Abstract
Johne's disease, caused by Mycobacterium avium, subspecies paratuberculosis (MAP) is becoming increasingly widespread on dairy farms worldwide, due in part, to the absence of vaccine/drug or curative modalities. This spread is of concern since MAP is at the center of a controversy as to its role in Crohn's disease. None of the methods presently available to define paratuberculosis in cattle have been examined for their ability to assess progression/regression of any treatment or intervention of this disease The research presented herein, therefore was designed to assess the reliability and accuracy of available ante-mortem assays to predict disease change of individual animals undergoing a probiotic, potentially therapeutic, treatment. Paratuberculosis positive (n = 75) and negative (n = 10) animals were longitudinally monitored over their natural lifetimes with specific serum antibody and fecal shedding assays, and for development of end-stage clinical disease. Longitudinal, increasing/decreasing serum ELISA values were associated with, and predictive of, progression/regression of disease. Changes in fecal shedding and serum AGID were of value at only specific stages. Documentation that ELISA-positive animals were positive for paratuberculosis was done by a compilation of ELISA-independent assays--succumbing with end-stage clinical disease, autopsy, AGID, and MAP fecal shedding.
Introduction
Johne's disease, caused by Mycobacterium avium, subspecies paratuberculosis (MAP), is becoming increasingly a worldwide problem due primarily to cattle movement from one farm to another, herd expansion, farm intensification and confinement, and to the absence of a preventive vaccine/drug or curative treatment. This spread maybe of importance since MAP is at the center of a controversy as to its role in Crohn's disease and since it is estimated to cost dairy farmers in the US up to 200 million dollars annually.Citation1,Citation2 Presently, sound management practices in conjunction with stringent culling are considered to be the best means to control the spread of MAP.Citation3–Citation9 Even though such practices have resulted in success, they have yet to, and most likely will not, eliminate MAP from herds; thus, other preventive and/or curative measures are needed.
Methods presently used to define paratuberculosis in cattle are:
Serum or milk antibodies specific for MAP, detected by an enzyme-linked immunosorbent assay (ELISA) and/or by serum agar gel immunodiffusion (AGID);
MAP detection in fecal material via culture and/or PCR;
Biopsy specimens obtained surgically and analyzed histologically;
Surveillance for clinical disease; and
Complete autopsy.
Which of these assays might best address specific questions have been proposed,Citation10 the choice is usually based on the question asked, since each assay has a somewhat non-overlapping basis. Even though a reference ante-mortem test against which these assays can be assessed does not exist,Citation11–Citation14 we undertook a field-trial investigation to determine which, if any, could reliably and accurately define disease progression/regression of individual, paratuberculosis animals. Once clarified, the bacterium, Dietzia subspecies C79793-74, which inhibited growth of MAP under specific in vitro culture conditions,Citation15 would be tested for any probiotic therapeutic value (see accompanying report).
Results
To assess any preferential alteration of ELISA values or fecal shedding by Dietzia per se, ELISA values were compared to concurrent fecal MAP cfu for treated animals only. shows the relationship (r2 = 0.19) for 206 pairs of data. Of these paired samples, only 2 (0.97%) were ELISA negative and fecal positive, whereas 80 (38.8%) were ELISA positive and fecal negative. MAP-negative samples were associated with ELISA values spanning the range from low to high (1.5 to 4.1). In contrast, most of the samples with MAP >300 cfu were associated with ELISA values ≥3.0; only 3 (4.5%) were <3.0.
Examples of longitudinal changes in ELISA and fecal shedding for individual Dietzia-treated cows are shown in . The reasonably consistent AGID and ELISA values for G-11 and Green-1 are shown in , whereas concurrent fecal shedding showed large fluctuations (), ranging from 0 to >300 cfu. Serum ELISA values of cow 212 (initially negative but eventually AGID positive) increased during treatment to a relatively stable, albeit low level. In contrast, to these stable or increasing values, many AGID-negative, ELISA positive, cows had longitudinally declining ELISA values (). Corresponding fecal MAP cfu for these animals are shown in . Considerable variability of shedding was found ( and D), as well as large differences in shedding from one animal to another. Animals in succumbed with end-stage clinical Johne's disease, whereas all of those in all succumbed from non-paratuberculosis problems.
Representative longitudinal changes of ELISA values for ELISA-negative, treated and non-treated cows (controls), and for non-treated paratuberculosis positive cows, are shown in , respectively. The mean best-fit ELISA changes for the two ELISA negative groups of −0.05 and −0.08 () did not differ significantly from one another (p = 0.664). Similar small changes (see ) were observed for animals with initially high, ELISA values. As shown for the nontreated, paratuberculosis animals, with the exception of cow 270 (a cow with potentially false-positive ELISAs—see Discussion), once ELISA values began to change, the trend was to increase. All animals in , except 270, succumbed with end-stage clinical Johne's disease. The mean yearly best-fit change was +1.51 ± 0.0.15 SE, which differed significantly from that of the paratuberculosis-free, treated and non-treated groups (p = <0.0001 and <0.0001, respectively).
Data for individual animals are provided in –. Various parameters of paratuberculosis-free animals treated with Dietzia (n = 5) were compared to those not treated (n = 5) to assess for any adverse reactions (). Daily treatment was for 8–12 months and survival was 3 to >12 years. None of these 10, developed any positive MAP parameters during their lifetime, showed any sign of clinical Johne's disease or adverse medical reactions to Dietzia and all were terminated for non-paratuberculosis reasons. The longitudinal ELISA values changed very little (), ranging from −0.27 to 0.05.
provides data for 27 treated and non-treated paratuberculosis positive animals with increasing longitudinal ELISA values. All were ELISA positive multiple times, including the three (29, B9, 227) that were never AGID positive and never shed detectable MAP. The longitudinal changes are arranged from essentially zero to a high of 4.74. Twenty of these animals were treated daily and seven were never treated. Fourteen were initially shedding MAP and 10 more (total of 88.9%) became detected shedders. Twenty-two (81.5%) were euthanized with end-stage clinical Johne's disease (Stage IV) and two with Stage III clinical disease. All, but one (227), was either a fecal shedder and/or succumbed with end stage-disease (these two parameters when considered together are strong indicators of paratuberculosis). Three were terminated for various medical conditions unrelated to paratuberculosis (mastitis, pneumonia, foot/leg problems). Cow 227 succumbed of heart failure, ELISA positive multiple times, but always, fecal MAP and AGID negative (extensive autolysis prevented meaningful autopsy). Six (21.4%) animals were AGID positive on their initial test and 13 more (total of 70.4%) became positive, all succumbed with Stage III or IV clinical disease. Only three (11.1%) animals succumbed or were terminated without any sign of Stage III or IV clinical disease. All non-treated animals in this group, except 33, succumbed with Stage IV clinical disease.
