could alleviate the LPS-induced intestinal damage.Citation167 VSL#3, a probiotic mixture, can stimulate the epithelial-derived TNF and prevents the onset of ileitis in SAMP mice by decreasing the ileal paracellular permeability through downregulation of claudin-2 and upregulation of occludin.Citation168 Butyrate improves the barrier function of GI epithelia and has a potential to treat IBD. It restores the TJs in IBD patients by downregulating the expression of claudin-1 and -2 and upregulating occludin, cingulin, ZO-1 and ZO-2.Citation169 Intestinal alkaline phosphatase (IAP) has been found to reduce the intestinal injury in a NEC rat model established by LPS administration and this effect is dose-dependent.Citation170 Bao et al. have reported that herbs-partitioned moxibustion (HPM) and mild-warm moxibustion (MWM) treatments significantly enhances the expression of occludin, claudin-1 and ZO-1 and improves the microstructure of the colonic epithelial barrier in a CD rat model established by TNBS treatment.Citation171 Recently, Moran et al. have found that Glucagon-like peptide-2 (GLP-2), an intestinotrophic enteroendocrine peptide, can increase the TER by upregulating the protein expression of occludin and ZO-1 in Caco-2 cells.Citation172 Using the HT-29/B6 cells as a model, Hering et al. assess the barrier-protective effects of TGF-β (isoforms 1–3) on intestines. TGF-β increases the claudin-4 expression at the transcriptional level by binding to the promoter of claudin-4.Citation173 Nicotine treatment on Caco-2 cell monolayers significantly improves TJ integrity as measured by TER and significantly upregulates occludin and claudin-1.Citation174 Kaempferol, a natural flavonoid present in fruits, vegetables and teas, provides beneficial effects for human health. Kaempferol enhances the TJ integrity in Caco-2 cells. The protein levels of claudin-1, -3 and -4 on the cell membrane are increased during the first 6 or 12 h after kaempferol administration. Confocal fluorescent microscopy reveals higher immunofluorescence intensities for claudin-3 at the intercellular junctions than the untreated controls after kaempferol treatment for 6 h.Citation175 Flavonoid quercetin enhances epithelial barrier function and increases claudin-4 expression in Caco-2 cells.Citation176 Methionine-restricted (MR) diet in rats results in the improved colonic epithelial barrier function compared with the rats on the control diet by altering TJ protein composition. MR diet increases TER with concomitant decrease in paracellular permeability. RT-PCR and western blot analysis show an 80% increase in claudin-3 mRNA and 5-fold increase in claudin-3 protein level.Citation177 IL-15 is able to enhance the TJ formation in intestinal epithelial cells. The recruitment of claudin-1 and -2 to the cell membrane are enhanced by IL-15 in the presence of IL-2Rβ subunit, a subunit of IL-15 receptor in T84 cells.Citation178 IL-17 upregulates claudin-1 and -2 at the transcriptional level, which induces the formation of TJs in T84 cells.Citation179 As a summary, the above several major factors affecting the intestinal barrier by either disrupting or improving TJ structure and function have been listed in .
Table 4. Factors affecting claudin expression and intestinal barrier
Conclusion
TJs in enterocytes separate the intestinal lumen from the underlying tissues, thus maintaining the homeostasis of intestines. Claudins are essential components of TJs regulating paracellular solute transport. Claudins can alter or be altered by a number of signaling molecules/pathways. Abnormal expression and/or mis-localization of claudins are associated with various pathophysiological conditions of intestines. Studying the molecules/chemical agents that regulate the claudins, either by enhancing or ameliorating the intestinal permeability, can provide an opportunity for exploring prospective approaches for treating the intestinal diseases or delivering drugs. More studies are needed in order to understand whether the changes in claudins are the causes or the consequences of the intestinal diseases and the underlying mechanisms related to the intestine disorders.
Acknowledgments
This work was supported by the National Institute of Health grants HL085752 and ES016888 to Y.-H.C. and the National Natural Science Foundation of China 31200581 and the Foundation from Hangzhou Science and Technology Bureau 20120633B26 to Z.L.
Disclosure of Potential Conflicts of Interest
No potential conflict of interest was disclosed.
References
- Niessen CM. Tight junctions/adherens junctions: basic structure and function. J Invest Dermatol 2007; 127:2525 - 32; http://dx.doi.org/10.1038/sj.jid.5700865; PMID: 17934504
- Van Itallie CM, Anderson JM. The molecular physiology of tight junction pores. Physiology (Bethesda) 2004; 19:331 - 8; http://dx.doi.org/10.1152/physiol.00027.2004; PMID: 15546850
- Krug SM, Günzel D, Conrad MP, Lee IF, Amasheh S, Fromm M, et al. Charge-selective claudin channels. Ann N Y Acad Sci 2012; 1257:20 - 8; http://dx.doi.org/10.1111/j.1749-6632.2012.06555.x; PMID: 22671585
- González-Mariscal L, Betanzos A, Nava P, Jaramillo BE. Tight junction proteins. Prog Biophys Mol Biol 2003; 81:1 - 44; http://dx.doi.org/10.1016/S0079-6107(02)00037-8; PMID: 12475568
- Lameris AL, Huybers S, Kaukinen K, Mäkelä TH, Bindels RJ, Hoenderop JG, et al. Expression profiling of claudins in the human gastrointestinal tract in health and during inflammatory bowel disease. Scand J Gastroenterol 2013; 48:58 - 69; http://dx.doi.org/10.3109/00365521.2012.741616; PMID: 23205909
- Bertiaux-Vandaële N, Youmba SB, Belmonte L, Lecleire S, Antonietti M, Gourcerol G, et al. The expression and the cellular distribution of the tight junction proteins are altered in irritable bowel syndrome patients with differences according to the disease subtype. Am J Gastroenterol 2011; 106:2165 - 73; http://dx.doi.org/10.1038/ajg.2011.257; PMID: 22008894
- Yang JJ, Ma YL, Zhang P, Chen HQ, Liu ZH, Qin HL. Histidine decarboxylase is identified as a potential biomarker of intestinal mucosal injury in patients with acute intestinal obstruction. Mol Med 2011; 17:1323 - 37; http://dx.doi.org/10.2119/molmed.2011.00107; PMID: 21915437
- Oshima T, Miwa H, Joh T. Changes in the expression of claudins in active ulcerative colitis. J Gastroenterol Hepatol 2008; 23:Suppl 2 S146 - 50; http://dx.doi.org/10.1111/j.1440-1746.2008.05405.x; PMID: 19120888
- Holmes JL, Van Itallie CM, Rasmussen JE, Anderson JM. Claudin profiling in the mouse during postnatal intestinal development and along the gastrointestinal tract reveals complex expression patterns. Gene Expr Patterns 2006; 6:581 - 8; http://dx.doi.org/10.1016/j.modgep.2005.12.001; PMID: 16458081
- Wang Y, Liu Y, Sidhu A, Ma Z, McClain C, Feng W. Lactobacillus rhamnosus GG culture supernatant ameliorates acute alcohol-induced intestinal permeability and liver injury. Am J Physiol Gastrointest Liver Physiol 2012; 303:G32 - 41; http://dx.doi.org/10.1152/ajpgi.00024.2012; PMID: 22538402
- Ding L, Lu Z, Foreman O, Tatum R, Lu Q, Renegar R, et al. Inflammation and disruption of the mucosal architecture in claudin-7-deficient mice. Gastroenterology 2012; 142:305 - 15; http://dx.doi.org/10.1053/j.gastro.2011.10.025; PMID: 22044670
- Fujita H, Chiba H, Yokozaki H, Sakai N, Sugimoto K, Wada T, et al. Differential expression and subcellular localization of claudin-7, -8, -12, -13, and -15 along the mouse intestine. J Histochem Cytochem 2006; 54:933 - 44; http://dx.doi.org/10.1369/jhc.6A6944.2006; PMID: 16651389
- Wada M, Tamura A, Takahashi N, Tsukita S. Loss of Claudins 2 and 15 From Mice Causes Defects in Paracellular Na(+) Flow and Nutrient Transport in Gut and Leads to Death from Malnutrition. Gastroenterology 2012.
