Abstract
In the present study, we examined prolonged infection after antigenic co-stimulation by inoculation of the fungus Fonsecaea pedrosoi at two different sites in three mouse strains (BALB/c, Swiss, and C57BL/6). Using this murine model of infection, we showed that antigen induction of infection at more than one site led to a local suppression of active lesions, which increased the time course of experimental chromoblastomycosis (CBM). Footpad infection with a simultaneous infection of the peritoneum or a mucosal site appeared to cause prolonged infection and frequent fungal disseminations. Using knockout (KO) mice, we observed that antigenic co-stimulation caused progressive illness in CD8-KO animals and an effective immune response in the absence of IL-10. In Xid mice, co-stimulation provoked chronic infection (not prolonged), suggesting that B1 B cells play an important role in the control of fungal infection. The tissue response to infection was similar in all co-stimulated mouse groups, as anatomopathologic sections revealed multifocal lesions (granuloma-like). In general, these mice had acute responses at primary antigenic sites with an intense migration of polymorphonuclear leukocytes (PMNs), whereas the distant infection sites (footpad) showed signs of chronic infection. The migration of PMNs to the secondary site (footpad) increased in the later periods of infection, especially after the disappearance of the primary antigenic focus. PMN migration was associated with lesion-dormancy breakage and fungal elimination. Our findings suggest that the host inflammatory/suppression mechanisms induced by antigenic co-stimulation to systemically fight the same pathogen act coordinately through responses that differ at the sites of infection between acute and chronic integrated healing processes that are more prolonged than an acute infection at a single site. However, the long persistence of fungal cells in the host may be linked to microbial adaptation to a parasitic infection as observed in co-stimulated Xid mice.
Introduction
Since the 20th century, the phenomenon of microbial immunosuppression and its relationship with host tolerance has been reviewed.Citation1 Mice infected on the footpad with Mycobacterium lepraemurium and simultaneously challenged with the same heat-killed microorganism were found to be hyporesponsive to infection.Citation2 Surprisingly, host inter-specific microbial costimuli have been associated with down-modulation of the immune response to helminthic infections, as well as to vaccines commonly used worldwide.Citation3–Citation6 This promiscuous immunity probably occurs because once the suppressive response is activated, it seems to be nonspecific.Citation7 More recently, however, a link between T suppressor cells and peripheral tolerance induced after Leishmania major inoculation at two sites has been described.Citation8,Citation9
Over the past 15 years, evidence has accumulated that suppressor cells such as CD4+CD25+ T regulatory cells (T regs) are important for peripheral tolerance and downregulation of various immune responses.Citation10,Citation11 The production of tolerant cells may be beneficial to hosts and parasites.Citation7,Citation12 For instance, the immunosuppressive response provoked by these cells can block the collateral tissue damage that results from excessive inflammation and can cause chronic infections, or even parasitic infection, as a result of microbial adaptation following an extended period of infection.Citation13 The control or regulation of the immune system to prevent or minimize the damage caused by immune reactivity to antigens or over-active immune responses to pathogens can be modulated either by immunosuppressive cellular contacts or by the production of cytokines.Citation7,Citation13 Suppressive cells produce interleukin-10 (IL-10) and exert strong anti-inflammatory effects.Citation13,Citation14 The severe human form of chromoblastomycosis (CBM) has been shown to correlate with high levels of IL-10, whereas low or moderate clinical manifestations correlate with a lower production of IL-10.Citation15
The local and systemic actions of lymphocytes may have different features for CBM disease. Fonsecaea pedrosoi infection in athymic mice with higher susceptibility regressed following the adoptive transfer of lymphocytes,Citation16 thus demonstrating the importance of these cells in fungal control. The absence of CD4 T cells has been reported as a factor that inhibits the ability of mice to defend against F. pedrosoi.Citation17 On the other hand, the significant migration of polymorphonuclear leukocytes (PMNs) to the inoculation site may be linked to resistance to F. pedrosoi infection.Citation16,Citation18,Citation19 Macrophages may induce neutrophil apoptosis through membrane tumor necrosis factor (TNF).Citation20 This apoptosis may lead to the release of complement proteins, microbicidal molecules, and oxidative enzymes, as well as the activation of phagocytes for subsequent clearance.Citation21,Citation22
The development of immune responses to antigens may depend on the interactions between reactive T cells and B cells.Citation23 Despite the professional antigen-presenting macrophages and dendritic cells, mature B lymphocytes play a pivotal role in autoimmunity as antigen-presenting cells (APCs) as well as in cellular responses to self-antigens.Citation24 Furthermore, the possible presence of B cells may also be important for the host “peripheral intolerant” response to non-self-antigens.