Twenty-six animals () had longitudinal declining ELISA values, ranging from −5.24 to an insignificant −0.02. All cows in this group, except cow 270, were Dietzia treated. Twelve were initially fecal positive, 8 more became positive (76.9%). Four (15.4%) were initially AGID positive, nine others (total of 50%) became positive, and all but two of these 13 succumbed with end-stage disease. Twelve (48%) treated-animals succumbed or were terminated clinically paratuberculosis asymptomatic and only 13 succumbed with end-stage clinical disease.
provides data for animals that succumbed prior to obtaining three or more ELISA tests (longitudinal changes not determinable). Only five animals survived sufficiently to obtain a second ELISA and/or fecal test. The three males were euthanized at 24–25 months of age, and 16 of the 19 cows succumbed with end-stage clinical disease (84.2%). Fourteen of the 19 (73.7%) tested for fecal MAP were shedders. Twenty-one (95.5%), including four of the five that were initially fecal negative, were either fecal shedders, positive for AGID, and/or succumbed with endstage disease.
Based on 80 routinely tested animals, out of more than 200 herdmates considered negative for paratuberculosis (data for only 10 are shown in ), plus the seven animals that were ELISA positive only (all other ELISA-independently determined parameters were negative), ELISA specificity was estimated to be 92%. This, however, is most likely an underestimation since 5 of the 7 “false positives” were ELISA positive multiple times. Based on the number of animals detected by any of the other tests, individually or combined, the diagnostic sensitivity of ELISA was estimated to be 100%. When compared to ELISA, the other parameters would have a sensitivity considerably less than 100%.
Animals that underwent complete autopsies (indicated by cow ID shown in bold font in the Tables) included 12 randomly chosen that were euthanized with end-stage Johne disease and the 3 steers. All 15 of these animals at autopsy had pathological and culture evidence consistent with paratuberculosis. In addition, three other animals (animals 13, B71 and N52) that tested paratuberculosis positive prior to treatment, but negative at termination (B71's ELISA of 1.4 was on the cut-off) were autopsied. Gross pathology detected no granulomatous enteritis in the intestinal sections of any of these three. There was also no culture evidence for MAP in multiple tissues analyzed from cows 13 and N52, supporting a status of being paratuberculosis negative posttreatment. MAP was, however, cultured from an intestinal lymph node of B71; thus even though still paratuberculosis positive, the final ante-mortem ELISA and AGID were less than those present at initiation of treatment.
Discussion
The validity of conclusions regarding progression/regression of any disease is dependent upon the reliability and accuracy of available ante-mortem assays. Our definition of clinical progressionCitation16 is “a change from clinical Stage II or IIICitation17 to end-stage clinical disease (Stage IV).” Regression will be defined as conversion from: (a) asymptomatic or clinically, early-stage disease (Stage II or III) to a state of complete remission, Stage I where all parameters are negative, or (b) end-stage clinical disease to clinical Stage I, II or III. Interestingly, none of the tests available to identify paratuberculosis in cattle—milk ELISA, serum ELISA, serum AGID, fecal shedding, clinical appearance, tissue biopsy and autopsy—have been evaluated as to their value for assessing potential preventive/therapeutic protocols. Further, a reference test against which ante-mortem assays can be assessed is not available,Citation11–Citation14 although, we would argue, based on the data presented herein, that end-stage clinical disease is a logical, reliable and irrefutable reference end point for animals that shed MAP or are ELISA positive.
Analysis of the 206-paired serum ELISA and fecal MAP data of only treated animals resulted in an r2 value of 0.19, suggesting a poor association of the two. Whether this is due to inherent variations or due to Dietzia treatment preferentially altering either is not clear. Irrespective, high fecal MAP samples (>300 cfu) were more likely associated with high serum ELISA values (≥3.0), which is similar to that reported for non-treated animals.Citation18–Citation22 On the other hand, 38.8% of ELISA-positive sera, detected at any stage of treatment, were from animals that tested concurrently MAP negative, again similar to that for non-treated animals that are at an early stage of disease.Citation18,Citation21,Citation23,Citation24
Our results with treated animals supports the lack of association of AGID and ELISA reported for non-treated cattle and goats.Citation24–Citation27 AGID was less sensitive (out of 75 ELISA positives, only 40 were AGID positive) and no serum was ever AGID positive and ELISA negative. Thirty-nine (97.5%) of the AGID-positive animals (the only exception was steer 262 terminated at 25 months of age) were either fecal shedders, succumbed with end-stage clinical disease or were MAP culture positive at autopsy. Thus, our results confirm that AGID is an accurate, but not very sensitive, indicator of paratuberculosis.Citation25,Citation26 In addition, positive to negative or negative to positive AGID changes were found during treatment and may have some value for monitoring progression/regression of disease. It should be emphasized that it was not our intention to specifically address associations or levels of agreement of various paratuberculosis assays since such assessments were recently reviewed.Citation12–Citation14
Considering the advantages and disadvantages of each antemortem assay, we concluded that even with multiple biological perturbations (such as the stage and parity of lactation) best-fit longitudinal increases/decreases in serum ELISA values were the most informative regarding changes in disease status of individual animals. This is especially true for the unanticipated findings that 25 of 45 (55.6%) treated animals had declining longitudinal ELISA changes, whereas all non-treated animals (except cow 270) had longitudinal increasing values. Milk ELISA was deemed inadequate because dry cows, heifers and males would be excluded, plus it is less sensitive and influenced more by parity and stage of lactation than serum ELISA.Citation19,Citation24,Citation28 Even though changes in AGID and fecal shedding were associated with later stages of disease, their use presented a major obstacle for monitoring progression/regression over a lifetime because (a) animals with positive ELISAs that became negative were almost always fecal and AGID negative and (b) 32 of the 71 (45.1%) ELISA-positive animals initially tested for fecal shedding MAP were negative; i.e., they would have been incorrectly classified as paratuberculosis negative (‘false positive’ ELISA) and excluded from the study. Indeed, 24 (75%) of these ‘false positives’ eventually succumbed with end-stage clinical disease, became AGID positive and/or harbored detectable MAP (fecal or necropsied tissue).