- Clark MA, Hirst BH. Expression of junction-associated proteins differentiates mouse intestinal M cells from enterocytes. Histochem Cell Biol 2002; 118:137 - 47; PMID: 12189517
- Tamagawa H, Takahashi I, Furuse M, Yoshitake-Kitano Y, Tsukita S, Ito T, et al. Characteristics of claudin expression in follicle-associated epithelium of Peyer’s patches: preferential localization of claudin-4 at the apex of the dome region. Lab Invest 2003; 83:1045 - 53; http://dx.doi.org/10.1097/01.LAB.0000078741.55670.6E; PMID: 12861044
- Patel RM, Myers LS, Kurundkar AR, Maheshwari A, Nusrat A, Lin PW. Probiotic bacteria induce maturation of intestinal claudin 3 expression and barrier function. Am J Pathol 2012; 180:626 - 35; http://dx.doi.org/10.1016/j.ajpath.2011.10.025; PMID: 22155109
- Markov AG, Veshnyakova A, Fromm M, Amasheh M, Amasheh S. Segmental expression of claudin proteins correlates with tight junction barrier properties in rat intestine. J Comp Physiol B 2010; 180:591 - 8; http://dx.doi.org/10.1007/s00360-009-0440-7; PMID: 20049600
- Karaki S, Kaji I, Otomo Y, Tazoe H, Kuwahara A. The tight junction component protein, claudin-4, is expressed by enteric neurons in the rat distal colon. Neurosci Lett 2007; 428:88 - 92; http://dx.doi.org/10.1016/j.neulet.2007.09.059; PMID: 17964719
- Rahner C, Mitic LL, Anderson JM. Heterogeneity in expression and subcellular localization of claudins 2, 3, 4, and 5 in the rat liver, pancreas, and gut. Gastroenterology 2001; 120:411 - 22; http://dx.doi.org/10.1053/gast.2001.21736; PMID: 11159882
- Ohta H, Yamaguchi T, Rajapakshage BK, Murakami M, Sasaki N, Nakamura K, et al. Expression and subcellular localization of apical junction proteins in canine duodenal and colonic mucosa. Am J Vet Res 2011; 72:1046 - 51; http://dx.doi.org/10.2460/ajvr.72.8.1046; PMID: 21801061
- Amasheh S, Milatz S, Krug SM, Bergs M, Amasheh M, Schulzke JD, et al. Na+ absorption defends from paracellular back-leakage by claudin-8 upregulation. Biochem Biophys Res Commun 2009; 378:45 - 50; http://dx.doi.org/10.1016/j.bbrc.2008.10.164; PMID: 19000657
- Fujita H, Sugimoto K, Inatomi S, Maeda T, Osanai M, Uchiyama Y, et al. Tight junction proteins claudin-2 and -12 are critical for vitamin D-dependent Ca2+ absorption between enterocytes. Mol Biol Cell 2008; 19:1912 - 21; http://dx.doi.org/10.1091/mbc.E07-09-0973; PMID: 18287530
- Christakos S, Dhawan P, Ajibade D, Benn BS, Feng J, Joshi SS. Mechanisms involved in vitamin D mediated intestinal calcium absorption and in non-classical actions of vitamin D. J Steroid Biochem Mol Biol 2010; 121:183 - 7; http://dx.doi.org/10.1016/j.jsbmb.2010.03.005; PMID: 20214989
- Tamura A, Kitano Y, Hata M, Katsuno T, Moriwaki K, Sasaki H, et al. Megaintestine in claudin-15-deficient mice. Gastroenterology 2008; 134:523 - 34; http://dx.doi.org/10.1053/j.gastro.2007.11.040; PMID: 18242218
- Tamura A, Hayashi H, Imasato M, Yamazaki Y, Hagiwara A, Wada M, et al. Loss of claudin-15, but not claudin-2, causes Na+ deficiency and glucose malabsorption in mouse small intestine. Gastroenterology 2011; 140:913 - 23; http://dx.doi.org/10.1053/j.gastro.2010.08.006; PMID: 20727355
- Vezzoli G, Soldati L, Gambaro G. Update on primary hypercalciuria from a genetic perspective. J Urol 2008; 179:1676 - 82; http://dx.doi.org/10.1016/j.juro.2008.01.011; PMID: 18343451
- Boudreau F, Lussier CR, Mongrain S, Darsigny M, Drouin JL, Doyon G, et al. Loss of cathepsin L activity promotes claudin-1 overexpression and intestinal neoplasia. FASEB J 2007; 21:3853 - 65; http://dx.doi.org/10.1096/fj.07-8113com; PMID: 17622569
- Miwa N, Furuse M, Tsukita S, Niikawa N, Nakamura Y, Furukawa Y. Involvement of claudin-1 in the beta-catenin/Tcf signaling pathway and its frequent upregulation in human colorectal cancers. Oncol Res 2001; 12:469 - 76; PMID: 11939410
- Meyer zum Büschenfelde D, Tauber R, Huber O. TFF3-peptide increases transepithelial resistance in epithelial cells by modulating claudin-1 and -2 expression. Peptides 2006; 27:3383 - 90; http://dx.doi.org/10.1016/j.peptides.2006.08.020; PMID: 17018241
- Sakaguchi T, Gu X, Golden HM, Suh E, Rhoads DB, Reinecker HC. Cloning of the human claudin-2 5′-flanking region revealed a TATA-less promoter with conserved binding sites in mouse and human for caudal-related homeodomain proteins and hepatocyte nuclear factor-1alpha. J Biol Chem 2002; 277:21361 - 70; http://dx.doi.org/10.1074/jbc.M110261200; PMID: 11934881
- Escaffit F, Boudreau F, Beaulieu JF. Differential expression of claudin-2 along the human intestine: Implication of GATA-4 in the maintenance of claudin-2 in differentiating cells. J Cell Physiol 2005; 203:15 - 26; http://dx.doi.org/10.1002/jcp.20189; PMID: 15389642
- Lei Z, Maeda T, Tamura A, Nakamura T, Yamazaki Y, Shiratori H, et al. EpCAM contributes to formation of functional tight junction in the intestinal epithelium by recruiting claudin proteins. Dev Biol 2012; 371:136 - 45; http://dx.doi.org/10.1016/j.ydbio.2012.07.005; PMID: 22819673
- Moeser AJ, Nighot PK, Ryan KA, Simpson JE, Clarke LL, Blikslager AT. Mice lacking the Na+/H+ exchanger 2 have impaired recovery of intestinal barrier function. Am J Physiol Gastrointest Liver Physiol 2008; 295:G791 - 7; http://dx.doi.org/10.1152/ajpgi.00538.2007; PMID: 18719001
- Pan W, Borovac J, Spicer Z, Hoenderop JG, Bindels RJ, Shull GE, et al. The epithelial sodium/proton exchanger, NHE3, is necessary for renal and intestinal calcium (re)absorption. Am J Physiol Renal Physiol 2012; 302:F943 - 56; http://dx.doi.org/10.1152/ajprenal.00504.2010; PMID: 21937605
- Zhang W, Xu Y, Chen Z, Xu Z, Xu H. Knockdown of aquaporin 3 is involved in intestinal barrier integrity impairment. FEBS Lett 2011; 585:3113 - 9; http://dx.doi.org/10.1016/j.febslet.2011.08.045; PMID: 21907710