In general, animals inoculated at a single site with high parasitic burdens of F. pedrosoi cells have proven unsuitable as models of experimental CBM, especially because of the acute course of infections and efficient fungal elimination by the host response.Citation18,Citation19,Citation25,Citation26 However, inoculation of viable cells from a CBM agent at different peritoneal sites in mice allows increased fungal persistence.Citation27–Citation29 Following studies that showed increased host susceptibility to F. pedrosoi, we have become interested in understanding the elementary immune mechanisms involved in the induction of persistent mycosis following antigenic stimulation at multiple sites. Therefore, the main goal of the present study was to outline the murine immune response to microbial stimulation at two distinct sites using different antigen-presenting routes, viable and nonviable inocula, specific animal knockouts, and Xid mice, which are deficient in B1 B cells.
Results and Discussion
One-site infection.
At 15 days after the inoculation of cells from broth cultures, the site of infection on the footpad showed the presence of conidia, fungal budding-cells that are oval or round in shape with a thick cell wall, and germinative tubes (small, thin hyphae probably resulting from conidia germination). Scleroticlike forms were observed an average of 25 days after infection.Citation30 In general, destruction of the conidia and small hyphae occurred as early as during the third week. The infected footpads showed an intense inflammatory response, which normally decreased 30 to 35 days after inoculation (). The disappearance F. pedrosoi infection at one site commonly regressed after 35 days due to an intense inflammatory response that was linked to an abundant PMN migration to the abscess. Acute infections with CBM agents and the production of neutrophilic abscesses or exudates after inoculation at a single site have previously been described.Citation18,Citation26,Citation31,Citation32 In a previous study, we observed that neutrophil degeneration in vivo may be a cause related to the control of F. pedrosoi population.Citation30 Recently, an antimicrobial complex termed the neutrophil extracellular trap (NET), which is released after neutrophil degranulation, was reported to efficiently bind and kill microorganisms.Citation33 It is likely that the fungicidal action in vivo may be a result of the cellular discharge of these complexes at sites of inflammation. Thus, the intensity of neutrophil infiltration may be a factor associated with host resistance, and it may interfere with the adaptive response of fungal pathogens, during which they may shift to parasitism.
Co-stimuli.
Inoculation of Leishmania major at two sites in BALB/c mice has been shown to induce the migration of regulatory T cells to one of the infection sites, resulting in greater infection persistence than in animals inoculated at a single site.Citation9 A type of chronic F. pedrosoi infection occurs following fungal inoculation at various peritoneal sites.Citation27,Citation28 Similarly, we observed that BALB/c mice stimulated at two sites (peritoneum and footpad) with viable F. pedrosoi cells developed prolonged infection. Further experiments have shown that this long course of infection has also arisen when animals were inoculated with viable and nonviable cells at the footpad (s.c.) and peritoneum (i.p.), respectively. Surprisingly, the increase of the footpad volume and fungal persistence in animals inoculated at two sites with viable and nonviable cells were similar to those in animals inoculated at two sites with only viable cells. This demonstrated that the presence of antigens at different sites can stimulate inflammatory reactions that are different from those triggered by unifocal stimuli (), regardless of the cellular viability of the parasite. This result was observed in all groups, including C57BL/6 and outbred mice.
Thus, double-stimulated animals developed a prolonged illness that we believe to be a type of chronic infection. In addition, we observed different tissue responses in anatomopathologic sections of animals stimulated at one and two sites. Mice inoculated at a single site generally produced a more localized, neutrophilrich abscess,Citation30 while groups concomitantly stimulated at more than one site showed multifocal lesions (granuloma-like) that were rich in histiocytes, especially on the footpad (). We frequently observed a neutrophilic abscess with the characteristics of intense acute inflammation in the peritoneal tissue of co-stimulated animals. In the late periods of infection at approximately 50 to 60 days, the peritoneal abscess disappeared (total absence of antigen), and it was correlated with a decrease in the number of fungal cells (sclerotic bodies) in the multifocal lesions on the footpad and an increase in neutrophil migration to these sites. For this, we suggest that the development of an efficient inflammatory response at one infectious site (footpad) depends on the elimination of the antigenic stimulus at another site (peritoneum). Probably, the simultaneous immune response to specific pathogens at multiple sites resolves the infection only one site at a time. The natural attempt of the immune response to exhibit particular actions (sectoral) to eliminate one focus of infection at a time may be beneficial to the host as a whole. Indeed, necrosis of multiple sites or a strong inflammatory response at several sites would be extremely harmful to the host. However, prolonged infections are problematic as the length of time that the infection continues may allow the pathogen to adapt to the host by becoming parasitic. Nevertheless, although prolonged infection was observed in the present study following co-stimulation, experimental chronic disease due to CBM agents probably also depends on pre-adapted virulent fungal forms that resist the stress of the host conditions, including neutrophil action. Hence, the chronic course of the disease may be a function of such factors as fungal forms and host immunity.