The major advantage of a sensitive ELISA is that it generally detects MAP-specific antibodies prior to detection of fecal MAPCitation13,Citation29,Citation30 and prior to the appearance of any other detectable parameter used to define paratuberculosis. Differences of Allied's ELISA and other ELISAs revolve around the question that occupies a great deal of attention in the literature—the diagnostic sensitivity and specificity of ELISAs per se. Because ELISA sensitivity is influenced by the procedure in which preparations of MAP-ELISA test-antigens are derivedCitation29,Citation31–Citation34 and because there is no standardized, uniform antigen preparation used by all investigators, it would not be expected that all ELISAs would detect paratuberculosis animals similarly at a given stage (age) of infection.Citation23,Citation32,Citation35,Citation36 For our investigations, the Allied ELISA detected MAP antibody at an early asymptomatic stage of disease, which is similar to that of other ELISAs,Citation29,Citation31,Citation33 but different from that of earlier less sensitive assaysCitation37 and from that obtained with certain commercially available ELISA kits.Citation22,Citation29,Citation38–Citation40 Use of an ELISA that resulted in early detection is the most likely explanation as to why all animals in our study (except 212) were detected ELISA positive prior to or simultaneous with the detection of fecal shedding, instead of the reverse. All animals in the present study, except the three steers, were maintained in the herd until humane considerations dictated they be euthanized or the experiment was terminated, a practice we consider essential for documentation of Johne disease.
The diagnostic specificity of Allied's ELISA was determined by comparing the number of cows (7) that were ELISA positive but negative for other paratuberculosis parameters to the 80 herdmates that were always negative for all parameters; i.e., ELISA identified 80 of 87 (92%) correctly. If animals with multiple positive ELISA values are considered paratuberculosis positive, specificity would be 80 divided by 82 or 97.5%. However, even the two “false ELISA positive” cows (228 and 3036) were most likely positive (see below). The only cow considered to possibly possess false positive ELISAs is cow 270, which is discussed later; thus specificity would be 98.8%. We view differences of 92 and 98.8% as not meaningful for our conclusions regarding treatment efficacy, and both are in agreement with the 95.4% reported by Allied on their web site.
The diagnostic sensitivity of Allied's ELISA, as used herein, was 100% since the number of ELISA-positive animals was equal to or greater than that detected by any of the other ELISA-independent test parameters, individually or combined. This value is higher than that reported by Allied (58.8%), and most other ELISAs, especially commercial kits. Does our small number of 75 animals with >350 positive and >400 negative ELISA and AGID determinations and >385 MAP culture determinations impact this sensitivity value? Almost certainly, but a longitudinal analysis of a minimum of the proposed 400 animals to establish statistical validity41 for a true sensitivity value is both time and financially unrealistic and, for our goals, irrelevant.
Of the 75 ELISA positive animals, only one (cow 270) had multiple ELISA values >2.0 that may be false positive. This uncertainty arises because the horizontal best-fit value () mimics those of paratuberculosis-free animals and because she has never been AGID or fecal positive or shown any clinical sign of disease. Since she remains in the herd at 7 years of age, it is anticipated that her status will become clarified by further tests and/or by autopsy. Of the remaining animals (3 steers excluded), 52 of 71 (73.2%) succumbed with end-stage clinical disease. This contrasts to the absence of any paratuberculosis-like, clinically end-stage animals (false clinically positive) in the more than 200 herdmates over an 8-year period. There were 40 (54.1%) animals that were AGID positive and, of the 71 tested for fecal shedding, 58 (81.7%) were positive at least once. Of the 14 “suspect” animals (OD of 1.5–2.0), nine were detected fecal shedders and 2 of the non-shedders succumbed with end-stage clinical disease; thus, 11 (78.6%) were positive for at least one non-ELISA parameter, which met our criterion for paratuberculosis. Of the three lacking ELISA independent positive criteria, two (228, B70) were in the treated group (B70 with multiple positive ELISAs >2) and the one not treated (3036) succumbed 3 months into the study. Cow 228, with a single positive ELISA, was considered positive based on: (a) treatment was initiated, and presumed successful, at a very early age of 18 months, (b) the best-fit analysis of her declining ELISA values during treatment (), (c) her dam succumbed with end-stage clinical Johne disease, ELISA positive and shedding MAP, and (d) her maternal sister (cow 231) was paratuberculosis ELISA and fecal positive. Although not conclusive, we consider cow 3036 also most likely paratuberculosis positive because her dam succumbed with end-stage disease, ELISA positive and MAP shedding. Therefore, the suspect category of Allied as used by othersCitation14 was excluded as a category for our analyses. Of 17 () that lacked multiple ELISA test-dates due to short survivals, all but 3036 either succumbed with end-stage disease, were fecal shedding or AGID positive.