- McCole DF. Regulation of epithelial barrier function by the inflammatory bowel disease candidate gene, PTPN2. Ann N Y Acad Sci 2012; 1257:108 - 14; http://dx.doi.org/10.1111/j.1749-6632.2012.06522.x; PMID: 22671596
- Gibson DL, Ma C, Rosenberger CM, Bergstrom KS, Valdez Y, Huang JT, et al. Toll-like receptor 2 plays a critical role in maintaining mucosal integrity during Citrobacter rodentium-induced colitis. Cell Microbiol 2008; 10:388 - 403; PMID: 17910742
- Chen HQ, Yang J, Zhang M, Zhou YK, Shen TY, Chu ZX, et al. Lactobacillus plantarum ameliorates colonic epithelial barrier dysfunction by modulating the apical junctional complex and PepT1 in IL-10 knockout mice. Am J Physiol Gastrointest Liver Physiol 2010; 299:G1287 - 97; http://dx.doi.org/10.1152/ajpgi.00196.2010; PMID: 20884889
- Zhou W, Cao Q, Peng Y, Zhang QJ, Castrillon DH, DePinho RA, et al. FoxO4 inhibits NF-kappaB and protects mice against colonic injury and inflammation. Gastroenterology 2009; 137:1403 - 14; http://dx.doi.org/10.1053/j.gastro.2009.06.049; PMID: 19560465
- Han X, Mann E, Gilbert S, Guan Y, Steinbrecher KA, Montrose MH, et al. Loss of guanylyl cyclase C (GCC) signaling leads to dysfunctional intestinal barrier. PLoS One 2011; 6:e16139; http://dx.doi.org/10.1371/journal.pone.0016139; PMID: 21305056
- Lin JE, Snook AE, Li P, Stoecker BA, Kim GW, Magee MS, et al. GUCY2C opposes systemic genotoxic tumorigenesis by regulating AKT-dependent intestinal barrier integrity. PLoS One 2012; 7:e31686; http://dx.doi.org/10.1371/journal.pone.0031686; PMID: 22384056
- Banan A, Zhang LJ, Shaikh M, Fields JZ, Choudhary S, Forsyth CB, et al. theta Isoform of protein kinase C alters barrier function in intestinal epithelium through modulation of distinct claudin isotypes: a novel mechanism for regulation of permeability. J Pharmacol Exp Ther 2005; 313:962 - 82; http://dx.doi.org/10.1124/jpet.105.083428; PMID: 15900076
- Allaire JM, Darsigny M, Marcoux SS, Roy SA, Schmouth JF, Umans L, et al. Loss of Smad5 leads to the disassembly of the apical junctional complex and increased susceptibility to experimental colitis. Am J Physiol Gastrointest Liver Physiol 2011; 300:G586 - 97; http://dx.doi.org/10.1152/ajpgi.00041.2010; PMID: 21212325
- Scharl M, Paul G, Weber A, Jung BC, Docherty MJ, Hausmann M, et al. Protection of epithelial barrier function by the Crohn’s disease associated gene protein tyrosine phosphatase n2. Gastroenterology 2009; 137:2030 - 40, e5; http://dx.doi.org/10.1053/j.gastro.2009.07.078; PMID: 19818778
- Langlois MJ, Bergeron S, Bernatchez G, Boudreau F, Saucier C, Perreault N, et al. The PTEN phosphatase controls intestinal epithelial cell polarity and barrier function: role in colorectal cancer progression. PLoS One 2010; 5:e15742; http://dx.doi.org/10.1371/journal.pone.0015742; PMID: 21203412
- Petit CS, Barreau F, Besnier L, Gandille P, Riveau B, Chateau D, et al. Requirement of cellular prion protein for intestinal barrier function and mislocalization in patients with inflammatory bowel disease. Gastroenterology 2012; 143:122 - , e15; http://dx.doi.org/10.1053/j.gastro.2012.03.029; PMID: 22446194
- Vetrano S, Ploplis VA, Sala E, Sandoval-Cooper M, Donahue DL, Correale C, et al. Unexpected role of anticoagulant protein C in controlling epithelial barrier integrity and intestinal inflammation. Proc Natl Acad Sci U S A 2011; 108:19830 - 5; http://dx.doi.org/10.1073/pnas.1107140108; PMID: 22109555
- Fredenburgh LE, Velandia MM, Ma J, Olszak T, Cernadas M, Englert JA, et al. Cyclooxygenase-2 deficiency leads to intestinal barrier dysfunction and increased mortality during polymicrobial sepsis. J Immunol 2011; 187:5255 - 67; http://dx.doi.org/10.4049/jimmunol.1101186; PMID: 21967897
- Lu P, Burger-van Paassen N, van der Sluis M, Witte-Bouma J, Kerckaert JP, van Goudoever JB, et al. Colonic gene expression patterns of mucin Muc2 knockout mice reveal various phases in colitis development. Inflamm Bowel Dis 2011; 17:2047 - 57; http://dx.doi.org/10.1002/ibd.21592; PMID: 21910166
- Schlegel N, Meir M, Heupel WM, Holthöfer B, Leube RE, Waschke J. Desmoglein 2-mediated adhesion is required for intestinal epithelial barrier integrity. Am J Physiol Gastrointest Liver Physiol 2010; 298:G774 - 83; http://dx.doi.org/10.1152/ajpgi.00239.2009; PMID: 20224006
- Buzza MS, Netzel-Arnett S, Shea-Donohue T, Zhao A, Lin CY, List K, et al. Membrane-anchored serine protease matriptase regulates epithelial barrier formation and permeability in the intestine. Proc Natl Acad Sci U S A 2010; 107:4200 - 5; http://dx.doi.org/10.1073/pnas.0903923107; PMID: 20142489
- Morita H, Katsuno T, Hoshimoto A, Hirano N, Saito Y, Suzuki Y. Connexin 26-mediated gap junctional intercellular communication suppresses paracellular permeability of human intestinal epithelial cell monolayers. Exp Cell Res 2004; 298:1 - 8; http://dx.doi.org/10.1016/j.yexcr.2004.03.046; PMID: 15242756
- Netzel-Arnett S, Buzza MS, Shea-Donohue T, Désilets A, Leduc R, Fasano A, et al. Matriptase protects against experimental colitis and promotes intestinal barrier recovery. Inflamm Bowel Dis 2012; 18:1303 - 14; http://dx.doi.org/10.1002/ibd.21930; PMID: 22081509
- Laukoetter MG, Nava P, Lee WY, Severson EA, Capaldo CT, Babbin BA, et al. JAM-A regulates permeability and inflammation in the intestine in vivo. J Exp Med 2007; 204:3067 - 76; http://dx.doi.org/10.1084/jem.20071416; PMID: 18039951
- Hossain Z, Hirata T. Molecular mechanism of intestinal permeability: interaction at tight junctions. Mol Biosyst 2008; 4:1181 - 5; http://dx.doi.org/10.1039/b800402a; PMID: 19396381
- Ivanov AI, Nusrat A, Parkos CA. The epithelium in inflammatory bowel disease: potential role of endocytosis of junctional proteins in barrier disruption. Novartis Foundation symposium 2004; 263:115-24; discussion 24-32, 211-8.