The lack of host immunity has been attributed to the dissemination of sclerotic cells in an experimental CBM model with a deficient cellular immune response.Citation16 Dissemination of sclerotic cells from the footpads to other sites, such as the scrotum, liver, tail, spleen, pancreas and viscera, has frequently been observed in co-stimulated mice (). The sclerotic cell disseminations were frequently observed 20 to 40 days post-infection. The cellular immune response is the most important mechanism of defense against fungi, and it is primarily mediated by neutrophils.Citation34,Citation35 Therefore, compromising or suppressing their function increases host vulnerability to fungal infection.Citation36 Infectious tolerance caused by humoral or cellular factors may suppress neutrophilic activity by means similar to infections by Aspergillus fumigatus.Citation37
Co-stimulation of mucosa and footpad.
The generation of systemic tolerance after oral antigen exposure is well established, and it generally requires high doses of antigen. To test whether fungal stimulation at two sites recognized to be routes of inducing tolerance can also cause an immunosuppressive response, we inoculated nonviable cells in the oral or pulmonary mucosa before infecting the footpad. At that point, the concept of “superinfection” as an inducer of immune tolerance seemed unclear because the responses were similar in animals that were costimulated independently if the primary site was inoculated with viable or nonviable fungal cells. Indeed, intense inflammatory responses and efficient fungal control have been shown to correlate with high inoculum concentrations in animals that received stimuli at one site.Citation30
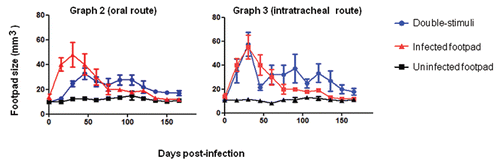
A total of 50 µL of 1 × 106 cells/mL heat-killed F. pedrosoi cells were given orally approximately three times weekly. In mice immunized orally prior to infection, footpad swelling was less intense, and the lesions stabilized two months after fungal inoculation (). This result suggests that antigenic co-stimulation can increase peripheral tolerance to F. pedrosoi infection, as evidenced by the lower initial inflammatory response at the site of secondary infection and the subsequent prolonged infection in mice that were pre-stimulated (v.o.). The other group of mice received a single dose of the same cellular antigen injected in the pulmonary mucosa following a tracheotomy. BALB/c mice were concurrently stimulated via tracheal immunization (i.t.) and subcutaneously infected on the footpad. In the early periods of infection, they showed swelling similar to that seen after unifocal infection, but the co-stimulated animals subsequently showed prolonged infection ().
The prolonged infection of the footpad caused by such oral and pulmonary stimuli suggests that the footpad infection and immunization of both mucosal routes can promote peripheral tolerance to fungal antigens. Such tolerance is more apparent in groups previously treated v.o. with antigen prior to infection than in mice challenged i.t.; this is likely due to the previous immunization. In general, in both co-stimulation groups, the healing phase of the infectious process started after five months, contrary to animal groups inoculated at single site, which often healed after two months. Histological characteristics were similar for mice co-stimulated subcutaneously (s.c.)/intraperitoneally (i.p.). The footpad lesions of mucosally stimulated mice were also lessened during the phase of increase in neutrophil migration to the infection site (footpad) generally after 4 months, as seen in the group challenged i.p.
One- and two-site immune stimulation of CD4 KO, CD8 KO or IL-10 KO mice.
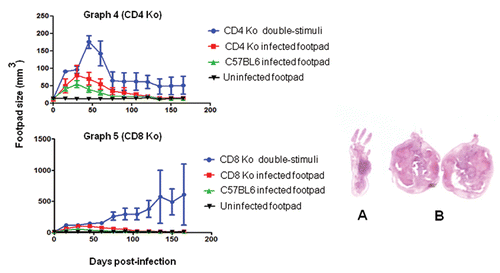
We studied various KO mice to verify the insights described above concerning murine peripheral tolerance to F. pedrosoi infection. Although KO animals infected at one site were generally more susceptible to fungal infection than wild-type mice (–), the KO groups showed similar tissue profiles and immune responses. However, the course of infection was different in the co-stimulated CD4 KO mice and CD8 KO mice. For example, the CD4 KO mice showed significant footpad swelling soon after infection, but this had regressed in most cases by 60 days (). Some of the co-stimulated CD4 KO mice healed normally after 165 days. In contrast, CD8 KO mice co-stimulated presented larger necrotic lesions with progressive footpad swelling and no signs of healing (). This suggests that a non-CD8 lymphocyte population might be responsible for exacerbating the peripheral tolerance. Suppressed host immune responses against chromoblastomycosis agents may be related to the induction of a specific population of T cells.Citation38 Thus, mice stimulated at two sites can display a late protective response in the absence of T cell CD4 receptors (healing after 165 days), whereas CD8 KO animals have severe, progressive infection. This suggests that there is an increase in suppressor activity that is specific to F. pedrosoi antigens at secondary infectious foci in the absence of the CD8 receptor. In histological sections, neutrophilic abscesses were seen on tissues from all animals infected at a single site (s.c.), and focal lesions rich in histiocytes were seen in groups stimulated at two sites (i.p./s.c).