The first positive ELISA detected for each animal, as well as those detected positive during treatment, cannot be attributed to Dietzia exposure since treatment was always started after an animal was detected positive and since ELISA-negative, adult animals () never became positive after prolonged, daily, oral treatment. Furthermore, the positive ELISA values reported herein are not likely false positives due to other environmental bacteriaCitation42 since:
All calves in the herd that developed positive parameters as adults were from dams with multiple positive parameters (unpublished),
None of over 150 calves in the herd whose dams tested paratuberculosis negative ever developed positive parameters (unpublished),
ELISA (Parachek) detected antibodies produced in goats against environmental Corynebacterium pseudotuberculosis were not detected positive by AGID.Citation27 Yet AGID was highly predictive in that 96.9% of cattle testing AGID positive were also culture or necropsy-confirmed positive for paratuberculosis.Citation25,Citation26 The similar status of AGID positive animals in the present study (39 of 40) confirm its predictive value,
Nineteen duplicate fecal samples from 15 animals that tested positive/negative by culture also tested positive/negative by specific MAP PCR, and
All but seven ELISA-positive animals had an additional, independently determined, positive paratuberculosis parameter and of these seven, five were ELISA positive multiple times.
The results presented herein indicate that a single positive ELISA value obtained with an appropriate antigen was sufficient to define an individual animal as paratuberculosis, including 11 of 14 “suspects” that were at an early subclinical stage. The only true test to establish an animal as ELISA false-positive is to either test them multiple times over their normal, natural (not terminated) lifetime or preferably, monitor other parameters, such as AGID, fecal shedding and most importantly, the development of end-stage clinical disease.Citation12,Citation19,Citation20,Citation43 Our results confirm those of others in that when ELISA and fecal shedding are analyzed longitudinally and in parallel, a much higher combined assurance of disease diagnosis is achieved.Citation11,Citation19–Citation21,Citation30,Citation43–Citation45 Our results suggest that AGID, and especially end-stage clinical disease, combined with fecal shedding, is an even more reliable “gold standard” by which to compare sensitivity and specificity of ELISA values. Undetectable MAP in tissue and/or manure is simply not sufficient to define an ELISA as false positive. In fact, we would argue that a sensitive ELISA is just as accurate as the gold standard, MAP fecal shedding, for identifying animals with Johne disease. Furthermore, 81.5% of those with increasing ELISA values and 84.2% of those with insufficient longitudinal survival times (three steers excluded) succumbed with end-stage disease, versus only 53.8% of those with declining longitudinal ELISA values; i.e., declining values were associated with therapeutic value of Dietzia, whereas increasing values were not. Fecal shedding and AGID status may be of predictive value for survival when either change from positive to negative or negative to positive; neither, however, was informative if they were consistently positive or negative.
Materials and Methods
Experimental design.
The present study was designed as a field trial with all aspects under our ownership and supervision and not as an academic, controlled laboratory experiment. A roughly 4:1 ratio of paratuberculosis-free to -positive dairy cows (see below for definition of each) were housed in a tie-stall facility as a single dairy herd under field conditions designed to mimic those of a “typical” small dairy farm. At any given time, the herd consisted of 50–60 cows. The diets were formulated for high production by Dr. Aitchison of Nutritional Professionals, Inc., (Hortonville, WI), and consisted of corn silage, haylage, dry hay, and a concentrate of high-moisture corn, protein, vitamins, minerals and 250 mg/day monensin sodium, a compound that inhibited MAP growth in culture,Citation46,Citation47 marginally reduced fecal shedding, and moderately halted/reversed pathologic lesions in specific, but not all, tissues.Citation48,Citation49 The common total-mixed-ration (TMR) was not used, i.e., all ingredients were fed separately to permit assessment of appetite. Dietzia was stored at −20°C at 1–3 × 1011 cfu/ml. Based on preliminary dosage experiments, small cows (Jerseys, Ayrshires, Jersey x Holstein crosses), and large cows (Holsteins and Guernseys) were treated by supplementation of their morning feed of corn with Dietzia at a daily dose of 2–3 × 1011 and 4–5 × 1011 cfu, respectively, or if an animal refused to eat, deposited directly into its mouth with a syringe. Because the focus of the present report is to define appropriate assays to monitor changes in disease during treatment, information relative to Dietzia is presented in the accompanying report.
Animals.
Paratuberculosis-free, adult dairy cows were obtained from a single local low prevalence Johne disease herd. Those that remained negative for the remainder of their lifetimes were considered negative. From this group, or their calves, only ten were chosen, based on breed, age and lactation, to provide comparative longitudinal data. Sixty-one (with Stage II or III clinical diseaseCitation16,Citation17) dairy cows, detected positive at dry-off by paratuberculosis-specific ELISAs (determined by the owners and veterinarians), were purchased (two Stage IV animals were donated) from seven local, well managed, moderately high-prevalence herds over a five-year period. Within a week of arriving at our facility, blood and feces were collected for retesting (ELISA, AGID and fecal shedding). In addition, three male calves (steers) and nine heifer calves (198, 228, 229, 231, 232, 234, 255, 266, 270) that developed paratuberculosis positive parameters (the heifers in their first or second lactation) were included in the study. All dams of these calves were ELISA positive and either succumbed with end-stage clinical Johne disease and/or were fecal shedders. As our goal was to investigate the effect of intervention rather than confirm the findings of others that non-treated paratuberculosis animals eventually succumb with clinical disease, more animals were assigned to the treated group (n = 51) than to the non-treated group (n = 24). Prior to initiation, 58 had Stage II, 15 had Stage III, and two had Stage IV clinical disease.Citation17 Most were in their second to fourth lactation and were or were not detectable fecal shedders. Over the five years, as animals were detected positive, two were assigned to the treated group and the next one to the control group; this was continued until all animals were in one of the two groups. Once assigned to a group, the different assays were repeated at various intervals over the remaining natural life of the animal. Our goal (although not always met due to early end-stage disease) was to achieve a minimum of three distinct test-times at 3–5 month intervals. Thus, animals were euthanized only for life threatening situations; the majority for Stage IV clinical Johne disease. The steers were tested only once and were euthanized at 24–25 months of age with no clinical signs of disease. Emaciated animals were defined as having end-stage clinical Johne disease (Stage IV) based on both “pipestream” diarrhea and reduced feed intake. Over the eight years (three years follow-up) of the study, none of more than 200 herdmates with negative paratuberculosis parameters ever became emaciated with diarrhea from other disease etiologic agents, although, as expected for any dairy herd, some had short episodes (days) of diarrhea without emaciation. Local veterinarians humanely euthanized recumbent, emaciated and/or cachectic, clinically end-stage animals when they no longer could get up and stand on their own by intravenous injection of a sodium pentobarbital solution (Fatal Plus, 6 gm/ml) at a dose of 1 ml per 4.5 kg body weight. Animals that showed potential life-threatening non-Johne disease ramifications (mastitis, pneumonia, displaced abomasums, foot/leg problems) were also euthanized (some died); others were terminated when the study was ended.