- Kucharzik T, Walsh SV, Chen J, Parkos CA, Nusrat A. Neutrophil transmigration in inflammatory bowel disease is associated with differential expression of epithelial intercellular junction proteins. Am J Pathol 2001; 159:2001 - 9; http://dx.doi.org/10.1016/S0002-9440(10)63051-9; PMID: 11733350
- Prasad S, Mingrino R, Kaukinen K, Hayes KL, Powell RM, MacDonald TT, et al. Inflammatory processes have differential effects on claudins 2, 3 and 4 in colonic epithelial cells. Lab Invest 2005; 85:1139 - 62; http://dx.doi.org/10.1038/labinvest.3700316; PMID: 16007110
- Zeissig S, Bürgel N, Günzel D, Richter J, Mankertz J, Wahnschaffe U, et al. Changes in expression and distribution of claudin 2, 5 and 8 lead to discontinuous tight junctions and barrier dysfunction in active Crohn’s disease. Gut 2007; 56:61 - 72; http://dx.doi.org/10.1136/gut.2006.094375; PMID: 16822808
- Bürgel N, Bojarski C, Mankertz J, Zeitz M, Fromm M, Schulzke JD. Mechanisms of diarrhea in collagenous colitis. Gastroenterology 2002; 123:433 - 43; http://dx.doi.org/10.1053/gast.2002.34784; PMID: 12145796
- Amasheh S, Dullat S, Fromm M, Schulzke JD, Buhr HJ, Kroesen AJ. Inflamed pouch mucosa possesses altered tight junctions indicating recurrence of inflammatory bowel disease. Int J Colorectal Dis 2009; 24:1149 - 56; http://dx.doi.org/10.1007/s00384-009-0737-8; PMID: 19488769
- Tang Y, Clayburgh DR, Mittal N, Goretsky T, Dirisina R, Zhang Z, et al. Epithelial NF-kappaB enhances transmucosal fluid movement by altering tight junction protein composition after T cell activation. Am J Pathol 2010; 176:158 - 67; http://dx.doi.org/10.2353/ajpath.2010.090548; PMID: 20008138
- Heller F, Florian P, Bojarski C, Richter J, Christ M, Hillenbrand B, et al. Interleukin-13 is the key effector Th2 cytokine in ulcerative colitis that affects epithelial tight junctions, apoptosis, and cell restitution. Gastroenterology 2005; 129:550 - 64; PMID: 16083712
- Mennigen R, Nolte K, Rijcken E, Utech M, Loeffler B, Senninger N, et al. Probiotic mixture VSL#3 protects the epithelial barrier by maintaining tight junction protein expression and preventing apoptosis in a murine model of colitis. Am J Physiol Gastrointest Liver Physiol 2009; 296:G1140 - 9; http://dx.doi.org/10.1152/ajpgi.90534.2008; PMID: 19221015
- Thuijls G, Derikx JP, de Haan JJ, Grootjans J, de Bruïne A, Masclee AA, et al. Urine-based detection of intestinal tight junction loss. J Clin Gastroenterol 2010; 44:e14 - 9; http://dx.doi.org/10.1097/MCG.0b013e31819f5652; PMID: 19525861
- Suzuki T, Yoshinaga N, Tanabe S. Interleukin-6 (IL-6) regulates claudin-2 expression and tight junction permeability in intestinal epithelium. J Biol Chem 2011; 286:31263 - 71; http://dx.doi.org/10.1074/jbc.M111.238147; PMID: 21771795
- Mankertz J, Schulzke JD. Altered permeability in inflammatory bowel disease: pathophysiology and clinical implications. Curr Opin Gastroenterol 2007; 23:379 - 83; http://dx.doi.org/10.1097/MOG.0b013e32816aa392; PMID: 17545772
- Hering NA, Schulzke JD. Therapeutic options to modulate barrier defects in inflammatory bowel disease. Dig Dis 2009; 27:450 - 4; http://dx.doi.org/10.1159/000233283; PMID: 19897959
- Rosen MJ, Frey MR, Washington MK, Chaturvedi R, Kuhnhein LA, Matta P, et al. STAT6 activation in ulcerative colitis: a new target for prevention of IL-13-induced colon epithelial cell dysfunction. Inflamm Bowel Dis 2011; 17:2224 - 34; http://dx.doi.org/10.1002/ibd.21628; PMID: 21308881
- Weber CR, Raleigh DR, Su L, Shen L, Sullivan EA, Wang Y, et al. Epithelial myosin light chain kinase activation induces mucosal interleukin-13 expression to alter tight junction ion selectivity. J Biol Chem 2010; 285:12037 - 46; http://dx.doi.org/10.1074/jbc.M109.064808; PMID: 20177070
- Utech M, Brüwer M, Nusrat A. Tight junctions and cell-cell interactions. Methods Mol Biol 2006; 341:185 - 95; PMID: 16799199
- Schulzke JD, Ploeger S, Amasheh M, Fromm A, Zeissig S, Troeger H, et al. Epithelial tight junctions in intestinal inflammation. Ann N Y Acad Sci 2009; 1165:294 - 300; http://dx.doi.org/10.1111/j.1749-6632.2009.04062.x; PMID: 19538319
- Das P, Goswami P, Das TK, Nag T, Sreenivas V, Ahuja V, et al. Comparative tight junction protein expressions in colonic Crohn’s disease, ulcerative colitis, and tuberculosis: a new perspective. Virchows Arch 2012; 460:261 - 70; http://dx.doi.org/10.1007/s00428-012-1195-1; PMID: 22297703
- Poritz LS, Harris LR 3rd, Kelly AA, Koltun WA. Increase in the tight junction protein claudin-1 in intestinal inflammation. Dig Dis Sci 2011; 56:2802 - 9; http://dx.doi.org/10.1007/s10620-011-1688-9; PMID: 21748286
- Fries W, Muja C, Crisafulli C, Cuzzocrea S, Mazzon E. Dynamics of enterocyte tight junctions: effect of experimental colitis and two different anti-TNF strategies. Am J Physiol Gastrointest Liver Physiol 2008; 294:G938 - 47; http://dx.doi.org/10.1152/ajpgi.00469.2007; PMID: 18258792
- Li Q, Zhang Q, Zhang M, Wang C, Zhu Z, Li N, et al. Effect of n-3 polyunsaturated fatty acids on membrane microdomain localization of tight junction proteins in experimental colitis. FEBS J 2008; 275:411 - 20; http://dx.doi.org/10.1111/j.1742-4658.2007.06210.x; PMID: 18167140
- Xia XM, Wang FY, Zhou J, Hu KF, Li SW, Zou BB. CXCR4 antagonist AMD3100 modulates claudin expression and intestinal barrier function in experimental colitis. PLoS One 2011; 6:e27282; http://dx.doi.org/10.1371/journal.pone.0027282; PMID: 22073304
- Reuter BK, Pizarro TT. Mechanisms of tight junction dysregulation in the SAMP1/YitFc model of Crohn’s disease-like ileitis. Ann N Y Acad Sci 2009; 1165:301 - 7; http://dx.doi.org/10.1111/j.1749-6632.2009.04035.x; PMID: 19538320
- Sapone A, Lammers KM, Casolaro V, Cammarota M, Giuliano MT, De Rosa M, et al. Divergence of gut permeability and mucosal immune gene expression in two gluten-associated conditions: celiac disease and gluten sensitivity. BMC Med 2011; 9:23; http://dx.doi.org/10.1186/1741-7015-9-23; PMID: 21392369
- Szakál DN, Gyorffy H, Arató A, Cseh A, Molnár K, Papp M, et al. Mucosal expression of claudins 2, 3 and 4 in proximal and distal part of duodenum in children with coeliac disease. Virchows Arch 2010; 456:245 - 50; http://dx.doi.org/10.1007/s00428-009-0879-7; PMID: 20143085
- Schumann M, Günzel D, Buergel N, Richter JF, Troeger H, May C, et al. Cell polarity-determining proteins Par-3 and PP-1 are involved in epithelial tight junction defects in coeliac disease. Gut 2012; 61:220 - 8; http://dx.doi.org/10.1136/gutjnl-2011-300123; PMID: 21865402