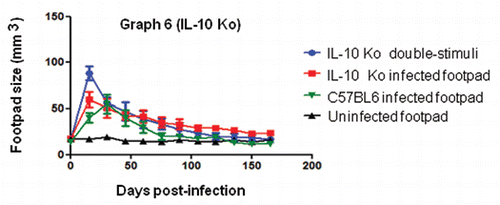
IL-10 has been described as an important cytokine in chronic infections. High levels of IL-10 have been detected after immune co-stimulations.Citation9,Citation39 In our investigation, the IL-10 KO groups showed a similar ability to eliminate fungal infection that was independent of the number of inoculation sites and histopathological profiles (in co-stimulated IL-10 KO mice, we also observed multifocal lesions). This indicates that infection is not prolonged in the absence of IL-10, especially in co-stimulated mice. The findings that co-stimulated IL-10 KO animals did not have prolonged infection () and that tissue reactions were similar to those of other groups of mice stimulated at two sites indicate that these cytokines can play an important role in modulating suppressive immunity to experimental infection with F. pedrosoi.
Infections of B1 B cell-deficient mice and irradiated mice.
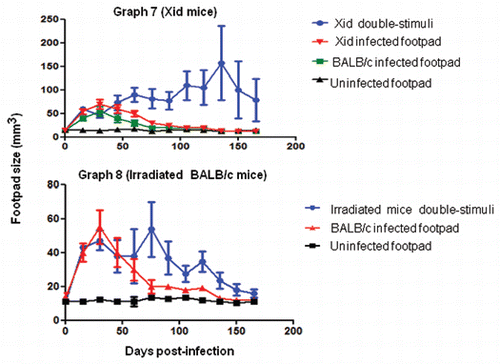
The footpad lesions of Xid mice infected at a single site decreased similarly to the infected BALB/c mice (). However, Xid mice stimulated at two sites (s.c. and i.p.) presented infections that generally lasted more than eight months, which we characterized as chronic infection (not prolonged), including lesion stability and the presence of only sclerotic cells after six months. After four months, disorganized and enlarged focal lesions with a great number of fungal cells, such as conidiogenic cells and small and thin hyphae, were observed in co-stimulated Xid mice ( and B). However, both lesions were small and encompassed with a low number of sclerotic cells and without thin hyphae eight months after infection ( and D). Fungal elimination in the footpad in irradiated, co-stimulated BALB/c mice took longer than in the group inoculated at a single site (). This host response may be the result of repopulation with peritoneal B1 B cells. Thus, multiple antigenic stimuli caused chronic infection in the absence of B1 B lymphocytes; in the presence of these cells, the outcomes were abrogated. It is therefore possible that the B cell population plays antagonistic roles in the suppressive response.
Finally, this model of “infectious tolerance” by antigenic costimulation with viable and nonviable antigens can be expanded to experiments with other fungal agents that are used to evoke prolonged or chronic infections. However, microbial cells or potentially infective forms should be employed for experimental murine infections, because these immunosuppressive tools as antigenic co-stimulation are only auxiliary means to increase the chances of obtaining prolonged infections or chronic diseases in certain immunocompromised hosts. The term “superinfection” has been used in many ways, including for infections at more than one site. We believe that this term should only be used when high parasite concentrations are administered at a single localized site or occasionally for endogenous infections. However, when a host receives the same stimuli at multiple sites, the terms “multifocal” or “multi-site infection” may be preferable. Another insight provided by this study is the possibility that APCs from different sites have different properties that contribute to immunosuppression in different ways in systemically dependent and locally independent fashions. Thus, our observations suggest that co-stimulation with antigen induces the immune system to respond differently at each local site, depending on the systemic whole to solve a multifocal problem. If this reasoning is accurate, the antigenic co-stimulation phenomenon involved in immunosuppressive responses may be important to our understanding of peripheral tolerance.
Materials and Methods
Mice.
Lineages of 6- to 8-week-old mice weighing approximately 23 g were used throughout this study. The specific-pathogenfree animals were purchased from the Center of Development for Experimental Models to Medicine and Biology (CEDEME/UNIFESP) and from the Biotery of Isogenic Mice of the Department of Immunology of Sao Paulo University (ICB/USP), Sao Paulo, Brazil. The protocol used was approved by the UNIFESP Ethics Committee (project 0808/05). Thirty mice were used for each group. A lethal dose of two cycles of 300 rad was administered individually to one group of BALB/c mice because of the radiosensitivity of peritoneal B1 B cells. All experimental procedures were conducted in accordance with standard guidelines on the use and care of laboratory animals.Citation40–Citation42
Microorganism.