An animal was considered to have paratuberculosis based on detection of at least one positive serum ELISA (OD >1.4) plus one of the following: (a) test positive for a different paratuberculosis parameter (fecal shedding, AGID), (b) develop end-stage clinical disease, (c) diagnosis at autopsy, or (d) test positive for ELISA multiple times. For the present investigation, 68 of the 75 ELISA positive animals possessed positive criteria (a), (b) or (c). Five of the remaining seven were ELISA positive multiple times, and arguments for why the other two were positive, were presented earlier.
Serum and fecal protocols.
Fecal material collected directly from the rectum using individual disposable gloves and blood obtained aseptically from the tail vein were transferred to sterile containers, coded and sent chilled on the day of collection to Allied Monitor, Inc., (Fayette, MO) for analysis. Allied Monitor is a USDA- and NVSL-approved laboratory that specializes in assays for Johne disease. The majority, but not all, of the fecal and blood samples were obtained concurrently. Occasionally, fecal samples were tested at the University Minnesota Veterinary School Diagnostic Laboratory (St. Paul, MN) for MAP using the ISMav2 TaqMan (Applied BioSystems #4324018) PCR protocol (PCR was positive only for concurrent fecal cultures of >5–8 cfu/slant). All serum ELISA and AGID assays and fecal MAP cultures were performed upon receipt as described below.
ELISA.
The ELISA for antibodies specific for MAP was performed using a crude, soluble, MAP protoplasmic antigen prepared by Allied. Test sera were preabsorbed with Mycobacterium phlei. Split-sample repeatability, as well as duplicate samples, varied less than 5% of the mean. The content of each well was read at a single wavelength (405 nm). ELISA values were calculated by dividing the test-sample OD by a value equivalent to ¼ the OD of a standard reference positive serum (range 0.13–0.14), and interpreted as follows: Negative ≤1.4 OD and Positive >1.4. Since 11 of 14 animals that would have been classified by Allied's criteria as suspect (OD of 1.5–2.0) were either, fecal shedders (9), succumbed with end-stage disease (5), or ELISA positive multiple times (1), this category was not used.
AGID.
The AGID testCitation25,Citation26 specific for antibodies specific for MAP was performed with the same crude MAP antigen used in the ELISA. The six peripheral wells were inoculated with 50 µl of test sera or reference serum. The central well was loaded with 30 µl of antigen. Final readings were performed after 48 hours. The reference paratuberculosis positive serum used in both the ELISA and AGID assays was obtained from a naturally MAP infected, adult paratuberculosis cow.
Fecal culture.
Fecal samples of 2 g were weighed into 50 ml sterile conical centrifugation tubes containing 35 ml of sterile 0.75% hexadecylpyridinium chloride (HPC) (Sigma Aldrich, St. Louis, MO). Tubes were shaken for 30 min by a horizontal shaker, after which the contents allowed to settle in the upright tubes for 2 h. Approximately 2 ml of supernatant, just above the sedimentation layer, was removed using a sterile transfer pipette. The loaded pipette containing supernatant was placed upright into a sterile culture tube (20 × 75 mm) at room temperature for overnight decontamination. Triplicate Herrold's egg yolk (HEY) agar slants containing 50 mg each Naladixic acid, Vancomycin and Amphotericin B (Becton Dickinson and Company, Sparks, MD) with mycobactin-J and one without (to evaluate mycobactin dependency of suspect colonies) were inoculated with four drops (∼0.1 ml) of the decontaminated supernatant. Slants were maintained at 37°C in a horizontal position for 1 week, after which they were placed in the upright position and incubated for a total of 13 weeks. Enumeration of slightly raised white-yellow colonies evaluated for typical acid fastness and morphological appearance of MAP of the triplicates was recorded and summed together. Slants with so many colonies that made it impossible to accurately count were estimated and expressed as >100 cfu and are shown in the Tables and Figures as >300 cfu (>100 × 3 slants). Any fecally-shed MAP (especially low cfu levels) was considered as pass-through only if ELISA values, both prior to and post MAP detection, were negative and all other test parameters also remained negative. Based on this criterion, only two cases of MAP shedding (both >300 cfu) were considered pass-through.
Postmortem analysis.
As an additional means to document Johne disease status of specific animals (identified by bold print in the Tables), gross pathology and tissues tested for MAP were undertaken at the University of Minnesota Veterinary School Diagnostic Laboratory (St. Paul, MN). Findings from necropsy, histopathology of multiple organs, bacteriology, parasitology, serology and molecular diagnostics performed on each animal were used to identify an animal as positive or negative for paratuberculosis. These postmortem findings were not used to define specific aspects or extent of disease,Citation12 nor compared to any ante-mortem parameter.
Statistical methods.
Analysis of concurrent serum ELISA and fecal MAP data was performed by entering the pairs of data into a Microsoft Excel® spreadsheet and calculating the r2 value using the RSQ worksheet function. The longitudinal, best-fit of ELISA values for each animal was estimated by entering the test dates and ELISA values into an Excel spreadsheet and calculating change/year using the SLOPE worksheet function.