- Schumann M, Kamel S, Pahlitzsch ML, Lebenheim L, May C, Krauss M, et al. Defective tight junctions in refractory celiac disease. Ann N Y Acad Sci 2012; 1258:43 - 51; http://dx.doi.org/10.1111/j.1749-6632.2012.06565.x; PMID: 22731714
- Wei J, Hemmings GP. Gene, gut and schizophrenia: the meeting point for the gene-environment interaction in developing schizophrenia. Med Hypotheses 2005; 64:547 - 52; http://dx.doi.org/10.1016/j.mehy.2004.08.011; PMID: 15617864
- Pizzuti D, Senzolo M, Buda A, Chiarelli S, Giacomelli L, Mazzon E, et al. In vitro model for IgE mediated food allergy. Scand J Gastroenterol 2011; 46:177 - 87; http://dx.doi.org/10.3109/00365521.2010.525716; PMID: 21028948
- Rimpiläinen R, Vakkala M, Rimpiläinen E, Jensen H, Rimpiläinen J, Erkinaro T, et al. Minimized and conventional cardiopulmonary bypass damage intestinal mucosal integrity. Scand Cardiovasc J 2011; 45:236 - 46; http://dx.doi.org/10.3109/14017431.2011.572996; PMID: 21495910
- Vaziri ND, Yuan J, Rahimi A, Ni Z, Said H, Subramanian VS. Disintegration of colonic epithelial tight junction in uremia: a likely cause of CKD-associated inflammation. Nephrol Dial Transplant 2012; 27:2686 - 93; http://dx.doi.org/10.1093/ndt/gfr624; PMID: 22131233
- Assimakopoulos SF, Tsamandas AC, Tsiaoussis GI, Karatza E, Triantos C, Vagianos CE, et al. Altered intestinal tight junctions’ expression in patients with liver cirrhosis: a pathogenetic mechanism of intestinal hyperpermeability. Eur J Clin Invest 2012; 42:439 - 46; http://dx.doi.org/10.1111/j.1365-2362.2011.02609.x; PMID: 22023490
- Natoli M, Felsani A, Ferruzza S, Sambuy Y, Canali R, Scarino ML. Mechanisms of defence from Fe(II) toxicity in human intestinal Caco-2 cells. Toxicol In Vitro 2009; 23:1510 - 5; http://dx.doi.org/10.1016/j.tiv.2009.06.016; PMID: 19540330
- Collins JF. Gene chip analyses reveal differential genetic responses to iron deficiency in rat duodenum and jejunum. Biol Res 2006; 39:25 - 37; PMID: 16629162
- Suzuki T, Hara H. Dietary fat and bile juice, but not obesity, are responsible for the increase in small intestinal permeability induced through the suppression of tight junction protein expression in LETO and OLETF rats. Nutr Metab (Lond) 2010; 7:19; http://dx.doi.org/10.1186/1743-7075-7-19; PMID: 20222989
- Wang N, Yu H, Ma J, Wu W, Zhao D, Shi X, et al. Evidence for tight junction protein disruption in intestinal mucosa of malignant obstructive jaundice patients. Scand J Gastroenterol 2010; 45:191 - 9; http://dx.doi.org/10.3109/00365520903406701; PMID: 20095884
- Charoenphandhu N, Wongdee K, Tudpor K, Pandaranandaka J, Krishnamra N. Chronic metabolic acidosis upregulated claudin mRNA expression in the duodenal enterocytes of female rats. Life Sci 2007; 80:1729 - 37; http://dx.doi.org/10.1016/j.lfs.2007.01.063; PMID: 17383680
- Charoenphandhu N, Tudpor K, Pulsook N, Krishnamra N. Chronic metabolic acidosis stimulated transcellular and solvent drag-induced calcium transport in the duodenum of female rats. Am J Physiol Gastrointest Liver Physiol 2006; 291:G446 - 55; http://dx.doi.org/10.1152/ajpgi.00108.2006; PMID: 16675746
- Thuijls G, de Haan JJ, Derikx JP, Daissormont I, Hadfoune M, Heineman E, et al. Intestinal cytoskeleton degradation precedes tight junction loss following hemorrhagic shock. Shock 2009; 31:164 - 9; http://dx.doi.org/10.1097/SHK.0b013e31817fc310; PMID: 18650780
- Beutheu Youmba S, Belmonte L, Galas L, Boukhettala N, Bôle-Feysot C, Déchelotte P, et al. Methotrexate modulates tight junctions through NF-κB, MEK, and JNK pathways. J Pediatr Gastroenterol Nutr 2012; 54:463 - 70; http://dx.doi.org/10.1097/MPG.0b013e318247240d; PMID: 22197938
- Wang H, Zhao JX, Hu N, Ren J, Du M, Zhu MJ. Side-stream smoking reduces intestinal inflammation and increases expression of tight junction proteins. World J Gastroenterol 2012; 18:2180 - 7; http://dx.doi.org/10.3748/wjg.v18.i18.2180; PMID: 22611310
- Deitch EA. Bacterial translocation or lymphatic drainage of toxic products from the gut: what is important in human beings?. Surgery 2002; 131:241 - 4; http://dx.doi.org/10.1067/msy.2002.116408; PMID: 11894026
- Magnotti LJ, Deitch EA. Burns, bacterial translocation, gut barrier function, and failure. J Burn Care Rehabil 2005; 26:383 - 91; http://dx.doi.org/10.1097/01.bcr.0000176878.79267.e8; PMID: 16151282
- Vreemann A, Qu H, Mayer K, Andersen LB, Stefana MI, Wehner S, et al. Cathepsin B release from rodent intestine mucosa due to mechanical injury results in extracellular matrix damage in early post-traumatic phases. Biol Chem 2009; 390:481 - 92; http://dx.doi.org/10.1515/BC.2009.055; PMID: 19335208
- Li Q, Zhang Q, Wang C, Liu X, Qu L, Gu L, et al. Altered distribution of tight junction proteins after intestinal ischaemia/reperfusion injury in rats. J Cell Mol Med 2009; 13:9B 4061 - 76; http://dx.doi.org/10.1111/j.1582-4934.2009.00975.x; PMID: 19929946
- Takizawa Y, Kishimoto H, Kitazato T, Tomita M, Hayashi M. Changes in protein and mRNA expression levels of claudin family after mucosal lesion by intestinal ischemia/reperfusion. Int J Pharm 2012; 426:82 - 9; http://dx.doi.org/10.1016/j.ijpharm.2012.01.023; PMID: 22285474
- Takizawa Y, Kishimoto H, Kitazato T, Tomita M, Hayashi M. Effects of nitric oxide on mucosal barrier dysfunction during early phase of intestinal ischemia/reperfusion. Eur J Pharm Sci 2011; 42:246 - 52; http://dx.doi.org/10.1016/j.ejps.2010.11.016; PMID: 21134443
- Chen C, Wang P, Su Q, Wang S, Wang F. Myosin light chain kinase mediates intestinal barrier disruption following burn injury. PLoS One 2012; 7:e34946; http://dx.doi.org/10.1371/journal.pone.0034946; PMID: 22529961
- Li X, Akhtar S, Choudhry MA. Alteration in intestine tight junction protein phosphorylation and apoptosis is associated with increase in IL-18 levels following alcohol intoxication and burn injury. Biochim Biophys Acta 2012; 1822:196 - 203; http://dx.doi.org/10.1016/j.bbadis.2011.09.019; PMID: 22001439
- Huo Q, Kinugasa T, Wang L, Huang J, Zhao J, Shibaguchi H, et al. Claudin-1 protein is a major factor involved in the tumorigenesis of colorectal cancer. Anticancer Res 2009; 29:851 - 7; PMID: 19414319
- Aung PP, Mitani Y, Sanada Y, Nakayama H, Matsusaki K, Yasui W. Differential expression of claudin-2 in normal human tissues and gastrointestinal carcinomas. Virchows Arch 2006; 448:428 - 34; http://dx.doi.org/10.1007/s00428-005-0120-2; PMID: 16328347
- Tatum R, Zhang Y, Salleng K, Lu Z, Lin JJ, Lu Q, et al. Renal salt wasting and chronic dehydration in claudin-7-deficient mice. Am J Physiol Renal Physiol 2010; 298:F24 - 34; http://dx.doi.org/10.1152/ajprenal.00450.2009; PMID: 19759267