Fonsecaea pedrosoi strain EPM-380/03, isolated from a patient with CBM examined in 2003 in the Dermatology Outpatient Department of the Federal University of Sao Paulo (UNIFESP), was cultivated on Sabouraud dextrose agar (SDA, DIFCO Laboratories, Detroit, MI) supplemented with 80 mg/L gentamicin at a temperature of 25°C. The F. pedrosoi strain was subcultured every 15 days into SDA with gentamicin. To enhance fungal virulence, the F. pedrosoi was intraperitoneally reinoculated three times in animals at concentrations of 1 × 108 cells/mL and recovered 12 days later in Mycosel™ agar (BD BBL, Franklin Lakes, NJ USA).Citation27
Inocula.
Fungal cultures were incubated in 300 mL potato dextrose broth (PDB, DIFCO Laboratories) supplemented with 80 mg/L gentamicin and 0.05 mg/mL chloramphenicol, pH 5.7, at 30°C for 30 days in cylindrical 1-L glass bottles (KIMAX® GL-45, DAIGGER Lab Equipments and Supplies) at 100 rpm on a gyratory shaker. These cultures were then filtered twice through 11 µm filter paper (Grade n°. 1, Whatman), and the cells were recovered by centrifuging the filtrate at 3,000 xg for 10 min. Conidia and yeast-like forms were the most abundant cell types based on light microscopy.
F. pedrosoi cells were washed and concentrated three times in phosphate-buffered saline (PBS). Inoculum viability was determined using a LIVE/DEAD® Cell Vitality Assay Kit (L34951, Invitrogen, probes.invitrogen.com/media/pis/mp34951.pdf) and fluorescent microscopy. The viability of inocula suspensions was generally more than 98% of total cells. The suspensions containing fungal forms were adjusted in PBS to concentrations of 1 × 106 cells/mL in a Neubauer chamber.
Mucosal antigenic and peritoneal stimulation.
Fungal inocula were autoclaved in 50-mL Falcon tubes for 20 min at 121°C. Approximately 50 µL of autoclaved inoculum containing dead cells were orally administered (v.o.) with a gavage needle three times a week to BALB/c mice, and after the last dose of the week, the footpad was simultaneously infected by the subcutaneous (s.c.) route with viable fungal cells. The other BALB/c group was tracheotomized for an injection of 50 µL of heat-killed inoculum into the pulmonary mucosa. The remaining groups were inoculated intraperitoneally (i.p.) with approximately 100 µL of heat-killed inoculum. Animal groups stimulated at two sites were immunized and subsequently infected, except for the ones previously treated v.o.
Infectious immune stimulation at one and two sites.
Ten minutes prior to infection, animals were anesthetized i.p. with 0.4 µL of Anasedan and 0.35 mL of Dopalen per kg body weight (www.vetbrands.com.br). Three groups of mice (BALB/c, Swiss, C57BL/6) were infected s.c. on the plantar side with 50 µL of viable inoculum. Another three groups of the same lineages were infected with viable inocula on the footpad and peritoneum. After distinct host reactions were observed among the BALB/c mice infected at one or two sites, we i.p. immunized the co-stimulated (two-site stimulation) group with 100 µL of heat-killed fungal cells, and subsequently infected the footpad s.c. with 50 µL of viable inoculum.
In this study, we also aimed to analyze cellular responses in vivo following double stimulation (i.p. and s.c.). We used groups of animals with knockouts of CD4, CD8 or IL-10, as well as BALB/c mice deficient in B1 B cells and mice that had been irradiated (see animal groups in “Mice”).
A 50 µL volume of PBS was administered to five mice in each of the three lineage groups (BALB/c, Swiss, C57BL/6) to serve as negative controls. Swelling was monitored every week, up to a maximum of 165 days, using a Mitutoyo digital caliper (www.mitutoyo.com). Footpad volume was calculated based on height and width measurements and the mathematic formula for the volume of a cylinder. Results are expressed as mean ± SE. Based on normality data, as defined by the omnibus normality test of D'Agostino and Pearson, significant differences were determined using the Student's t and ANOVA tests (Prism software, www.prism-software.com). Three animals from each group were sacrificed and organs were removed weekly from groups infected unifocally or monthly from mice inoculated at two sites. A portion of each specimen of infected tissue was cultivated on SDA at 37°C for 20 days. Tissue fragments of peritoneal abscesses and infected footpads were clarified with 20% KOH Parker ink for observation by direct microscopy.
Histopathology.