ELISA specificity was determined by dividing the number of paratuberculosis-free cows by the sum of this number plus the number of cows testing ELISA positive only, but negative for all other parameters. For this calculation, paratuberculosis-free animals were defined as those that tested ELISA, fecal MAP and AGID negative, never developed end-stage clinical disease, and where applicable, were confirmed negative at autopsy. An unrelated herd, with a high probability of being paratuberculosis negative, was not used as the source of negative animals since a potential etiological agent that may cause false positive ELISAs in our herd may or may not exist in any unrelated herd. ELISA sensitivity was calculated by dividing the number of ELISA positive animals by the total number of paratuberculosis animals. For this calculation, positive animals were defined as those that were either MAP and/or AGID positive, developed end-stage clinical Johne disease or confirmed paratuberculosis positive at autopsy.
Conflict of interest
Both authors are members of Paralab, LLC, Eau Claire, WI. which is the assignee of a patent application covering the Dietzia technology presented in this paper. R.E.C. is a partner in Altick, Associates, River Falls, WI.
Figures and Tables
Figure 1 Scatter graph of ELISA and fecal MAP cfu observed during treatment of positive cows only. Serum ELISA OD values (y-axis) for Dietzia treated animals only are plotted against fecal MAP cfu (x-axis) obtained concurrently for all test-dates at which either ELISA or fecal shedding was positive.
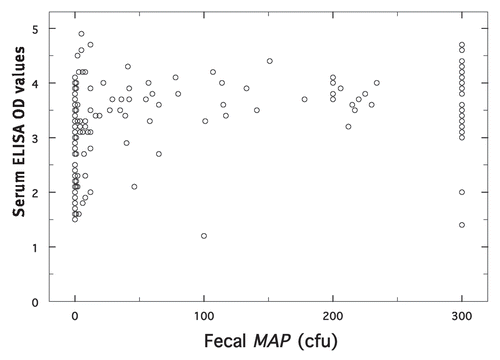
Figure 2 Representative longitudinal changes in ELISA and fecal shedding for paratuberculosis cows that were treated with Dietzia. (A and B) show serum ELISA OD values (y-axis) and (C and D) corresponding fecal MAP cfu (y-axis) for six different cows over their treatment interval—two with stable or increasing ELISA values and four with declining ELISA values as shown by the best-linear-fit of the data (thin line). Please note y-axis scales for (C and D) are different. Each cow's identification name/number is shown by a specific color detailed in each Panel. Longitudinal changes in each cow's ELISA and fecal values are shown in the same color as their ID color. ELISA values shown as (+) indicate that at that time point, the serum was also AGID positive. BL indicates the cutoff value of 1.4 used to define ELISA positive from negative.
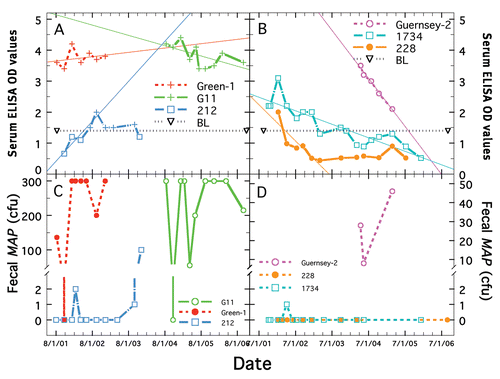
Figure 3 Longitudinal changes in ELISA and fecal shedding for Dietzia treated and non-Dietzia treated, ELISA negative cows and for ELISA positive, paratuberculosis cows that were not treated. (A) shows examples of longitudinal changes in ELISA values for 3 non-treated, ELISA negative animals, (B) for 2 treated, ELISA negative animals, and (C) for 4 non-treated paratuberculosis animals. Each cow's identification name/number is shown by a specific color detailed in each Panel. Longitudinal changes in each cow's ELISA and fecal values are shown in the same color as their ID color. Also shown in (C) is the best-linear-fit of the data (thin line) and fecal shedding changes for the only cow shedding, cow 266. ELISA values shown as (+) indicate that at that time point, the serum was also AGID positive. BL indicates the cutoff value of 1.4 used to define ELISA positive from negative.
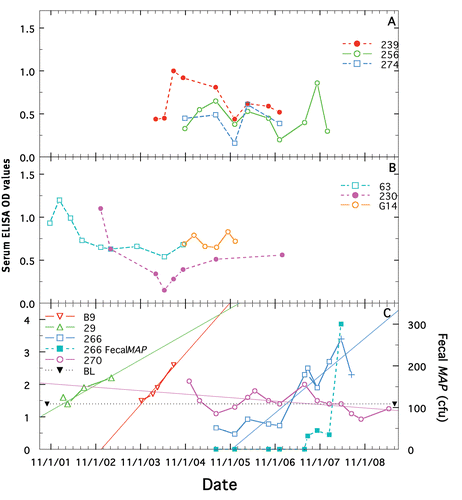
Table 1 Parameters for paratuberculosis-negative cows
Table 2 Parameters for cows with longitudinally increasing ELISAs
Table 3 Parameters for cows with longitudinal declining ELISAs
Table 4 Parameters of cows with 2 or less test-days
Acknowledgements
This research was funded, in part, by NIH Grant R01AI027331, by Paralab, LLC, Eau Claire, WI and by Altick Associates, River Falls, WI. The sponsors, outside their funding, had no involvement in any part of the study. We wish to thank William Richards for the initial ICON 6 isolate (Dietzia).