- Mees ST, Mennigen R, Spieker T, Rijcken E, Senninger N, Haier J, et al. Expression of tight and adherens junction proteins in ulcerative colitis associated colorectal carcinoma: upregulation of claudin-1, claudin-3, claudin-4, and beta-catenin. Int J Colorectal Dis 2009; 24:361 - 8; http://dx.doi.org/10.1007/s00384-009-0653-y; PMID: 19184060
- Darido C, Buchert M, Pannequin J, Bastide P, Zalzali H, Mantamadiotis T, et al. Defective claudin-7 regulation by Tcf-4 and Sox-9 disrupts the polarity and increases the tumorigenicity of colorectal cancer cells. Cancer Res 2008; 68:4258 - 68; http://dx.doi.org/10.1158/0008-5472.CAN-07-5805; PMID: 18519685
- Galamb O, Spisák S, Sipos F, Tóth K, Solymosi N, Wichmann B, et al. Reversal of gene expression changes in the colorectal normal-adenoma pathway by NS398 selective COX2 inhibitor. Br J Cancer 2010; 102:765 - 73; http://dx.doi.org/10.1038/sj.bjc.6605515; PMID: 20087348
- Derikx JP, van Waardenburg DA, Thuijls G, Willigers HM, Koenraads M, van Bijnen AA, et al. New Insight in Loss of Gut Barrier during Major Non-Abdominal Surgery. PLoS One 2008; 3:e3954; http://dx.doi.org/10.1371/journal.pone.0003954; PMID: 19088854
- Wilmore DW, Smith RJ, O’Dwyer ST, Jacobs DO, Ziegler TR, Wang XD. The gut: a central organ after surgical stress. Surgery 1988; 104:917 - 23; PMID: 3055397
- Leaphart CL, Tepas JJ 3rd. The gut is a motor of organ system dysfunction. Surgery 2007; 141:563 - 9; http://dx.doi.org/10.1016/j.surg.2007.01.021; PMID: 17462455
- Zhang Q, Li Q, Wang C, Li N, Li J. Redistribution of tight junction proteins during EPEC infection in vivo. Inflammation 2012; 35:23 - 32; http://dx.doi.org/10.1007/s10753-010-9285-1; PMID: 21170673
- Liu Z, Ma Y, Yang J, Zhang P, Moyer MP, Qin H. Expression of the Lactobacillus plantarum surface layer MIMP protein protected NCM460 epithelial cells from enteroinvasive Escherichia coli infection. Cell Physiol Biochem 2011; 27:99 - 108; PMID: 21325827
- Muza-Moons MM, Schneeberger EE, Hecht GA. Enteropathogenic Escherichia coli infection leads to appearance of aberrant tight junctions strands in the lateral membrane of intestinal epithelial cells. Cell Microbiol 2004; 6:783 - 93; http://dx.doi.org/10.1111/j.1462-5822.2004.00404.x; PMID: 15236645
- Strauman MC, Harper JM, Harrington SM, Boll EJ, Nataro JP. Enteroaggregative Escherichia coli disrupts epithelial cell tight junctions. Infect Immun 2010; 78:4958 - 64; http://dx.doi.org/10.1128/IAI.00580-10; PMID: 20823198
- Roxas JL, Koutsouris A, Bellmeyer A, Tesfay S, Royan S, Falzari K, et al. Enterohemorrhagic E. coli alters murine intestinal epithelial tight junction protein expression and barrier function in a Shiga toxin independent manner. Lab Invest 2010; 90:1152 - 68; http://dx.doi.org/10.1038/labinvest.2010.91; PMID: 20479715
- Hering NA, Richter JF, Krug SM, Günzel D, Fromm A, Bohn E, et al. Yersinia enterocolitica induces epithelial barrier dysfunction through regional tight junction changes in colonic HT-29/B6 cell monolayers. Lab Invest 2011; 91:310 - 24; http://dx.doi.org/10.1038/labinvest.2010.180; PMID: 20956974
- Nusrat A, Parkos CA, Verkade P, Foley CS, Liang TW, Innis-Whitehouse W, et al. Tight junctions are membrane microdomains. J Cell Sci 2000; 113:1771 - 81; PMID: 10769208
- Chen ML, Ge Z, Fox JG, Schauer DB. Disruption of tight junctions and induction of proinflammatory cytokine responses in colonic epithelial cells by Campylobacter jejuni. Infect Immun 2006; 74:6581 - 9; http://dx.doi.org/10.1128/IAI.00958-06; PMID: 17015453
- Nielsen HL, Nielsen H, Ejlertsen T, Engberg J, Günzel D, Zeitz M, et al. Oral and fecal Campylobacter concisus strains perturb barrier function by apoptosis induction in HT-29/B6 intestinal epithelial cells. PLoS One 2011; 6:e23858; http://dx.doi.org/10.1371/journal.pone.0023858; PMID: 21887334
- Humen MA, Pérez PF, Liévin-Le Moal V. Lipid raft-dependent adhesion of Giardia intestinalis trophozoites to a cultured human enterocyte-like Caco-2/TC7 cell monolayer leads to cytoskeleton-dependent functional injuries. Cell Microbiol 2011; 13:1683 - 702; http://dx.doi.org/10.1111/j.1462-5822.2011.01647.x; PMID: 21790940
- Lejeune M, Moreau F, Chadee K. Prostaglandin E2 produced by Entamoeba histolytica signals via EP4 receptor and alters claudin-4 to increase ion permeability of tight junctions. Am J Pathol 2011; 179:807 - 18; http://dx.doi.org/10.1016/j.ajpath.2011.05.001; PMID: 21683675
- Khailova L, Dvorak K, Arganbright KM, Halpern MD, Kinouchi T, Yajima M, et al. Bifidobacterium bifidum improves intestinal integrity in a rat model of necrotizing enterocolitis. Am J Physiol Gastrointest Liver Physiol 2009; 297:G940 - 9; http://dx.doi.org/10.1152/ajpgi.00141.2009; PMID: 20501441
- Ewaschuk JB, Diaz H, Meddings L, Diederichs B, Dmytrash A, Backer J, et al. Secreted bioactive factors from Bifidobacterium infantis enhance epithelial cell barrier function. Am J Physiol Gastrointest Liver Physiol 2008; 295:G1025 - 34; http://dx.doi.org/10.1152/ajpgi.90227.2008; PMID: 18787064
- Zhou Y, Qin H, Zhang M, Shen T, Chen H, Ma Y, et al. Lactobacillus plantarum inhibits intestinal epithelial barrier dysfunction induced by unconjugated bilirubin. Br J Nutr 2010; 104:390 - 401; http://dx.doi.org/10.1017/S0007114510000474; PMID: 20412608
- Qin H, Zhang Z, Hang X, Jiang YL. L. plantarum prevents enteroinvasive Escherichia coli-induced tight junction proteins changes in intestinal epithelial cells. BMC Microbiol 2009; 9:63; http://dx.doi.org/10.1186/1471-2180-9-63; PMID: 19331693
- Köhler H, Sakaguchi T, Hurley BP, Kase BA, Reinecker HC, McCormick BA. Salmonella enterica serovar Typhimurium regulates intercellular junction proteins and facilitates transepithelial neutrophil and bacterial passage. Am J Physiol Gastrointest Liver Physiol 2007; 293:G178 - 87; http://dx.doi.org/10.1152/ajpgi.00535.2006; PMID: 17615177
- Troeger H, Epple HJ, Schneider T, Wahnschaffe U, Ullrich R, Burchard GD, et al. Effect of chronic Giardia lamblia infection on epithelial transport and barrier function in human duodenum. Gut 2007; 56:328 - 35; http://dx.doi.org/10.1136/gut.2006.100198; PMID: 16935925
- Sakaguchi T, Köhler H, Gu X, McCormick BA, Reinecker HC. Shigella flexneri regulates tight junction-associated proteins in human intestinal epithelial cells. Cell Microbiol 2002; 4:367 - 81; http://dx.doi.org/10.1046/j.1462-5822.2002.00197.x; PMID: 12067320