Tissue specimens were left in 10% formalin for more than 12 hours, irrespective of whether they contained macroscopic black spots or not. Bone tissue from the feet was decalcified with 5% ethylenediaminetetraacetic acid (EDTA).Citation43 The specimens were embedded in paraffin, and serial sections with a thickness of 3.5 µm were stained with hematoxylin-eosin (H&E).Citation28
Abbreviations
| CBM | = | chromoblastomycosis |
| SDA | = | Sabouraud dextrose agar |
| PMNs | = | polymorphonuclear leucocytes |
| H&E | = | hematoxylin-eosin |
| KO | = | knockout |
| IL | = | interleukin |
| CD4 | = | cluster of differentiation 4 |
| CD8 | = | cluster of differentiation 8 |
| APC | = | antigen-presenting cell |
| Xid | = | X-linked immunodeficiency |
Figures and Tables
Figure 1 Common histopathological findings of footpad tissue from mice stimulated at two sites; H&E. Focal lesions that were granuloma-like (A, 40x) and rich in fungal cells (B, 400x) observed 30 days post-infection. (C) After 60 days of infection, lesions presented low numbers of sclerotic-like cells (arrows; 200x and insert 1000x), regions rich in histiocytes*H (clear cytoplasms due to cell-cell junctions) and organized focal lesions surrounded by fibroblasts*F (cells with elongated nucleus). (D) 90 days post-infection, high numbers of polymorphonuclear cells(*) were verified in focal lesions without the presence of fungal cells.
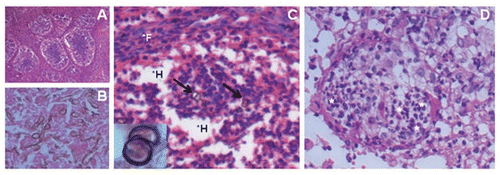
Figure 2 Metastasis of sclerotic-like cells in animals stimulated at two sites. (A) Mouse infected in the footpad with F. pedrosoi cells; the animal received a simultaneous immunization i.p. with heat-killed fungal cells. (B) Common morphology of sclerotic-like cells that migrated to other sites, 1000x.
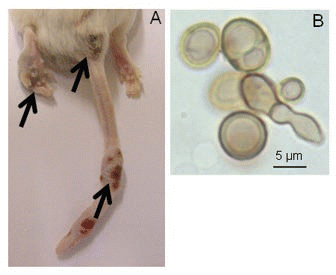
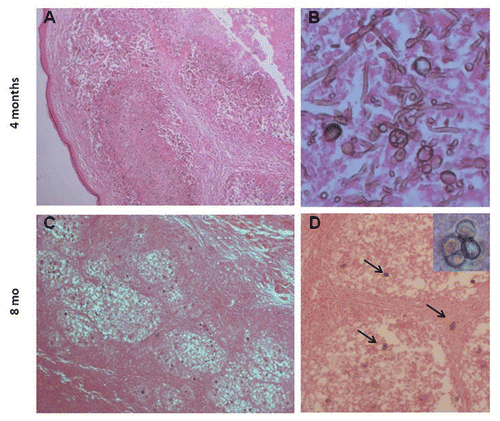
Figure 3 Histopathological characteristics of focal lesions in Xid mice 4 and 8 months post-costimulation; H&E. Larger and irregular lesions of the footpad (A, 100x) with high numbers of thin, small hyphae, conidia and conidiogenous cells (B, 400x) four months post-infection. Small and well-organized granuloma-like lesions (C, 100x) with a small number of sclerotic-like cells (D, 200x and insert 1000x) were observed after eight months.
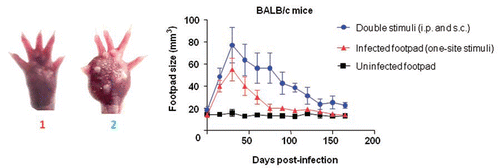
Graph 1 Co-stimulation of the footpad and the peritoneum caused an increase in footpad swelling as well as a prolonged infection in BALB/c mice. The footpads of mice were measured every 15 days for 165 days after inoculation with F. pedrosoi. Animals infected s.c. in the footpad with viable F. pedrosoi cells (triangles) generally presented an infection peak after 30 days and were healed after 60 days post-infection, whereas the lesions of mice co-stimulated by immunization i.p. with nonviable cells and a s.c. footpad infection with fungus had strong swelling and a slower healing rate (circles). The control group of animals was treated with 50 µL of PBS (uninfected footpad, squares). Data are shown with mean and SE values; Student's t test (p < 0.001). Images of the footpads of mice stimulated at a single site (1) and at two sites (2) are shown at 30 days post-infection (left of the graph).
Graphs 2 and 3 Kinetics of footpad lesions in BALB/c mice infected with viable F. pedrosoi cells that received oral (graph 2) or pulmonary (graph 3) mucosal stimuli with nonviable fungal cells. Data are shown as the mean +/- SE. The Student's t test was used to verify the significance between co-stimulation and footpad infection (one site) data; p > 0.05.
Graphs 4 and 5 CD4 KO and CD8 KO mice that were footpad infected with viable F. pedrosoi cells and co-stimulated i.p. with heat-killed fungal cells (co-stimulated). In the early periods post-infection, increased swelling was observed in the footpads of the two animal groups. Animals in the CD4 KO group had a decrease in inflammation after 60 days (). CD8 KO mice did not heal and showed a strong progressive enlargement of the footpad (). Data are shown with mean and SE values; an ANOVA test was used for and ; p < 0.05. In the right panel are pictures of histopathological sections from the footpad of a one-site infected CD8 KO mouse at 30 days (A) and enlarged footpads after 150 days from CD8 KO mice that were co-stimulated (B).