References
- Ott SI, Wells SJ, Wagner BA. Herd-level economic losses associated with Johne's disease on US dairy operations. Prev Vet Med 1999; 40:179 - 192
- Chi J, VanLeeuwen JA, Weersink A, Keefe GP. Direct production losses and treatment costs from bovine viral diarrhoea virus, bovine leucosis virus, Mycobacterium avium subsp. paratuberculosis, and Neorsporta caninum. Prev Vet Med 2002; 55:137 - 153
- Wells SJ, Wagner BA. Herd-level risk factors for infection with Mycobacterium paratuberculosis in US dairies and association between familiarity of the herd manager with the disease or prior diagnosis of the disease in that herd and use of preventive measures. J Am Vet Med Assoc 2000; 216:1450 - 1457
- Kennedy DJ, Benedictus G. Control of Mycobacterium avium subspecies paratuberculosis infection in agricultural species. Rev Sci Tech 2001; 20:151 - 179
- McKenna SL, Keefe GP, Tiwari A, VanLeeuwen J, Barkema HW. Johne's disease in Canada part II: disease impacts, risk factors and control programs for dairy producers. Can Vet J 2006; 47:1089 - 1099
- Lu Z, Mitchell RM, Smith RL, Van Kessel JS, Chapagain PP, Schukken YH, et al. The importance of culling in Johne's disease control. J Theor Biol 2008; 254:135 - 146
- Tavornpanich S, Johnson WO, Anderson RJ, Gardner IA. Herd characteristics and management practices associated with seroprevalence of Mycobacterium avium subspecies paratuberculosis infection in dairy herds. Am J Vet Res 2008; 69:904 - 911
- Benedictus A, Mitchell RM, Linde-Widmann M, Sweeney R, Fyock T, Schukken YH, et al. Transmission parameters of Mycobacterium avium subspecies paratuberculosis infections in a dairy herd going through a control program. Prev Vet Med 2008; 83:215 - 227
- Ferrouillet C, Wells SJ, Hartmann WL, Godden SM, Carrier J. Increase of Johne's disease prevalence and incidence in six Minnesota USA, dairy cattle herds on a long-term management program. Prev Vet Med 2009; 88:128 - 137
- Collins MT, Gardner IA, Garry FB, Roussel AJ, Wells SJ. Consensus recommendations on diagnostic testing for the detection of paratuberculosis in cattle in the United States. J Am Vet Assoc 2006; 229:1912 - 1919
- Nielsen SS, Toft N. Age-specific characteristics of ELISA and fecal culture for purpose-specific testing for paratuberculosis. J Dairy Sci 2006; 89:569 - 579
- Antognoli MC, Garry FB, Hirst HL, Lombard JE, Dennis MM, Gould DH, et al. Characterization of Mycobacterium avium subspecies paratuberculosis disseminated infection in dairy cattle and its association with antemortem test results. Vet Microbiol 2008; 127:300 - 308
- Nielsen SS, Toft N. Ante mortem diagnosis of paratuberculosis: A review of accuracies of ELISA, interferon-gamma assay and faecal culture techniques. Vet Microbiol 2008; 129:217 - 235
- Pinedo PJ, Rae DO, Williams JE, Donovan GA, Melendez P, Buergelt CD. Association among results of serum ELISA, faecal culture and nested PCR on milk and feces for the detection of paratuberculosis in dairy cows. Transbound Emer Dis 2008; 55:125 - 133
- Richards WD. Milner AR, Wood PR. In vitro and in vivo inhibition of Mycobacterium paratuberculosis by iron deprivation: A hypothesis. Proc Conf. Johne's Disease Australia 1988; 87 - 94
- Chiodini RJ, Van Kruiningen HJ, Merkal RS. Ruminant paratuberculosis (Johne's disease)—the current status and future prospects. Cornell Vet 1984; 74:218 - 262
- Whitlock RH, Buergelt C. Preclinical and clinical manifestations of paratuberculosis (including pathology). Vet Clin North Am Food Anim Pract 1996; 12:345 - 356
- Whitlock RH, Wells SJ, Sweeney RW, Van Tiem J. ELISA and fecal culture for paratuberculosis (Johne's disease): sensitivity and specificity of each method. Vet Microbiol 2000; 77:387 - 398
- Nielsen SS, Grohn YT, Enevoldsen C. Variation of the milk antibody response to paratuberculosis in naturally infected dairy cows. J Dairy Sci 2002; 85:2795 - 2802
- van Schaik G, Rossiter CR, Stehman SM, Shin SJ, Schukken YH. Longitudinal study to investigate variation in results of repeated ELISA and culture of fecal samples for Mycobacterium avium subspecies paratuberculosis in commercial dairy herds. A J Vet Res 2003; 64:479 - 484
- Nielsen SS, Ersboll AK. Age at occurrence of Mycobacterium avium subspecies paratuberculosis in naturally infected dairy cows. J Dairy Sci 2006; 89:4557 - 4566
- Clark DL, Koziczkowski JJ, Radcliff RP, Carlson RA, Ellingson JLE. Detection of Mycobacterium avium subspecies paratuberculosis: comparing fecal culture versus serum enzyme-linked immunosorbent assay and direct fecal polymerase chain reaction. J Dairy Sci 2008; 91:2620 - 2627
- Colgrove GS, Thoen CO, Blackburn BO, Murphy CD. Paratuberculosis in cattle: a comparison of three serologic tests with results of fecal culture. Vet Microbiol 1989; 19:183 - 187
- Hendrick SH, Duffield TE, Kelton DE, Leslie KE, Lissemore KD, Archambault M. Evaluation of enzymelinked immunosorbent assays performed on milk and serum samples for detection of paratuberculosis in lactating dairy cows. J Am Vet Med Assoc 2005; 226:424 - 428
- Sherman DM, Markham RJ, Bates F. Agar gel immunodiffusion test for diagnosis of clinical paratuberculosis in cattle. J Am Vet Med Assoc 1984; 185:179 - 182