- Li Q, Zhang Q, Wang C, Liu X, Li N, Li J. Disruption of tight junctions during polymicrobial sepsis in vivo. J Pathol 2009; 218:210 - 21; http://dx.doi.org/10.1002/path.2525; PMID: 19235836
- Zhang Y, Li J. Carbachol ameliorates lipopolysaccharide-induced intestinal epithelial tight junction damage by down-regulating NF-κβ and myosin light-chain kinase pathways. Biochem Biophys Res Commun 2012; 428:321 - 6; http://dx.doi.org/10.1016/j.bbrc.2012.10.056; PMID: 23098909
- Dickman KG, Hempson SJ, Anderson J, Lippe S, Zhao L, Burakoff R, et al. Rotavirus alters paracellular permeability and energy metabolism in Caco-2 cells. Am J Physiol Gastrointest Liver Physiol 2000; 279:G757 - 66; PMID: 11005763
- Troeger H, Loddenkemper C, Schneider T, Schreier E, Epple HJ, Zeitz M, et al. Structural and functional changes of the duodenum in human norovirus infection. Gut 2009; 58:1070 - 7; http://dx.doi.org/10.1136/gut.2008.160150; PMID: 19036950
- Epple HJ, Schneider T, Troeger H, Kunkel D, Allers K, Moos V, et al. Impairment of the intestinal barrier is evident in untreated but absent in suppressively treated HIV-infected patients. Gut 2009; 58:220 - 7; http://dx.doi.org/10.1136/gut.2008.150425; PMID: 18936106
- Xu LF, Teng X, Guo J, Sun M. Protective effect of intestinal trefoil factor on injury of intestinal epithelial tight junction induced by platelet activating factor. Inflammation 2012; 35:308 - 15; http://dx.doi.org/10.1007/s10753-011-9320-x; PMID: 21452036
- McLaughlin J, Padfield PJ, Burt JP, O’Neill CA. Ochratoxin A increases permeability through tight junctions by removal of specific claudin isoforms. Am J Physiol Cell Physiol 2004; 287:C1412 - 7; http://dx.doi.org/10.1152/ajpcell.00007.2004; PMID: 15229101
- Diesing AK, Nossol C, Dänicke S, Walk N, Post A, Kahlert S, et al. Vulnerability of polarised intestinal porcine epithelial cells to mycotoxin deoxynivalenol depends on the route of application. PLoS One 2011; 6:e17472; http://dx.doi.org/10.1371/journal.pone.0017472; PMID: 21364771
- Pinton P, Braicu C, Nougayrede JP, Laffitte J, Taranu I, Oswald IP. Deoxynivalenol impairs porcine intestinal barrier function and decreases the protein expression of claudin-4 through a mitogen-activated protein kinase-dependent mechanism. J Nutr 2010; 140:1956 - 62; http://dx.doi.org/10.3945/jn.110.123919; PMID: 20861219
- De Walle JV, Sergent T, Piront N, Toussaint O, Schneider YJ, Larondelle Y. Deoxynivalenol affects in vitro intestinal epithelial cell barrier integrity through inhibition of protein synthesis. Toxicol Appl Pharmacol 2010; 245:291 - 8; http://dx.doi.org/10.1016/j.taap.2010.03.012; PMID: 20362602
- Nakao T, Kurita N, Komatsu M, Yoshikawa K, Iwata T, Utusnomiya T, et al. Irinotecan injures tight junction and causes bacterial translocation in rat. J Surg Res 2012; 173:341 - 7; http://dx.doi.org/10.1016/j.jss.2010.10.003; PMID: 21176921
- Alhamoruni A, Lee AC, Wright KL, Larvin M, O’Sullivan SE. Pharmacological effects of cannabinoids on the Caco-2 cell culture model of intestinal permeability. J Pharmacol Exp Ther 2010; 335:92 - 102; http://dx.doi.org/10.1124/jpet.110.168237; PMID: 20592049
- McCall IC, Betanzos A, Weber DA, Nava P, Miller GW, Parkos CA. Effects of phenol on barrier function of a human intestinal epithelial cell line correlate with altered tight junction protein localization. Toxicol Appl Pharmacol 2009; 241:61 - 70; http://dx.doi.org/10.1016/j.taap.2009.08.002; PMID: 19679145
- Charoenphandhu N, Nakkrasae LI, Kraidith K, Teerapornpuntakit J, Thongchote K, Thongon N, et al. Two-step stimulation of intestinal Ca(2+) absorption during lactation by long-term prolactin exposure and suckling-induced prolactin surge. Am J Physiol Endocrinol Metab 2009; 297:E609 - 19; http://dx.doi.org/10.1152/ajpendo.00347.2009; PMID: 19567804
- Teerapornpuntakit J, Wongdee K, Thongbunchoo J, Krishnamra N, Charoenphandhu N. Proliferation and mRNA expression of absorptive villous cell markers and mineral transporters in prolactin-exposed IEC-6 intestinal crypt cells. Cell Biochem Funct 2012; 30:320 - 7; http://dx.doi.org/10.1002/cbf.2807; PMID: 22281785
- Nagumo Y, Han J, Bellila A, Isoda H, Tanaka T. Cofilin mediates tight-junction opening by redistributing actin and tight-junction proteins. Biochem Biophys Res Commun 2008; 377:921 - 5; http://dx.doi.org/10.1016/j.bbrc.2008.10.071; PMID: 18952063
- Pannequin J, Delaunay N, Darido C, Maurice T, Crespy P, Frohman MA, et al. Phosphatidylethanol accumulation promotes intestinal hyperplasia by inducing ZONAB-mediated cell density increase in response to chronic ethanol exposure. Mol Cancer Res 2007; 5:1147 - 57; http://dx.doi.org/10.1158/1541-7786.MCR-07-0198; PMID: 18025260
- Oshima T, Sasaki M, Kataoka H, Miwa H, Takeuchi T, Joh T. Wip1 protects hydrogen peroxide-induced colonic epithelial barrier dysfunction. Cell Mol Life Sci 2007; 64:3139 - 47; http://dx.doi.org/10.1007/s00018-007-7268-7; PMID: 17965834
- Wisner DM, Harris LR 3rd, Green CL, Poritz LS. Opposing regulation of the tight junction protein claudin-2 by interferon-gamma and interleukin-4. J Surg Res 2008; 144:1 - 7; http://dx.doi.org/10.1016/j.jss.2007.03.059; PMID: 17640674
- Sander GR, Cummins AG, Henshall T, Powell BC. Rapid disruption of intestinal barrier function by gliadin involves altered expression of apical junctional proteins. FEBS Lett 2005; 579:4851 - 5; http://dx.doi.org/10.1016/j.febslet.2005.07.066; PMID: 16099460
- Yeh TH, Hsu LW, Tseng MT, Lee PL, Sonjae K, Ho YC, et al. Mechanism and consequence of chitosan-mediated reversible epithelial tight junction opening. Biomaterials 2011; 32:6164 - 73; PMID: 21641031
- Uskoković V, Lee PP, Walsh LA, Fischer KE, Desai TA. PEGylated silicon nanowire coated silica microparticles for drug delivery across intestinal epithelium. Biomaterials 2012; 33:1663 - 72; http://dx.doi.org/10.1016/j.biomaterials.2011.11.010; PMID: 22116000
- Kutuzova GD, Deluca HF. Gene expression profiles in rat intestine identify pathways for 1,25-dihydroxyvitamin D(3) stimulated calcium absorption and clarify its immunomodulatory properties. Arch Biochem Biophys 2004; 432:152 - 66; http://dx.doi.org/10.1016/j.abb.2004.09.004; PMID: 15542054
- Menez C, Buyse M, Chacun H, Farinotti R, Barratt G. Modulation of intestinal barrier properties by miltefosine. Biochem Pharmacol 2006; 71:486 - 96; http://dx.doi.org/10.1016/j.bcp.2005.11.008; PMID: 16337152
- Li N, Neu J. Glutamine deprivation alters intestinal tight junctions via a PI3-K/Akt mediated pathway in Caco-2 cells. J Nutr 2009; 139:710 - 4; http://dx.doi.org/10.3945/jn.108.101485; PMID: 19211824