Graph 6 Co-stimulated IL-10 KO mice presented with progressive inflammation of the footpads early after infection and healed after 60 days; this was similar in all animal groups. A one-way ANOVA was performed for the IL-10 group; p > 0.05.
Graphs 7 and 8 Deficiency in B1 B cells causes an increase of host susceptibility to F. pedrosoi infection in co-stimulated mice. Co-stimulated Xid were chronically infected (). Immune control of the fungus in irradiated animals generally occurs after 90 days post-infection (). Data are shown with mean and SE values. An ANOVA was used for analyses of significance for Xid mice (p < 0.05), and the Student's t test was used for the irradiated mice as well as the BALB/c footpad infection data (p = 0.0304).
Acknowledgements
This study was financially supported in part by the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq).
References
- Schwab JH. Suppression of the immune response by microorganisms. Bacteriol Rev 1975; 39:121 - 143
- Lefford MJ, Mackaness GB. Suppression of immunity to Mycobacterium lepraemurium infection. Infect Immun 1977; 18:363 - 369
- Borkow G, Bentwich Z. Chronic immune activation associated with chronic helminthic and human immunodeficiency virus infections: role of hyporesponsiveness and anergy. Clin Microbiol Rev 2004; 17:1012 - 1030
- Cooper PJ, Espinel I, Wieseman M, Paredes W, Espinel M, Guderian RH, Nutman TB. Human onchocerciasis and tetanus vaccination: impact on the postvaccination antitetanus antibody response. Infect Immun 1999; 67:5951 - 5957
- Elias D, Akuffo H, Pawlowski A, Haile M, Schon T, Britton S. Schistosoma mansoni infection reduces the protective efficacy of BCG vaccination against virulent Mycobacterium tuberculosis. Vaccine 2005; 23:1326 - 1334
- Stewart GR, Boussinesq M, Coulson T, Elson L, Nutman T, Bradley JE. Onchocerciasis modulates the immune response to mycobacterial antigens. Clin Exp Immunol 1999; 117:517 - 523
- Cools N, Ponsaerts P, Van Tendeloo VF, Berneman ZN. Balancing between immunity and tolerance: an interplay between dendritic cells, regulatory T cells and effector T cells. J Leukoc Biol 2007; 82:1365 - 1374
- Belkaid Y, Piccirillo CA, Mendez S, Shevach EM, Sacks DL. CD4+CD25+ regulatory T cells control Leishmania major persistence and immunity. Nature 2002; 420:502 - 507
- Mendez S, Reckling SK, Piccirillo CA, Sacks D, Belkaid Y. Role for CD4(+) CD25(+) regulatory T cells in reactivation of persistent leishmaniasis and control of concomitant immunity. J Exp Med 2004; 200:201 - 210
- Pan PY, Ozao J, Zhou Z, Chen SH. Advancements in immune tolerance. Adv Drug Deliv Rev 2008; 60:91 - 105
- Piccirillo CA, Shevach EM. Naturally-occurring CD4+CD25+ immunoregulatory T cells: central players in the arena of peripheral tolerance. Semin Immunol 2004; 16:81 - 88
- Belkaid Y, Rouse BT. Natural regulatory T cells in infectious disease. Nat Immunol 2005; 6:353 - 360
- O'arra A, Vieira PL, Vieira P, Goldfeld AE. IL-10-producing and naturally occurring CD4+ Tregs: limiting collateral damage. J Clin Invest 2004; 114:1372 - 1378
- Trinchieri G. Interleukin-10 production by effector T cells: Th1 cells show self control. J Exp Med 2007; 204:239 - 243
- Sousa MG, de Maria Pedrozo e Silva Azevedo C, Nascimento RC, Ghosn EE, Santiago KL, Noal V, et al. Fonsecaea pedrosoi infection induces differential modulation of costimulatory molecules and cytokines in monocytes from patients with severe and mild forms of chromoblastomycosis. J Leukoc Biol 2008; 84:864 - 870
- Ahrens J, Graybill JR, Abishawl A, Tio FO, Rinaldi MG. Experimental murine chromomycosis mimicking chronic progressive human disease. Am J Trop Med Hyg 1989; 40:651 - 658
- Teixeira de Sousa M, Ghosn E, Almeida S. Absence of CD4+ T cells impairs host defence of mice infected with Fonsecaea pedrosoi. Scand J Immunol 2006; 64:595 - 600
- Gugnani HC, Obiefuna MN, Ikerionwu SE. Studies on pathogenic dematiaceous fungi, II. Pathogenicity of Fonsecaea pedrosoi and Phialophora verrucosa for laboratory mice. Mykosen 1986; 29:505 - 515