- Sherman DM, Bray B, Gay JM, Bates F. Evaluation of the agar gel immunodiffusion test for diagnosis of subclinical paratuberculosis in cattle. Am J Vet Res 1989; 50:525 - 530
- Manning EJB, Cushing HF, Hietala S, Wolf CB. Impact of Corynebacterium pseudotuberculosis infection of serologic surveillance for Johne's disease in goats. J Vet Diagn Invest 2007; 19:187 - 190
- Lombard JE, Byrem TM, Wagner BA, McCluskey BJ. Comparison of milk and serum enzyme-liked immunosorbent assays for diagnosis of Mycobacterium avium subspecies paratuberculosis infection in dairy cattle. J Vet Diagn Invest 2006; 18:448 - 458
- Waters WR, Miller JM, Palmer MV, Stabel JR, Jones DE, Koistinen KA, et al. Early induction of humoral and cellular immune responses during experimental Mycobacterium avium subspecies paratuberculosis infection of calves. Infect Immun 2003; 71:5130 - 5138
- Nielsen SS. Transition in diagnostic tests used for detection of Mycobacterium avium subspecies paratuberculosis infections in cattle. Vet Microbiol 2008; 132:274 - 282
- Koets AP, Rutten VP, de Boer M, Bakker D, Valentin-Weigand P, van Eden W. Differential changes in heat shock protein, lipoarabinomannan-, and purified protein derivative-specific immunoglobulin G1 and G2 isotype responses during bovine Mycobacterium avium subspecies paratuberculosis infection. Infect Immun 2001; 69:1492 - 1498
- Eda S, Bannantine JP, Waters WR, Mori Y, Whitlock RH, Scott MC, et al. A highly sensitive and subspeciesspecific surface antigen enzyme-linked immunosorbent assay for diagnosis of Johne's disease. Clin Vaccine Immunol 2006; 13:837 - 844
- Bannantine JP, Bayles DO, Waters WR, Palmer MV, Stabel JR, Paustian ML. Early antibody response against Mycobacterium avium subspecies paratuberculosis antigens in subclinical cattle. Proteome Sci 2008; 6:5 - 9
- Shin SJ, Cho D, Collins MT. Diagnosis of bovine paratuberculosis by a novel enzyme-linked immunosorbent assay based on early-secreted antigens of Mycobacterium avium subspecies paratuberculosis. Clin Vaccine Immunol 2008; 15:1277 - 1281
- Collins MT, Wells SJ, Petrini KR, Collins JE, Schultz RD, Whitlock RH. Evaluation of five antibody detection tests for diagnosis of bovine paratuberculosis. Clin Diagn Lab Immunol 2005; 12:685 - 692
- McKenna SL, Sockett DC, Keefe GP, McClure J, VanLeeuwen JA, Barkema HW. Comparison of two enzyme-linked immunosorbent assays for diagnosis of Mycobacterium avium subspecies paratuberculosis. J Vet Diagn Invest 2005; 17:463 - 466
- Stabel JR, Wells SJ, Wagner BA. Relationships between fecal culture, ELISA and bulk tank milk results for Johne's disease in US dairy herds. J Dairy Sci 2002; 85:525 - 531
- Paolicchii FA, Zumarraga MJ, Gioffre A, Zamorano P, Morsella C, Verna A, et al. Application of different methods for the diagnosis of paratuberculosis in a dairy cattle herd in Argentina. J Vet Med B Infec Dis Vet Public Health 2003; 50:20 - 26
- Gumber S, Eamens G, Whittington RJ. Evaluation of a Pourquier ELISA kit in relation to agar gel immunodiffusion (AGID) test for assessment of the humoral immune response in sheep and goats with and without Mycobacterium paratuberculosis infection. Vet Microbiol 2006; 115:91 - 101
- Fry MP, Kruze J, Collins MT. Evaluation of four commercial enzyme-linked immunosorbent assays for the diagnosis of bovine paratuberculosis in Chilean dairy herds. J Vet Diagn Invest 2008; 20:329 - 332
- Collins MT. Proper estimation of sensitivity and specificity. Clin Vaccine Immunol 2006; 13:1373 - 1374
- Osterstock JB, Fosgate GT, Norby B, Manning EJ, Collins MT, Roussel AJ. Contribution of environmental mycobacterium to false-positive serum ELISA results for paratuberculosis. J Am Vet Med Assoc 2007; 230:896 - 901
- Sweeney RW, Whitlock RH, McAdams S, Fyock T. Longitudinal study of ELISA seroreactivity to Mycobacterium avium subspecies paratuberculosis in infected cattle and culture-negative herd mates. J Vet Diagn Invest 2006; 18:2 - 6
- van Schaik G, Stehman SM, Jacobson RH, Schukken YH, Shin SJ, Lein DH. Cow-level evaluation of a kinetics ELISA with multiple cutoff values to detect fecal shedding of Mycobacterium avium subspecies paratuberculosis in New York State dairy cows. Prev Vet Med 2005; 72:221 - 236
- Barrington GM, Gay JM, Eriks IS, Davis WC, Evermann JF, Emerson C, et al. Temporal patterns of diagnostic results in serial samples from cattle with advanced paratuberculosis infections. J Vet Diagn Invest 2003; 15:195 - 200
- Brumbaugh GW, Simpson RB, Edwards JF, Anders DR, Thomson TD. Susceptibility of Mycobacterium avium subsp paratuberculosis to monensin sodium or tilmicosin phosphate in vitro and resulting infectivity in a murine model. Can J Vet Res 2004; 68:175 - 181
- Greenstein RJ, Su L, Whitlock RH, Brown ST. Monensin causes dose dependent inhibition of Mycobacterium avium subspecies paratuberculosis in radiometric culture. Gut Pathog 2009; 9:4 - 9
- Brumbaugh GW, Edwards JF, Roussel AJ, Thomson TD. Effect of monensin sodium on histological lesions of naturally occurring bovine paratuberculosis. J Comp Pathol 2000; 123:22 - 28
- Hendrick SH, Kelton DF, Leslie KE, Lissemore KD, Archambault M, Bagg R, et al. Efficacy of monensin sodium for the reduction of fecal shedding of Mycobacterium avium subsp. paratuberculosis in infected dairy cattle. Prev Vet Med 2006; 75:206 - 220