- Shrestha A, McClane BA. Human claudin-8 and -14 are receptors capable of conveying the cytotoxic effects of Clostridium perfringens enterotoxin. MBio 2013; 4; http://dx.doi.org/10.1128/mBio.00594-12; PMID: 23322640
- Uchida H, Kondoh M, Hanada T, Takahashi A, Hamakubo T, Yagi K. A claudin-4 modulator enhances the mucosal absorption of a biologically active peptide. Biochem Pharmacol 2010; 79:1437 - 44; http://dx.doi.org/10.1016/j.bcp.2010.01.010; PMID: 20096266
- Matsuhisa K, Kondoh M, Suzuki H, Yagi K. Comparison of mucosal absorption-enhancing activity between a claudin-3/-4 binder and a broadly specific claudin binder. Biochem Biophys Res Commun 2012; 423:229 - 33; http://dx.doi.org/10.1016/j.bbrc.2012.05.060; PMID: 22659740
- Smedley JG 3rd, Saputo J, Parker JC, Fernandez-Miyakawa ME, Robertson SL, McClane BA, et al. Noncytotoxic Clostridium perfringens enterotoxin (CPE) variants localize CPE intestinal binding and demonstrate a relationship between CPE-induced cytotoxicity and enterotoxicity. Infect Immun 2008; 76:3793 - 800; http://dx.doi.org/10.1128/IAI.00460-08; PMID: 18505809
- Katahira J, Sugiyama H, Inoue N, Horiguchi Y, Matsuda M, Sugimoto N. Clostridium perfringens enterotoxin utilizes two structurally related membrane proteins as functional receptors in vivo. J Biol Chem 1997; 272:26652 - 8; http://dx.doi.org/10.1074/jbc.272.42.26652; PMID: 9334247
- Katahira J, Inoue N, Horiguchi Y, Matsuda M, Sugimoto N. Molecular cloning and functional characterization of the receptor for Clostridium perfringens enterotoxin. J Cell Biol 1997; 136:1239 - 47; http://dx.doi.org/10.1083/jcb.136.6.1239; PMID: 9087440
- Takahashi A, Komiya E, Kakutani H, Yoshida T, Fujii M, Horiguchi Y, et al. Domain mapping of a claudin-4 modulator, the C-terminal region of C-terminal fragment of Clostridium perfringens enterotoxin, by site-directed mutagenesis. Biochem Pharmacol 2008; 75:1639 - 48; http://dx.doi.org/10.1016/j.bcp.2007.12.016; PMID: 18342294
- Ebihara C, Kondoh M, Harada M, Fujii M, Mizuguchi H, Tsunoda S, et al. Role of Tyr306 in the C-terminal fragment of Clostridium perfringens enterotoxin for modulation of tight junction. Biochem Pharmacol 2007; 73:824 - 30; http://dx.doi.org/10.1016/j.bcp.2006.11.013; PMID: 17169334
- Takahashi A, Kondoh M, Uchida H, Kakamu Y, Hamakubo T, Yagi K. Mutated C-terminal fragments of Clostridium perfringens enterotoxin have increased affinity to claudin-4 and reversibly modulate tight junctions in vitro. Biochem Biophys Res Commun 2011; 410:466 - 70; http://dx.doi.org/10.1016/j.bbrc.2011.05.161; PMID: 21672529
- Shi Y, Bao CH, Wu HG, Ma XP, Yu LQ, Zhang R, et al. [Effect of moxibustion on colonic TNF-alpha content and influence of colonic supernatant of crohn’s disease rats undergoing moxibustion on expression of occludin, claudin-1 and zonula occludens-1 proteins and genes in cultured colonic epithelial cells]. Zhen Ci Yan Jiu 2011; 36:235 - 41; PMID: 21942174
- Liu Y, Chen F, Odle J, Lin X, Jacobi SK, Zhu H, et al. Fish oil enhances intestinal integrity and inhibits TLR4 and NOD2 signaling pathways in weaned pigs after LPS challenge. J Nutr 2012; 142:2017 - 24; http://dx.doi.org/10.3945/jn.112.164947; PMID: 23014495
- Corridoni D, Pastorelli L, Mattioli B, Locovei S, Ishikawa D, Arseneau KO, et al. Probiotic bacteria regulate intestinal epithelial permeability in experimental ileitis by a TNF-dependent mechanism. PLoS One 2012; 7:e42067; http://dx.doi.org/10.1371/journal.pone.0042067; PMID: 22848704
- Plöger S, Stumpff F, Penner GB, Schulzke JD, Gäbel G, Martens H, et al. Microbial butyrate and its role for barrier function in the gastrointestinal tract. Ann N Y Acad Sci 2012; 1258:52 - 9; http://dx.doi.org/10.1111/j.1749-6632.2012.06553.x; PMID: 22731715
- Rentea RM, Liedel JL, Welak SR, Cassidy LD, Mayer AN, Pritchard KA Jr., et al. Intestinal alkaline phosphatase administration in newborns is protective of gut barrier function in a neonatal necrotizing enterocolitis rat model. J Pediatr Surg 2012; 47:1135 - 42; http://dx.doi.org/10.1016/j.jpedsurg.2012.03.018; PMID: 22703783
- Bao CH, Wu LY, Shi Y, Wu HG, Liu HR, Zhang R, et al. Moxibustion down-regulates colonic epithelial cell apoptosis and repairs tight junctions in rats with Crohn’s disease. World J Gastroenterol 2011; 17:4960 - 70; http://dx.doi.org/10.3748/wjg.v17.i45.4960; PMID: 22174545
- Moran GW, O’Neill C, McLaughlin JT. GLP-2 enhances barrier formation and attenuates TNFα-induced changes in a Caco-2 cell model of the intestinal barrier. Regul Pept 2012; 178:95 - 101; http://dx.doi.org/10.1016/j.regpep.2012.07.002; PMID: 22809889
- Hering NA, Andres S, Fromm A, van Tol EA, Amasheh M, Mankertz J, et al. Transforming growth factor-β, a whey protein component, strengthens the intestinal barrier by upregulating claudin-4 in HT-29/B6 cells. J Nutr 2011; 141:783 - 9; http://dx.doi.org/10.3945/jn.110.137588; PMID: 21430244
- McGilligan VE, Wallace JM, Heavey PM, Ridley DL, Rowland IR. The effect of nicotine in vitro on the integrity of tight junctions in Caco-2 cell monolayers. Food Chem Toxicol 2007; 45:1593 - 8; http://dx.doi.org/10.1016/j.fct.2007.02.021; PMID: 17399881
- Suzuki T, Tanabe S, Hara H. Kaempferol enhances intestinal barrier function through the cytoskeletal association and expression of tight junction proteins in Caco-2 cells. J Nutr 2011; 141:87 - 94; http://dx.doi.org/10.3945/jn.110.125633; PMID: 21068182
- Amasheh M, Schlichter S, Amasheh S, Mankertz J, Zeitz M, Fromm M, et al. Quercetin enhances epithelial barrier function and increases claudin-4 expression in Caco-2 cells. J Nutr 2008; 138:1067 - 73; PMID: 18492835
- Ramalingam A, Wang X, Gabello M, Valenzano MC, Soler AP, Ko A, et al. Dietary methionine restriction improves colon tight junction barrier function and alters claudin expression pattern. Am J Physiol Cell Physiol 2010; 299:C1028 - 35; http://dx.doi.org/10.1152/ajpcell.00482.2009; PMID: 20739626
- Nishiyama R, Sakaguchi T, Kinugasa T, Gu X, MacDermott RP, Podolsky DK, et al. Interleukin-2 receptor beta subunit-dependent and -independent regulation of intestinal epithelial tight junctions. J Biol Chem 2001; 276:35571 - 80; http://dx.doi.org/10.1074/jbc.M106013200; PMID: 11466322
- Kinugasa T, Sakaguchi T, Gu X, Reinecker HC. Claudins regulate the intestinal barrier in response to immune mediators. Gastroenterology 2000; 118:1001 - 11; http://dx.doi.org/10.1016/S0016-5085(00)70351-9; PMID: 10833473