- Nishimura KaM M. Defense mechanisms of mice against Fonsecaea pedrosoi infection. Mycopathologia 1981; 76:155 - 166
- Allenbach C, Zufferey C, Perez C, Launois P, Mueller C, Tacchini-Cottier F. Macrophages induce neutrophil apoptosis through membrane TNF, a process amplified by Leishmania major. J Immunol 2006; 176:6656 - 6664
- Nuutila J, Lilius EM. Distinction between bacterial and viral infections. Curr Opin Infect Dis 2007; 20:304 - 310
- Ribeiro-Gomes FL, Silva MT, Dosreis GA. Neutrophils, apoptosis and phagocytic clearance: an innate sequence of cellular responses regulating intramacrophagic parasite infections. Parasitology 2006; 132:61 - 68
- Yu S, Maiti PK, Dyson M, Jain R, Braley-Mullen H. B cell-deficient NOD.H-2 h4 mice have CD4+CD25+ T regulatory cells that inhibit the development of spontaneous autoimmune thyroiditis. J Exp Med 2006; 203:349 - 358
- Meyer-Bahlburg A, Rawlings DJ. B cell autonomous TLR signaling and autoimmunity. Autoimmun Rev 2008; 7:313 - 316
- Kurita N. Cell-mediated immune responses in mice infected with Fonsecaea pedrosoi. Mycopathologia 1979; 68:9 - 15
- Kurup PV. Pathogenicity of Phialophora pedrosoi. Mykosen 1971; 14:41 - 44
- Cardona-Castro N, Agudelo-Florez P. Development of a chronic chromoblastomycosis model in immunocompetent mice. Med Mycol 1999; 37:81 - 83
- Cardona-Castro N, Agudelo-Florez P, Restrepo-Molina R. Chromoblastomycosis murine model and in vitro test to evaluate the sensitivity of Fonsecaea pedrosoi to ketoconazole, itraconazole and saperconazole. Mem Inst Oswaldo Cruz 1996; 91:779 - 784
- Xie Z, Zhang J, Xi L, Li X, Wang L, Lu C, Sun J. A chronic chromoblastomycosis model by Fonsecaea monophora in Wistar rat. Med Mycol 2009; 1 - 6
- Machado AP, Regis Silva MR, Fischman O. Local responses mediated by phagocytes after murine infection with different forms of Fonsecaea pedrosoi and transformation in vivo of conidiogenous cells. Mycoses 2009; In press
- Polak A. Experimental infection of mice by Fonsecaea pedrosoi and Wangiella dermatitidis. Sabouraudia 1984; 22:167 - 169
- Walsh TJ, Dixon DM, Polak A, Salkin IF. Comparative histopathology of Dactylaria constricta, Fonsecaea pedrosoi, Wangiella dermatitidis and Xylohypha bantiana in experimental phaeohyphomycosis of the central nervous system. Mykosen 1987; 30:215 - 225
- Fuchs TA, Abed U, Goosmann C, Hurwitz R, Schulze I, Wahn V, et al. Novel cell death program leads to neutrophil extracellular traps. J Cell Biol 2007; 176:231 - 241
- Murphy JW. Mechanisms of natural resistance to human pathogenic fungi. Annu Rev Microbiol 1991; 45:509 - 538
- Segal AW. How neutrophils kill microbes. Annu Rev Immunol 2005; 23:197 - 223
- Andres E, Zimmer J, Affenberger S, Federici L, Alt M, Maloisel F. Idiosyncratic drug-induced agranulocytosis: Update of an old disorder. Eur J Intern Med 2006; 17:529 - 535
- Aguirre J, Hansberg W, Navarro R. Fungal responses to reactive oxygen species. Med Mycol 2006; 44:101 - 107
- Esterre P, Richard-Blum S. Chromoblastomycosis: new concepts in physiopathology and treatment. J Mycol Med 2002; 12:21 - 24
- Suffia IJ, Reckling SK, Piccirillo CA, Goldszmid RS, Belkaid Y. Infected site-restricted Foxp3+ natural regulatory T cells are specific for microbial antigens. J Exp Med 2006; 203:777 - 788
- CCAC. Guide to the care and use of experimental animals 1993; http://www.ccac.ca/en/CCAC_Programs/Guidelines_Policies/PDFs/ExperimentalAnimals_GDL.pdf
- CGUANBR. Guidelines for the care and use of mammals in neuroscience and behavioral research 2003; http://grants.nih.gov/grants/olaw/National_Academies_Guidelines_for_Use_and_Care.pdf
- NACLAR. Guidelines on the care and use of animals for scientific purposes 2004; http://www.ava.gov.sg/NR/rdonlyres/C64255C0-3933-4EBC-B869-84621A9BF682/8338/Attach3_AnimalsforScientificPurposes.PDF
- Bourque WT, Gross M, Hall BK. A histological processing technique that preserves the integrity of calcified tissues (bone, enamel), yolky amphibian embryos, and growth factor antigens in skeletal tissue. J Histochem Cytochem 1993; 41:1429 - 1434